Back to Journals » International Journal of General Medicine » Volume 16
Prognostic Factors of Adrenocortical Carcinoma: Experience from a Regional Medical Center in Eastern China
Authors Li P, Su X, Zhang X, Sun L, Zhang G
Received 28 November 2022
Accepted for publication 24 January 2023
Published 3 February 2023 Volume 2023:16 Pages 453—465
DOI https://doi.org/10.2147/IJGM.S399473
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Peng Li,1 Xiaonan Su,2 Xiaotong Zhang,3 Lijiang Sun,1 Guiming Zhang1
1Department of Urology, The Affiliated Hospital of Qingdao University, Qingdao, 266003, People’s Republic of China; 2Department of Urology, Zoucheng People’s Hospital, Jining, 273500, People’s Republic of China; 3Department of Vascular Surgery, Qingdao Eighth People’s Hospital, Qingdao, 266100, People’s Republic of China
Correspondence: Guiming Zhang, Email [email protected]
Objective: This study aimed to summarize and analyze the clinical and pathological features and prognostic risk factors of adrenocortical carcinoma (ACC).
Methods: We retrospectively analyzed clinical and pathological data and the prognoses of 39 adult ACC patients confirmed by pathologic diagnosis at the Affiliated Hospital of Qingdao University between August 2009 and October 2021. Kaplan–Meier curves and univariate and multivariate Cox regression models were used to analyze correlations between clinical and pathological parameters and prognosis. A nomogram prediction model was constructed for overall survival (OS) based on the independent prognostic factors and externally validated it with The Cancer Genome Atlas (TCGA) dataset.
Results: The mean age of the patient cohort was 53.87 ± 11.1 years (range: 29– 80 years), which included 17 men and 22 women. The 1-, 2-, and 5-year OS rates were 83.7%, 64.4%, and 59.8%, respectively; the recurrence-free survival (RFS) rates at the same time points were 76.1%, 45.8%, and 23.5%, respectively. Kaplan–Meier curves showed that patients with poor OS were associated with M1 stage (P = 0.008), late ENSAT stage (P = 0.017), presence of venous tumor thrombus (P = 0.015), Ki67 > 20% (P = 0.006), R1/R2 status (P = 0.018), and poorly differentiated tumors (P = 0.047). Patients with late ENSAT stage (P = 0.017), combined with venous tumor thrombus (P = 0.008), Ki67 > 20% (P = 0.022) were more likely to have tumor recurrence. However, age, gender, BMI, tumor diameter, clinical symptoms and postoperative treatment were not correlated with OS or RFS (P > 0.05). Univariate and multivariate COX analyses showed that Ki67 > 20% (P = 0.013) and R1/2 status (P = 0.040) were independent risk factors for OS, while only Ki67 > 20% (P = 0.032) was an independent risk factor for RFS. A nomogram for predicting OS was constructed based on the above factors, and the area under the receiver characteristic curve (ROC)-1, 3, and 5-year survival were 0.8, 0.825 and 0.902, respectively. The C-index of the predicted nomogram was 0.813 and a high C-index value of 0.846 could still be achieved in the external validation of TCGA.
Conclusion: ACC is a rare and deadly endocrine malignancy with a high rate of recurrence. High Ki67 index (> 20%) and R1/R2 resection status were independent risk factors for poor prognosis in ACC patients. A novel nomogram with a relatively good accuracy was established to assist clinicians in assessing the risk of OS in patients with ACC.
Keywords: adrenocortical carcinoma, clinical diagnosis, survival, recurrence, risk factors, prognosis, nomogram
Background
Adrenocortical carcinoma (ACC) is a rare and aggressive malignant tumor originating from adrenocortical cells with an annual incidence of approximately (0.7–2.0)/1,000,000.1,2 Diagnoses of ACC are primarily made on the basis of local symptoms caused by abnormal hormone secretion or mass enlargement, such as Cushing syndrome, hypertension, menopause, abdominal fullness, et al, as well as occasionally by physical examination. The prognosis of ACC is generally poor, with a median survival period of approximately 4 years.1 Radical surgical resection is an effective therapy for ACC,3 but more than 50% of patients experience recurrence, even after complete R0 resection.4 For these patients, there are a number of options for subsequent treatment including salvage surgery, mitotane therapy, chemotherapy, radiotherapy, immunotherapy, and targeted therapy.5–8 However, these adjuvant therapies have shown limited impact. In this study, we analyzed the clinical, pathological, postoperative treatment, and follow-up data of 39 adult ACC patients to provide references for clinical early diagnosis, selection of operation technique, postoperative treatment, and prognostic assessment of ACC patients.
Methods
Patient Cohort and Data Collection
We retrospectively analyzed clinical data of patients with a pathologically confirmed diagnosis of ACC who presented to the Affiliated Hospital of Qingdao University between August 2009 and October 2021. Clinical data were collected from the Hospital Information System including gender, age, clinical manifestations, past medical history, pre- and post-operative laboratory and radiological findings, surgical method, postoperative pathology results, pathological stage, and postoperative treatment modalities. Additionally, prognostic information was collected by reviewing the medical records and telephone follow-up. The date of the last follow-up was January 25, 2022. Overall survival (OS) was defined as time from primary resection to death of any cause or the last follow‐up. Recurrence-free survival (RFS) was defined as the time from surgery to the first recurrence (ie, local recurrence or distant metastases) on the basis of evidence obtained through clinical examination, radiographic analysis, and laboratory findings, death or the last follow-up. The clinical information of ACC for external validation was downloaded from The Cancer Genome Atlas (TCGA).
Tumor, Node, Metastasis (TNM) Staging and Diagnostic Criteria
Currently, the most commonly used staging system for ACC is the European Network for the Study of Adrenal (ENSAT) system, which has replaced the Union for International Cancer Control (UICC) system. Both are based on the established TNM classification system proposed by the UICC and the American Joint Committee on Cancer (AJCC).9–11 Table 1 shows the ENSAT and UICC staging systems.
 |
Table 1 Multiple Staging Systems of Adrenocortical Carcinoma |
R Status
R status depended on surgical notes and pathological analysis: R0, complete resection; R1, microscopically positive margin; R2, macroscopically positive margin; Rx, resection status unknown.
Statistical Analysis
Data were analyzed using SPSS version 25.0 software (IBM Corp., Armonk, NY, USA) and R version 4.2.2 software. Measurement data are expressed as mean ± standard deviation (SD), and count data are expressed as numbers (%). Survival analysis was performed using the Kaplan–Meier method and Log rank tests, with median survival times and 95% confidence intervals (95% CIs) reported. Receiver operating characteristic (ROC) curves were plotted to measure the sensitivity and specificity of the observation indicators for predicting prognosis and determining cut-off points.
The optimum cut-off points were calculated with the maximum of the Youden index (Youden index = sensitivity + specificity − 1). Patients were divided into two groups: those above and those below the cut-points. Univariate and multivariate Cox regression analysis were performed to determine the significant risk factors for OS and RFS. Gender, age, BMI, tumor size, tumor stage, venous tumor thrombus, clinical symptoms, hormone secretion, surgical method, Ki67 status, surgical resection margins (R status), degree of tumor differentiation, and postoperative adjuvant therapy were entered into the univariate Cox proportional hazards regression analysis. Variables with P < 0.1 in the univariate Cox regression analysis were included in the multivariable Cox model. Independent risk factors affecting OS were identified and a prediction nomogram model was developed. The ROC was plotted, and the predictive effect of the model was assessed by C-index and Area Under Curve (AUC). External validation was performed in TCGA. P < 0.05 was regarded as statistically significant.
Results
General Information and Clinical Data
This study collected clinical data of 39 adult patients with ACC, including 17 men (43.6%) and 22 women (56.4%). The age range of the patients was from 29 to 80 years, and the mean age was 53.87 ± 11.1 years. Among the 39 patients, tumors were found incidentally on physical examination in 17 cases, 15 presented with tumor-related symptoms, five presented with hormone-related symptoms, and two presented for unknown reasons. Twelve patients were found to have abnormal hormone secretion during preoperative laboratory tests: five showed abnormal cortisol secretion, four showed abnormal sex hormone secretion, and three showed abnormal aldosterone secretion. All patients with hormone-related symptoms were found to have abnormal hormone secretion by laboratory examination. However, compared with symptomatic patients, patients with incidentalomas rarely showed abnormal hormone secretion. The mean diameter of the ACC tumors was 10.14 ± 4.73 cm. Distant metastases occurred in seven patients, of whom five had metastases to the lung, four to the liver, two to the bone, two to the cervical lymph node, and one to the oral mucosal. Among the 39 patients, 20 underwent laparoscopic surgery, 14 underwent open surgery, and five underwent puncture pathological biopsy. All the patients who did not receive surgical treatment were diagnosed with multiple metastases at the time of inclusion. Of the 10 patients complicated with venous tumor thrombus, seven successfully underwent open surgery for tumor resection and removal of the inferior vena cava tumor thrombus (three were not treated surgically). Only seven patients underwent adjuvant therapy with mitotane, with other postoperative adjuvant therapies including six patients who received chemotherapy, three who received targeted therapy, one who received immunotherapy, one who received radiotherapy, two who received interventional embolization therapy for pulmonary metastatic cancer, and one who received particle implantation.
Survival Analysis
The median follow-up time was 39.5 months. The 1-, 2-, and 5-year OS rates were 83.7%, 64.4%, and 59.8%, respectively. The 1-, 2-, and 5-year RFS rates were 76.1%, 45.8%, and 23.5%, respectively. Clinical and pathological data of the patients are presented in Table 2.
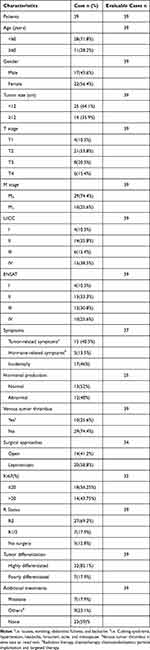 |
Table 2 Clinicopathological Characteristics of Adrenocortical Carcinoma Patients |
We used Log rank tests and Kaplan–Meier survival curves to detect differences in survival for each parameter presented in Table 2. Figure 1 shows Kaplan–Meier curves for M1 stage (P = 0.08), late ENSAT stage (P = 0.017), cases with tumor thrombus (P = 0.015), Ki67 >20% (P = 0.006), R1/2 status (P = 0.018), and poorly differentiated tumors (P = 0.047), which were all associated with worse OS, with median survival times of 17, 26, 12.5, 17, 42.5, and 13.5 months, respectively. These median OS durations were significantly lower than the median OS of all included ACC patients (65 months).
Figure 2 shows Kaplan–Meier curves for late ENSAT stage (P = 0.017), cases with tumor thrombus (P = 0.008), and Ki67 >20% (P = 0.022), which were all associated with worse RFS. Moreover, the median RFS for ENSAT stages I–IV was 62, 33, 10, and 7 months, respectively.
 |
Figure 2 Kaplan–Meier curve of recurrence-free survival for ENSAT stage (A), cases with tumor thrombus (B), Ki67 index (C) and gender (D). |
Cox Regression Analyses
The univariate Cox regression analyses individually examined the impact of each parameter on OS (Table 2). Decreased OS was significantly associated with late M stage (hazard ratio [HR]: 3.954, 95% CI: 1.309–11.942, P = 0.015), ENSAT stage III/IV (HR: 5.036, 95% CI: 1.384–18.322, P = 0.014), cases with tumor thrombus (HR: 3.814, 95% CI: 1.189–12.235, P = 0.024), Ki67 >20% (HR: 5.485, 95% CI: 1.426–21.101, P = 0.013), and R1/2 status (HR: 2.000, 95% CI: 1.075–3.723, P = 0.029) in the univariate analyses, so these were then taken as significant factors (P < 0.05) into the following multivariate analysis, which revealed that Ki67 >20% (HR: 7.490, 95% CI: 1.518–36.947, P = 0.013) and R1/2 status (HR: 3.793, 95% CI: 1.015–13.956, P = 0.040) were independent risk factors for OS (Table 3).
 |
Table 3 Univariate and Multivariate Cox Analysis of Clinicopathological Parameters for OS |
Table 4 shows that decreased RFS was significantly associated with ENSAT stage III/IV (HR: 2.874, 95% CI: 1.155–7.154, P = 0.023), cases with tumor thrombus (HR: 3.335, 95% CI: 1.292–8.609, P = 0.013), and Ki67 >20% (HR: 2.840, 95% CI: 1.121–7.195, P = 0.028) in the univariate analysis. Multivariate analysis subsequently revealed that only Ki67 >20% (HR: 2.952, 95% CI: 1.100–7.921, P = 0.032) was an independent risk factor for RFS.
 |
Table 4 Univariate and Multivariate Cox Analysis of Clinicopathological Parameter for RFS |
We constructed a nomogram to predict OS based on the significant factors in the multivariate analysis (Figure 3). The ROCs of 1, 3, and 5-year OS were plotted, and the AUC was 0.8, 0.825 and 0.902, respectively (Figure 4). The C-index of this predicted nomogram was 0.813 and a high C-index value of 0.846 could still be achieved in the external validation of TCGA.
 |
Figure 3 Nomogram-predicted OS of ACC. |
 |
Figure 4 ROC curves evaluated the predictive value of this nomogram in 1-year, 3-year, and 5-year OS. |
Discussion
ACC is a highly aggressive and malignant epithelial tumor that shows rapid clinical progression. In most countries with modern medical systems, the diagnosis rate of ACC is low and there is a lack of centralized reporting. Compared with other common cancers, there are fewer studies and less research data related to ACC. In China, ACC has primarily been reported via case reports and literature reviews. Therefore, obtaining and analyzing clinical and pathological characteristics of ACC patients still has a certain guiding significance for clarifying diagnoses, disease assessments, patient management, surgical plan selection, postoperative treatments, and assessments of prognostic risk.
Among the 39 adult ACC patients included in this study, the mean age was 53.87 years and the male/female ratio was 1:1.3; this age and sex distribution are consistent with those reported in previous studies.11–13 Due to the low incidence of ACC and lack of experience, and only 5 patients visited for abnormal hormonal symptoms, so many patients did not undergo relevant hormone detection before surgery. Among the 39 patients preoperative hormone assays were completed in 25. Of these, 48% (12/25) had abnormal hormone secretion, including abnormal sex hormone secretion (16%, 4/25), abnormal cortisol secretion (20%, 5/25), and abnormal aldosterone secretion (12%, 3/25), which was consistent with the report by Ayala-Ramirez et al.13 However, Berruti et al reported a lower proportion of ACC tumors with endocrine function (20–30%) than in this study. Furthermore, they found that patients with preoperative symptoms of hypercortisolism had lower RFS and OS even after complete resection of the lesion, suggesting that excess cortisol may be associated with more aggressive ACC.14 However, abnormal hormone secretion was not found to affect prognosis in this study.
The preferred treatment for ACC patients with indications for surgical resection is radical surgery, especially for ENSAT stage I–III patients without extensive metastasis.15 Open adrenalectomy (OA) was recommended as the standard operation in the 2020 Edition of Chinese Guidelines for the Diagnosis and Treatment of Urology and Andrology Diseases. Laparoscopic adrenalectomy (LA) can be selected according to the specific situation of the tumor.9 For this skilled technique, it is recommended to select early cases with tumor diameter <6 cm and no surrounding tissue invasion, but this may lead to a high postoperative recurrence rate (40%).16,17 LA is not recommended for stage III or higher cases with suspected involvement of peripheral tissue or lymph nodes.9
In this study, most of the patients who underwent LA had T2 stage disease (13/20), with a maximum tumor diameter of 16 cm, and none were complicated with venous tumor thrombus. Among the 14 OA patients, 50% (7/14) were associated with venous tumor thrombus, which needed to be removed concomitantly; the other five patients had tumors greater than 10 cm in diameter; the remaining two patients included a patient diagnosed with ACC in the pathology department of our hospital after open surgery at a lower-level hospital and a patient whose tumor invaded surrounding tissues. Most patients with stage T2 disease with no surrounding tissue or lymph node invasion had no difficulty receiving LA at our hospital, which is also consistent with the surgical recommendations in the guideline. In a small single-center study by Donatini et al, LA did not affect the prognosis of ACC patients with EASAT stage I/II disease or of patients with tumors less than 10 cm in diameter.18 A large multicenter study in Italy of 156 ACC patients with ENSAT stage I/II disease also found no significant difference in 5-year disease free survival (DFS) or OS between patients who received LA and OA.19 In this study, Kaplan–Meier survival analysis showed a better OS for patients who received LA than for patients who received OA, but there was no statistically significant difference between the two groups(Figure 1H). Therefore, we consider that the efficacy of LA may not be inferior to that of OA. However, compared with OA, patients who received LA had a relatively smaller tumor volume but a significantly higher incidence of tumor dissemination and/or positive margin and local recurrence or peritoneal recurrence.20 Cooper et al also confirmed that LA was associated with an increased risk of ACC recurrence in a retrospective study of 302 patients; their multivariate analysis also demonstrated that LA was significantly associated with shorter RFS and OS compared with OA.21 Thus, overall, the prognosis of LA surgery at our institution is not worse than OA, which may be due to technological advances (eg, the da Vinci robot) and adequate preoperative evaluation.
Currently, most ACC patients are diagnosed at advanced stages, and current treatments do not provide effective outcomes.22 Even after complete tumor resection, the median survival time is less than 12 months.23,24 In this study, the median survival time of stage IV patients was 17 months. Additionally, the high recurrence rate of ACC is a noteworthy problem. In advanced and recurrent cases of distant metastases, adjuvant therapy such as targeted therapy, chemotherapy, and radiotherapy may be necessary. However, the limited chemotherapy options and efficacy as well as the poor understanding of the efficacy and safety of radiotherapy increase the difficulty of treating ACC patients.25,26 According to the United States Food and Drug Administration and the European Medicines Agency, Mitotane is the only approved treatment for advanced ACC. It has been reported that mitotane prolongs OS and RFS in surgically treated patients, and it can also be used to treat inoperable patients or patients with extensive metastasis after surgery. Nevertheless, only seven patients postoperatively received mitotane in this study, which may be due to the fact that mitotane has not yet been marketed in China and there is limited experience with its use for ACC treatment in China. Moreover, we did not find that oral mitotane significantly improved patient prognosis, which may be related to our limited case numbers.
For patients with aggressive tumor features who are capable of tolerating systemic chemotherapy, the four-drug regimen of etoposide, doxorubicin, and cisplatin with mitotane (EDP-M) has been shown to be superior to the alternative regimen of streptozocin-mitotane.27,28 Notably, the mean survival, even with EDP-M, was still dismal, underscoring the limitations of the gold standard systemic treatment and the importance of finding other therapeutic options when possible, such as sequential surgical resection, targeted ablation, and radiotherapy. Among the surgical patients included in this study, only five received adjuvant chemotherapy for postoperative tumor recurrence and metastasis, and seven received other adjuvant treatments such as radiotherapy, chemoembolization, particle implantation, and targeted therapy. Therefore, this study does not have much information about postoperative adjuvant therapy in ACC, and there remains a lack of experience with these modalities in this field.
The ENSAT staging system was proposed by Fassnacht et al in 2009 and is based on a retrospective study of 492 ACC patients in Germany with a mean follow-up of 36 months.29 Following validation in 573 North American subjects, it was found to be more accurate at predicting survival and recurrence than the UICC system. Similarly, our study found a significant association between the ENSAT staging system and OS (P = 0.014) and RFS (P = 0.023) in COX univariate analysis. Furthermore, our Kaplan–Meier survival analysis showed that the EASNT staging system provided a good prognostic stratification for OS (P = 0.017), while the differences in Kaplan–Meier curves drawn on the basis of the UICC staging system among different stages were not statistically significant.
In a study involving 569 stage I–III ACC patients, Bueschlein et al identified Ki-67 as the single most effective predictor of RFS and OS.14 In a recent single-center retrospective study by Else et al,30 multivariate analysis revealed that age at diagnosis, increased cortisol secretion, and high tumor stage and grade were significantly associated with decreased OS. The grade, R status, age, symptoms (GRAS) score classification was recently reported to be significantly associated with ACC prognosis in a study involving data from ACC patients from the United States. High GRAS scores were also associated with OS and DFS in postoperative patients according to a study by Jordan et al.31 A study of 444 patients with stages III/IV ACC showed that the modified ENSAT (mENSAT) staging and GRAS parameters were the best methods for stratifying the prognosis of patients with advanced ACC.32 A cohort study of 65 ACC patients conducted by at the West China Hospital of Sichuan University in 2019 also showed that symptoms, higher tumor grade, and positive/unknown R-resection status were independent risk factors for stage I–III ACC patients.33 Our multivariate regression analysis that Ki67 >20% and residual tumor (R1/2 status) were independent risk factors for shorter OS; Ki67 >20% was also an independent risk factor for shorter RFS, which was consistent with previous studies. Our prediction nomogram based on Ki67, R status and venous cancer thrombus had high C-index and AUC values, and high C-index was also obtained in the external validation of TCGA, which ensured that the nomogram established in this study was more accurate and credible. Moreover, Kaplan–Meier survival analysis of our cohort showed that M1 stage (P = 0.008), late ENSAT stage (P = 0.017), cases with venous tumor thrombus (P = 0.015) and poorly differentiated tumors (P = 0.047) were associated with worse OS; furthermore, late ENSAT stage (P = 0.017) accompanied with tumor thrombus (P = 0.008) was associated with worse RFS.
In a French study of 253 patients, the 5-year OS rate of the entire cohort was 38%, and stage I, II, III, and IV patients showed 5-year OS rates of 66%, 58%, 24%, and 0,34 respectively. These data from our study were 100%, 75.5%, 50.9%, and 25.9%, respectively. A previous study of 3982 ACC patients found that among all patients who underwent surgical resection, the 5-year OS rate was 38.6% (median survival: 31.9 months), which was lower than the 59.8% in this study. The better overall prognosis of the patients in this study may be related to the small number of patients and selection bias. Additionally, Kaplan–Meier survival analysis showed that the OS and RFS of female patients were lower than those of men (Figures 1G and 2D), suggesting that gender might be a risk factor affecting prognostic stratification, but the differences between men and women were not statistically significant (P = 0.115, P = 0.097). Further verification in large samples is expected in future studies.
Conclusions
ACC is a rare and deadly endocrine malignancy with a high rate of recurrence. Radical surgery with complete tumor resection can prolong patient survival. Ki67 index >20% and R1/R2 resection status are independent risk factors for adverse prognosis in ACC patients. A novel nomogram with a relatively good accuracy was established to assist clinicians in assessing the risk of OS in patients with ACC.
Ethics Approval and Consent to Participate
This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and approved by the ethics committee at The Affiliated Hospital of Qingdao University. All patients involved in the present study provided signed informed consent.
Acknowledgments
We thank James P. Mahaffey, PhD, from Liwen Bianji (Edanz) for editing the English text of a draft of this manuscript.
Funding
This study was partly funded by the Natural Science Foundation of Shandong Province (ZR2021MH354), Medical and health research program of Qingdao (2021-WJZD170).
Disclosure
The authors declare no competing interest exists for this work.
References
1. Else T, Kim AC, Sabolch A, et al. Adrenocortical carcinoma. Endocr Rev. 2014;35(2):282–326. doi:10.1210/er.2013-1029
2. Kerkhofs TM, Verhoeven RH, Van der Zwan JM, et al. Adrenocortical carcinoma: a population-based study on incidence and survival in the Netherlands since 1993. Eur J Cancer. 2013;49(11):2579–2586. doi:10.1016/j.ejca.2013.02.034
3. Asare EA, Wang TS, Winchester DP, et al. A novel staging system for adrenocortical carcinoma better predicts survival in patients with stage I/II disease. Surgery. 2014;156(6):1378–1385. doi:10.1016/j.surg.2014.08.018
4. Puglisi S, Perotti P, Cosentini D, et al. Decision-making for adrenocortical carcinoma: surgical, systemic, and endocrine management options. Expert Rev Anticancer Ther. 2018;18(11):1125–1133. doi:10.1080/14737140.2018.1510325
5. Terzolo M, Berruti A. Adjunctive treatment of adrenocortical carcinoma. Curr Opin Endocrinol Diabetes Obes. 2008;15(3):221–226. doi:10.1097/MED.0b013e3282fdf4c0
6. Fassnacht M, Terzolo M, Allolio B, et al. Combination chemotherapy in advanced adrenocortical carcinoma. N Engl J Med. 2012;366(23):2189–2197. doi:10.1056/NEJMoa1200966
7. Alyateem G, Nilubol N. Current status and future targeted therapy in adrenocortical cancer. Front Endocrinol. 2021;12:613248. doi:10.3389/fendo.2021.613248
8. Erdogan I, Deutschbein T, Jurowich C, et al. The role of surgery in the management of recurrent adrenocortical carcinoma. J Clin Endocrinol Metab. 2013;98(1):181–191. doi:10.1210/jc.2012-2559
9. McDuffie LA, Aufforth RD. Adrenocortical carcinoma: modern management and evolving treatment strategies. Int J Endocr Oncol. 2016;3(2):161–174. doi:10.2217/ije-2015-0003
10. Gaujoux S, Mihai R, Carnaille B. European Society of Endocrine Surgeons (ESES) and European Network for the Study of Adrenal Tumours (ENSAT) recommendations for the surgical management of adrenocortical carcinoma. Br J Surg. 2017;104(4):358–376. doi:10.1002/bjs.10414
11. Shah MH, Goldner WS, Halfdanarson TR, et al. NCCN guidelines insights: neuroendocrine and adrenal tumors, version 2.2018. J Natl Compr Canc Netw. 2018;16(6):693–702. doi:10.6004/jnccn.2018.0056
12. Ayala-Ramirez M, Jasim S, Feng L, et al. Adrenocortical carcinoma: clinical outcomes and prognosis of 330 patients at a tertiary care center. Eur J Endocrinol. 2013;169(6):891–899. doi:10.1530/EJE-13-0519
13. Şişman P, Şahin AB, Peynirci H, et al. Adrenocortical carcinoma: single center experience. Turk J Urol. 2017;43(4):462–469. doi:10.5152/tud.2017.81598
14. Berruti A, Fassnacht M, Haak H, et al. Prognostic role of overt hypercortisolism in completely operated patients with adrenocortical cancer. Eur Urol. 2014;65(4):832–838. doi:10.1016/j.eururo.2013.11.006
15. Amini N, Margonis GA, Kim Y, et al. Curative resection of adrenocortical carcinoma: rates and patterns of postoperative recurrence. Ann Surg Oncol. 2016;23(1):126–133. doi:10.1245/s10434-015-4810-y
16. Cobb WS, Kercher KW, Sing RF, Heniford BT. Laparoscopic adrenalectomy for malignancy. Am J Surg. 2005;189(4):405–411. doi:10.1016/j.amjsurg.2005.01.021
17. Gonzalez RJ, Shapiro S, Sarlis N, et al. Laparoscopic resection of adrenal cortical carcinoma: a cautionary note. Surgery. 2005;138(6):1078–1085. doi:10.1016/j.surg.2005.09.012
18. Donatini G, Caiazzo R, Do Cao C, et al. Long-term survival after adrenalectomy for stage I/II adrenocortical carcinoma (ACC): a retrospective comparative cohort study of laparoscopic versus open approach. Ann Surg Oncol. 2014;21(1):284–291. doi:10.1245/s10434-013-3164-6
19. Lombardi CP, Raffaelli M, De Crea C, et al. Open versus endoscopic adrenalectomy in the treatment of localized (stage I/II) adrenocortical carcinoma: results of a multiinstitutional Italian survey. Surgery. 2012;152(6):1158–1164. doi:10.1016/j.surg.2012.08.014
20. Miller BS, Ammori JB, Gauger PG, et al. Laparoscopic resection is inappropriate in patients with known or suspected adrenocortical carcinoma. World J Surg. 2010;34(6):1380–1385. doi:10.1007/s00268-010-0532-2
21. Miller BS, Gauger PG, Hammer GD, Doherty GM. Resection of adrenocortical carcinoma is less complete and local recurrence occurs sooner and more often after laparoscopic adrenalectomy than after open adrenalectomy. Surgery. 2012;152(6):1150–1157. doi:10.1016/j.surg.2012.08.024
22. Kerkhofs TM, Ettaieb MH, Hermsen IG, Haak HR. Developing treatment for adrenocortical carcinoma. Endocr Relat Cancer. 2015;22(6):R325–338. doi:10.1530/ERC-15-0318
23. Sidhu S, Sywak M, Robinson B, Delbridge L. Adrenocortical cancer: recent clinical and molecular advances. Curr Opin Oncol. 2004;16(1):13–18. doi:10.1097/00001622-200401000-00004
24. Kebebew E, Reiff E, Duh QY, Clark OH, McMillan A. Extent of disease at presentation and outcome for adrenocortical carcinoma: have we made progress? World J Surg. 2006;30(5):872–878. doi:10.1007/s00268-005-0329-x
25. Hermsen IG, Haak HR, de Krijger RR, et al. Mutational analyses of epidermal growth factor receptor and downstream pathways in adrenocortical carcinoma. Eur J Endocrinol. 2013;169(1):51–58. doi:10.1530/EJE-13-0093
26. Hermsen IG, Groenen YE, Dercksen MW, Theuws J, Haak HR. Response to radiation therapy in adrenocortical carcinoma. J Endocrinol Invest. 2010;33(10):712–714. doi:10.1007/BF03346675
27. Schulick RD, Brennan MF. Long-term survival after complete resection and repeat resection in patients with adrenocortical carcinoma. Ann Surg Oncol. 1999;6(8):719–726. doi:10.1007/s10434-999-0719-7
28. Vaidya A, Nehs M, Kilbridge K. Treatment of adrenocortical carcinoma. Surg Pathol Clin. 2019;12(4):997–1006. doi:10.1016/j.path.2019.08.010
29. Fassnacht M, Johanssen S, Quinkler M, et al. Limited prognostic value of the 2004 international union against cancer staging classification for adrenocortical carcinoma: proposal for a revised TNM classification. Cancer. 2009;115(2):243–250. doi:10.1002/cncr.24030
30. Else T, Williams AR, Sabolch A, et al. Adjuvant therapies and patient and tumor characteristics associated with survival of adult patients with adrenocortical carcinoma. J Clin Endocrinol Metab. 2014;99(2):455–461. doi:10.1210/jc.2013-2856
31. Baechle JJ, Marincola Smith P, Solórzano CC, et al. Cumulative GRAS score as a predictor of survival after resection for adrenocortical carcinoma: analysis from the U.S. adrenocortical carcinoma database. Ann Surg Oncol. 2021;28(11):6551–6561. doi:10.1245/s10434-020-09562-8
32. Libé R, Borget I, Ronchi CL, et al. Prognostic factors in stage III-IV adrenocortical carcinomas (ACC): an European Network for the Study of Adrenal Tumor (ENSAT) study. Ann Oncol. 2015;26(10):2119–2125. doi:10.1093/annonc/mdv329
33. Liang J, Liu Z, Zhou L, et al. The clinical utility of ‘GRAS’ parameters in stage I-III adrenocortical carcinomas: long-term data from a high-volume institution. Endocrine. 2020;67(2):449–456. doi:10.1007/s12020-019-02141-2
34. Icard P, Goudet P, Charpenay C, et al. Adrenocortical carcinomas: surgical trends and results of a 253-patient series from the French Association Of Endocrine Surgeons study group. World J Surg. 2001;25(7):891–897. doi:10.1007/s00268-001-0047-y
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

