Back to Journals » Journal of Inflammation Research » Volume 14
Prognostic and Predictive Value of Circulating Inflammation Signature in Non-Metastatic Nasopharyngeal Carcinoma: Potential Role for Individualized Induction Chemotherapy
Authors Lv SH, Li WZ , Liang H, Liu GY, Xia WX, Xiang YQ
Received 7 March 2021
Accepted for publication 29 April 2021
Published 25 May 2021 Volume 2021:14 Pages 2225—2237
DOI https://doi.org/10.2147/JIR.S310017
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Monika Sharma
Shu-Hui Lv,1– 3,* Wang-Zhong Li,1,2,* Hu Liang,1,2 Guo-Ying Liu,1,2 Wei-Xiong Xia,1,2 Yan-Qun Xiang1,2
1State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangdong Key Laboratory of Nasopharyngeal Carcinoma Diagnosis and Therapy, Sun Yat-Sen University Cancer Center, Guangzhou, China; 2Department of Nasopharyngeal Carcinoma, Sun Yat-Sen University Cancer Center, Guangzhou, China; 3Medical Affairs Office, The Fifth Affiliated Hospital of Sun Yat-Sen University, Guangzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yan-Qun Xiang; Wei-Xiong Xia
Department of Nasopharyngeal Carcinoma, Sun Yat-Sen University Cancer Center, Guangzhou, 510060, China
Tel\Fax +86-1866-6096-623 Email [email protected]; [email protected]
Purpose: We sought to assess the prognostic and predictive value of a circulating inflammation signature (CISIG) and develop CISIG-based tools for predicting prognosis and guiding individualized induction chemotherapy (ICT) in non-metastatic nasopharyngeal carcinoma (NPC).
Patients and Methods: We retrospectively collected a candidate inflammatory biomarker panel from patients with NPC treated with definitive radiotherapy between 2012 and 2017. We developed the CISIG using candidate biomarkers identified by a least absolute shrinkage and selection operator (LASSO) Cox regression model. The Cox regression analyses were used to evaluate the CISIG prognostic value. A CISIG-based prediction model was constructed, validated, and assessed. Potential stratified ICT treatment effects were examined.
Results: A total of 1149 patients were analyzed. Nine biomarkers selected by LASSO regression in the training cohort were used to construct the CISIG, including hyaluronidase, laminin, procollagen III, neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, lymphocyte-to-monocyte ratio, high-density lipoprotein, lactate dehydrogenase, and C-reactive protein-to-albumin ratio. CISIG was an independent prognostic factor for disease-free survival (DFS; hazard ratio: 2.65, 95% confidence interval: 1.93– 3.64; P < 0.001). High CISIG group (>− 0.2) was associated with worse 3-year DFS than low CISIG group in both the training (67.5% vs 88.3%, P < 0.001) and validation cohorts (72.3% vs 85.1%, P < 0.001). We constructed and validated a CISIG-based nomogram, which showed better performance than the clinical stage and Epstein–Barr virus DNA classification methods. A significant interaction between CISIG and the ICT treatment effect was observed (P for interaction = 0.036). Patients with high CISIG values did not benefit from ICT, whereas patients with low CISIG values significantly benefited from ICT.
Conclusion: The developed CISIG, based on a circulating inflammatory biomarker panel, adds prognostic information for patients with NPC. The proposed CISIG-based tools offer individualized risk estimation to facilitate suitable ICT candidate identification.
Keywords: nasopharyngeal carcinoma, inflammatory biomarkers, circulating inflammation signature, prognostic value, predictive value, induction chemotherapy
Introduction
Nasopharyngeal carcinoma (NPC) is a malignancy arising from the nasopharyngeal epithelia.1 The past decades have witnessed remarkable progress in imaging modalities and radiotherapy techniques. Non-metastatic NPC has become a highly curable disease, especially in patients with early-stage NPC. However, 70–80% of NPC patients are not diagnosed until they have progressed to advanced disease stages.2 In the era of intensity-modulated radiotherapy (IMRT), induction chemotherapy (ICT) combined with concurrent chemoradiotherapy (CCRT) has emerged as the standard treatment strategy for patients with advanced-stage NPC.3–5
Current clinical decision-making for NPC is determined primarily according to the anatomical-based tumor node metastasis (TNM) stage.6 However, this strategy is insufficient for predicting survival outcomes or treatment benefits and cannot reflect the cancer properties that drive biological aggressiveness and metastatic potential.1 Patients with identical TNM stages who are treated with the same standard care could present heterogeneous survival outcomes. Identifying those patients who are most suitable for intensified ICT would allow for the early modification of treatment strategies, which is vital for maximizing the curative benefits, minimizing therapeutic toxicities,7,8 and represents a crucial approach for promoting the goal of precision oncology. We are in urgent need of effective tools that can identify which patients would most likely benefit from additional ICT.
Cancer is typically recognized as an inflammatory disease.9 Cancer-related inflammation biomarkers serve as hallmark features of cancer initiation and progression.10–13 In addition to plasma Epstein Barr virus (EBV) DNA, many circulating inflammatory biomarkers have been identified as significant prognostic factors, independent of current clinicopathological NPC characteristics, such as lactic dehydrogenase (LDH), the C-reactive protein (CRP)/albumin (ALB) ratio, and the neutrophil-to-lymphocyte ratio (NLR).14–22 These biomarkers are commonly used to predict prognosis and improve the performance of predictive NPC models. The limitation of these studies was that they typically investigated only one or a few biomarkers at a time. Insufficient sample sizes and the application of different cutoff values across these studies may result in inconsistent results. Although various parameters can be used to measure systemic inflammation, no study has investigated these biomarkers in aggregate or developed models to incorporate multiple biomarker findings into clinical decision-making processes. Previous studies conducted in NPC have demonstrated that converting multiple biomarkers into a single prognostic signature can improve prediction performance compared to individual biomarker analyses.23–26
In this study, we sought to evaluate the prognostic and predictive value of a circulating inflammation signature (CISIG), determined using a panel of inflammation-related biomarkers. We also utilized the CISIG to develop a potential prognostic model for predicting disease-free survival (DFS) in patients with non-metastatic NPC. Finally, we investigated the potential role of the CISIG to guide the application of individualized ICT.
Methods
Study Participants
The participants included in this study were patients with histologically confirmed NPC who were treated with definitive radiotherapy using the IMRT technique between 2012 and 2017. We excluded patients with metastatic disease at presentation and patients with previous or synchronous malignant tumors. Patient data regarding demographics, clinicopathological characteristics, and treatment information were extracted from the digital medical database. This retrospective cohort study was conducted in accordance with the Declaration of Helsinki. We obtained approval from the Institutional Ethics Committee of Sun Yat-Sen University Cancer Center. Informed consent was waived due to this study’s retrospective nature and the anonymized processing of patient data.
Laboratory Measurements
Baseline hematological and biochemical tests were measured with fasting blood samples in all patients before the initiation of any treatments. Routine biochemical measures were performed using an automated immunoturbidimetric analyzer 7600–020 (Hitachi High-Technologies, Tokyo, Japan). Blood cell counting and classification were detected using the fully automated hematology analyzer Sysmex XE-5000 (Sysmex, Kobe, Japan). The circulating EBV DNA concentrations were measured using quantitative polymerase chain reaction (PCR). The classifications of EBV DNA were performed as described in a previous study.15 In this study, we focused on ten candidate circulating inflammation-related biomarkers, including hyaluronidase (HA), collagen IV (CIV), laminin (LN), procollagen III (PIIINP), NLR, platelet-to-lymphocyte ratio (PLR), lymphocyte-to-monocyte ratio (LMR), high-density lipoprotein (HDL), LDH, and the CRP/ALB ratio. These selected circulating inflammatory biomarkers have been extensively investigated in NPC or other types of cancers and are highly correlated with survival outcomes.14–22,27–31 These candidate biomarkers were cost-effective and easily measured.
Treatment and Outcome
The disease stage was categorized according to the American Joint Committee on Cancer TNM Manual (7th edition). All patients were treated with radical IMRT as the primary treatment. The prescribed radiation doses were 66–72 Gy for the primary tumor and 60–70 Gy for the involved cervical region, at fractions of 30 to 35. Most of the patients (91.5%) received platinum-based chemotherapy, including 579 (50.4%) patients who received ICT plus CCRT and 472 (41.1%) patients who received CCRT alone. Detailed information on treatment protocol or the treatment allocation was described in a previous publication.32 The primary endpoint of the current study was DFS. DFS was defined as the interval between diagnosis and disease progression or death due to any cause. Patients lost to follow-up were censored at the date of the last contact.
Statistical Analysis
Participants were randomly assigned to the training and validation cohorts, aiming for a crude ratio of 6:4, using the R package “caret.” Maximally selected rank statistics were used to determine the optimal cutoff values for inflammation-related biomarkers, using the function provided in R package “survminer.” The least absolute shrinkage and selection operator (LASSO) Cox regression, using 10-fold cross-validation as provided in the R package “glmnet,” was applied to select the most useful prognostic markers among the ten considered inflammation-related biomarkers in the training cohort. The selected prognostic markers and their respective LASSO regression coefficients were further used to construct the CISIG. We estimated survival probabilities using the Kaplan–Meier method. The prognostic value of CISIG and clinicopathologic factors were assessed using Cox proportional hazards models. Variables with P < 0.05 in the univariate analyses were entered into the further multivariate analysis. Forward-backward stepwise multivariate analyses were applied to explore independent predictors. The final multivariate model was selected based on the minimum Akaike Information Criterion (AIC) and used to construct the CISIG-based nomogram. The performance of the nomogram was assessed based on discrimination, calibration, and clinical usefulness. Discrimination was measured using the concordance index (C-index), calibration was assessed using the calibration curve, and clinical usefulness was evaluated by decision curves analysis (DCA).33 The bootstrap resampling method with 1000 repetitions was applied for internal validation. Another independent cohort was subsequently evaluated to validate the nomogram. To exclude underlying selection bias in the treatment assignments, a 1:1 ratio propensity score matching (PSM) paradigm was applied to the training and validation cohorts to derive balanced treatment cohorts (CCRT vs ICT + CCRT and limited to Stage III/IV; patients with radiotherapy alone were excluded). To examine the potential role played by the CISIG in guiding individualized ICT, we tested the interaction between CISIG and the ICT treatment effect. The stratified treatment effects of ICT were explored in the matched training and validation cohorts.
All statistical analyses and model productions were performed in R version 3.6.3 (R Foundation for statistical computing, Vienna, Austria). A two-tailed P < 0.05 was considered significant.
Results
Participant Characteristics
A schematic illustration of the study design is presented in Figure 1. In total, 1149 patients who received radical IMRT for non-metastatic NPC were included. The study population was randomized into training and validation cohorts. Patient characteristics for the training and validation cohorts are shown in Table 1. The median follow-up time of the study population was 47.5 months (interquartile range [IQR], 46.1–49.4 months). Overall, 134 (11.7%) patients died from all causes, and 261 (22.7%) patients experienced treatment failure. The 3-year DFS in the training and validation cohorts were 79.9% and 80.2%, respectively.
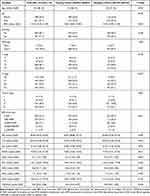 |
Table 1 Patient Characteristics |
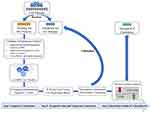 |
Figure 1 Schematic illustration of the study design. |
Construction of the CISIG
The optimal cutoffs for the examined inflammatory markers determined by maximally selected rank statistics and the DFS curves stratified by these cutoffs are presented in Figure S1a–j. The ten considered markers were reduced to the nine most useful prognostic markers using LASSO Cox regression, including HA, LN, PIIINP, NLR, PLR, LMR, HDL, LDH, and CRP/ALB (Figure 2A and B). The optimal cutoffs and respective LASSO regression coefficients of the selected markers are presented in Table S1.
Prognostic Value of CISIG
The distribution of CISIG in the training cohort is presented in Figure 3A. In the univariate Cox analysis, CISIG was identified as an unfavorable prognostic factor (hazard ratio [HR]: 3.08, 95% confidence interval [CI]: 2.27–4.19; P < 0.001; Table 2). An increase in the CISIG was associated with a decrease in the estimated 3-year and 5-year DFS probabilities (Figure 3B). We classified patients into low- or high-value CISIG groups using a cutoff point (−0.2) determined by the maximally selected rank statistics (Figure S2). Patients with high CISIG values were associated with unfavorable clinicopathological characteristics in both the training (Table S2) and validation (Table S3) cohorts. Kaplan–Meier analyses revealed that patients with high CISIG group were associated with worse 3-year DFS than those in the low CISIG group in both the training (67.5% vs 88.3%, P < 0.001, Figure 4A) and validation cohorts (72.3% vs 85.1%, P < 0.001, Figure 4B).
 |
Table 2 The Results of Univariate and Stepwise Multivariate Analyses in the Training Cohort |
 |
Figure 4 Crude survival curves, stratified by the circulating inflammation signature (CISIG) values in the training cohort (A) and validation cohort (B). |
Predictive Value of CISIG
Both CISIG and clinicopathological variables were considered during the development of the prediction model. The final model, which featured the minimal AIC value (1892.32) in the stepwise multivariate Cox regression analysis for the training cohort, was selected to construct a CISIG-based nomogram. The variables included in the nomogram were age, smoking status, histology, clinical stage, EBV DNA level, and CISIG value (Figure 5A). The nomogram showed good discrimination in the training cohort, with a C-index of 0.726 (95% CI: 0.693–0.779, Table 3), which was significantly higher than those for other traditional prognostic factors. The calibration curves plotted at the 1-, 3-, and 5-year time points showed good agreement between the nomogram-predicted DFS probability and the observed DFS rate (Figure 5B). DCA at the 3- and 5-year time points revealed that the developed nomogram conferred more significant benefits than both the treat-all-patients scheme and the treat-no-patients scheme if the threshold probabilities of a patient or physician ranged from 0.1 to 0.7 (Figure S3a and b). The independent validation cohort provided external confirmation that the developed nomogram featured good discrimination (C-index: 0.695; 95% CI: 0.640–0.750, Table 3), excellent calibration (Figure 5C), and clinical usefulness (Figure S3c and d).
 |
Table 3 C-Indices and Corresponding 95% CIs of All Prognostic Models |
Potential Role for Individualized ICT
The analysis of the potential role of CISIG in identifying patients who may benefit from ICT was limited to patients with Stage III or IV disease who received CCRT or ICT plus CCRT (n = 1010). To exclude potential underlying selection bias during treatment assignments, we applied PSM at a 1:1 ratio for both the training and validation cohorts to obtain balanced treatment cohorts (CCRT vs ICT + CCRT). The PSM procedure resulted in two well-balanced cohorts (Table S4 and S5). We found a significant interaction (P for interaction = 0.036) between CISIG and the ICT treatment effect in the matched population derived from the training cohort. We further stratified the treatment effects of ICT in the two matched cohorts and examined the associations with CISIG. Interestingly, in the PSM cohorts, CISIG was able to identify individuals with the potential to benefit from ICT. Patients with high CISIG values did not benefit from ICT (training cohort: P = 0.720; validation cohort: P = 0.660; Figure S4). By contrast, patients with low CISIG values significantly benefited from the ICT (training cohort: P = 0.020; validation cohort: P = 0.042).
Discussion
The accurate estimation of benefits derived from intensified ICT is crucial for decision-making when designing treatment strategies for patients with advanced-stage NPC. In the current study, which involved more than 1000 participants, we found that the CISIG value obtained from a panel of circulating inflammatory biomarkers correlated with NPC progression. Elevated CISIG was associated with unfavorable clinical characteristics and worse prognoses. We developed and validated a CISIG-based prediction model that can be used for the individual prediction of DFS in patients with NPC. The CISIG level can be used to distinguish a subpopulation of NPC patients that might benefit from additional ICT and might serve as a helpful tool that contributes to treatment decisions in daily clinical practice.
Plasma EBV DNA is the most widely used ctDNA marker in current clinical practice. Plasma EBV DNA has been shown to serve as an independent prognostic biomarker in NPC.1,34 The combination of EBV DNA levels with clinicopathological variables added more value to both prognostic prediction and clinical application.15,35 Evidence from the reported literature has shown that pre-treatment EBV DNA levels can be used to perform risk stratification and might help identify patients who might benefit from ICT.35–40 However, these studies failed to provide a consistent and accurate predictive estimation of the benefit derived from additional ICT when using EBV DNA as a stratification tool. This inconsistency may be due to several reasons. First, not all NPC patients have detectable plasma EBV DNA levels, and the proportion of EBV-related NPC varies with geographic location. In endemic regions, 17.2–29.3% of all newly diagnosed NPC patients have undetectable circulating EBV.41,42 Second, large interlaboratory variability in the detection of circulating EBV DNA is also a factor, even when using the same PCR assay and identical procedures without harmonization.43 Third, although pre-treatment plasma EBV DNA levels reflect the tumor burden and correlate with prognosis, the kinetics of circulating EBV DNA during chemoradiotherapy might add more prognostic value and identify distinct prognostic subtypes.34,44,45 A longitudinal baseline EBV DNA lacks the ability to reflect the response to treatment. These issues demonstrate the considerable need to identify additional biomarkers that can be used to predict prognosis or estimate treatment effects. In this study, the CISIG value was shown to be a promising prognostic factor, independent of a variety of known prognostic factors. CISIG showed more accuracy for the prediction of DFS probability than traditional prognostic factors. The integration of CISIG with traditional prognostic factors exhibited the potential for a more comprehensive assessment of tumor status, which could supplement traditional clinical stratification.
The association between cancer-related inflammation markers and poor survival outcomes has been well-studied in previous studies.14–22,46 Published biomarker-related studies have been designed specifically for the assessment of one or a few markers. However, estimates of the risk associated with specific inflammatory markers are not always equal. For example, NLR has been shown to be an independent prognostic factor in several studies;16,21,22 however, in a pooled analysis of two clinical trials, Chua and colleagues found that NLR failed to add prognostic value to conventional clinical variables when identifying patients with unfavorable prognoses.47 Similar phenomena have been observed for other inflammatory markers in various contexts, such as LMR, CRP, and LDH. These inconsistent results could be partially explained by heterogeneous study populations, the application of different cutoff values, and variations in sample sizes. The variability associated with the validation of specific biomarkers as independent prognostic factors and the similarity in the biological landscape of markers has raised great interest in the integration of multiple biomarkers into a single model. Compared with one or few markers, the integration of multiple markers into a single model improves prognostic value substantially.14,48–51 Our results were in line with the reported literature. In this study, we developed a CISIG based on a panel of inflammatory biomarkers, which was more potent than traditional models for the estimation of DFS in patients with NPC.
The mechanism of cancer-related inflammation-induced NPC progression is not clearly understood. Many basic scientists view cancer-related inflammation as a local immunoreaction observed at the tumor site, which usually precedes tumor formation and contributes to its progression.11 By contrast, clinicians regard cancer-related inflammation as a continuing and inappropriate systemic reaction to malignancy that leads to a series of paraneoplastic symptoms. In the present study, we demonstrated that the developed CISIG, which was based on various inflammatory biomarkers, could be used as a versatile predictor of NPC progression. The developed CISIG provides the potential to determine NPC aggressiveness and the likely responses to treatment. Notably, the developed CISIG could identify those patients who might benefit from the additional ICT. Patients with low CISIG values might represent the optimal ICT candidates.
Several limitations relevant to the interpretation of this study should be noted. First, although the results were internally validated, future collaborations to perform multicenter analyses and the external validation of these findings would strengthen the transparency and robustness of these associations. Second, systemic inflammation consists of a range of markers, including circulating cytokines, small inflammatory proteins, circulating immune cells, and acute-phase proteins.11 Not all these markers were considered in the construction of the CISIG in this study due to cost considerations. However, the selected inflammatory markers were prospectively collected and were identified as the most clinically relevant and low-cost biomarkers, which added to the validity of the study design. Third, the study population was derived from EBV endemic areas, where EBV-associated NPC and nonkeratinizing NPC are common.1 The generalization of our results to patients from low-risk regions should be applied with caution, and further study is warranted.
Conclusion
In conclusion, we demonstrated that the developed CISIG, based on a panel of circulating inflammatory biomarkers, adds prognostic information for patients with non-metastatic NPC. The proposed CISIG-based tools could offer individualized risk estimation that may help identify those patients who might benefit from intensified ICT.
Data Sharing Statement
All data generated or analyzed during the present study are available from the corresponding author upon reasonable request.
Ethics Approval and Informed Consent
This retrospective observational cohort study was performed in accordance with the Declaration of Helsinki. Approval was obtained from the Institutional Ethics Committee of Sun Yat-Sen University Cancer Center. Informed consent was waived due to this study’s retrospective nature and the anonymized processing of patient data.
Funding
This study is supported by the National Natural Science Foundation of China [No. 81672680 and 81802712], the Guangdong Medical Research Foundation [No. A2017492], and the Young Teacher Training Project of Sun Yat-Sen University [No. 19ykpy188].
Disclosure
The authors declare that they have no competing interests.
References
1. Chen Y, Chan A, Le Q, Blanchard P, Sun Y, Ma J. Nasopharyngeal carcinoma. Lancet (Lond). 2019;394(10192):64–80. doi:10.1016/S0140-6736(19)30956-0
2. Mao Y-P, Xie F-Y, Liu L, et al. Re-evaluation of 6th edition of AJCC staging system for nasopharyngeal carcinoma and proposed improvement based on magnetic resonance imaging. Int J Radiat Oncol Biol Phys. 2009;73(5):1326–1334. doi:10.1016/j.ijrobp.2008.07.062
3. Chen Y-P, Tang L-L, Yang Q, et al. Induction chemotherapy plus concurrent chemoradiotherapy in endemic nasopharyngeal carcinoma: individual patient data pooled analysis of four randomized trials. Clin Cancer Res. 2018;24(8):1824–1833. doi:10.1158/1078-0432.CCR-17-2656
4. Chua DT, Ma J, Sham JS, et al. Long-term survival after cisplatin-based induction chemotherapy and radiotherapy for nasopharyngeal carcinoma: a pooled data analysis of two Phase III trials. J Clin Oncol. 2005;23(6):1118–1124. doi:10.1200/JCO.2005.12.081
5. Hong R, Hsiao C, Ting L, et al. Final results of a randomized phase III trial of induction chemotherapy followed by concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in patients with stage IVA and IVB nasopharyngeal carcinoma-Taiwan Cooperative Oncology Group (TCOG) 1303 Study. Ann Oncol. 2018;29(9):1972–1979. doi:10.1093/annonc/mdy249
6. National Comprehensive Cancer Network Head and Neck Cancers, version 2.2021 — march 26, 2021. Available from: http://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf.
7. Lei Y, Li Y, Jiang W, et al. A gene-expression predictor for efficacy of induction chemotherapy in locoregionally advanced nasopharyngeal carcinoma. J Natl Cancer Inst. 2021;113(4):471–480. doi:10.1093/jnci/djaa100
8. Peng H, Chen L, Li W, et al. Tumor response to neoadjuvant chemotherapy predicts long-term survival outcomes in patients with locoregionally advanced nasopharyngeal carcinoma: a secondary analysis of a randomized Phase 3 clinical trial. Cancer. 2017;123(9):1643–1652. doi:10.1002/cncr.30520
9. ML Disis. Immune regulation of cancer. J Clin Oncol. 2010;28(29):4531–4538.
10. Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. 2014;15(11):e493–503. doi:10.1016/S1470-2045(14)70263-3
11. Shinko D, Diakos CI, Clarke SJ, Charles KA. Cancer-related systemic inflammation: the challenges and therapeutic opportunities for personalized medicine. Clin Pharmacol Ther. 2017;102(4):599–610. doi:10.1002/cpt.789
12. Varkaris A, K A, Davis JS, et al. Circulating inflammation signature predicts overall survival and relapse-free survival in metastatic colorectal cancer. Br J Cancer. 2019;120(3):340–345. doi:10.1038/s41416-018-0360-y
13. A Mantovani, P Allavena, A Sica,et al. Cancer-related inflammation. Nature. 2008;454(7203):436–444. doi:10.1038/nature07205
14. Li W, Hua X, Lv S, et al. A scoring system based on nutritional and inflammatory parameters to predict the efficacy of first-line chemotherapy and survival outcomes for de novo metastatic nasopharyngeal carcinoma. J Inflamm Res. 2021;14:817–828. doi:10.2147/JIR.S296710
15. Tang LQ, Li CF, Li J, et al. Establishment and validation of prognostic nomograms for endemic nasopharyngeal carcinoma. J Natl Cancer Inst. 2016;108(1).
16. Li JP, Chen SL, Liu XM, et al. A novel inflammation-based stage (I stage) predicts overall survival of patients with nasopharyngeal carcinoma. Int J Mol Sci. 2016;17(11):1900.
17. Xia X, Zhang H-B, Shi J-L, et al. A prognostic model predicts the risk of distant metastasis and death for patients with nasopharyngeal carcinoma based on pre-treatment serum C-reactive protein and N-classification. Eur J Cancer. 2013;49(9):2152–2160. doi:10.1016/j.ejca.2013.03.003
18. Yang S, Zhao K, Ding X, Jiang H, Lu H. Prognostic Significance of hematological markers for patients with nasopharyngeal carcinoma: a meta-analysis. J Cancer. 2019;10(11):2568–2577. doi:10.7150/jca.26770
19. Lu A, Li H, Zheng Y, et al. Prognostic significance of neutrophil to lymphocyte ratio, lymphocyte to monocyte ratio, and platelet to lymphocyte ratio in patients with nasopharyngeal carcinoma. Biomed Res Int. 2017;2017:3047802.
20. Li W, Lv S, Liu G, et al. Development of a prognostic model to identify the suitable definitive radiation therapy candidates in de novo metastatic nasopharyngeal carcinoma: a Real-World Study. Int J Radiat Oncol Biol Phys. 2021;109(1):120–130. doi:10.1016/j.ijrobp.2020.08.045
21. Yao -J-J, Zhu F-T, D J, et al. Prognostic value of neutrophil-to-lymphocyte ratio in advanced nasopharyngeal carcinoma: a large institution-based cohort study from an endemic area. BMC Cancer. 2019;19(1):37. doi:10.1186/s12885-018-5236-2
22. Li X-H, C H, Xu B-Q, et al. An inflammatory biomarker-based nomogram to predict prognosis of patients with nasopharyngeal carcinoma: an analysis of a prospective study. Cancer Med. 2017;6(1):310–319. doi:10.1002/cam4.947
23. Li J, Chen S, Peng S, et al. Prognostic nomogram for patients with nasopharyngeal carcinoma incorporating hematological biomarkers and clinical characteristics. Int J Biol Sci. 2018;14(5):549–556. doi:10.7150/ijbs.24374
24. Peng H, Dong D, Fang M-J, et al. Prognostic value of deep learning PET/CT-based radiomics: potential role for future individual induction chemotherapy in advanced nasopharyngeal carcinoma. Clin Cancer Res. 2019;25(14):4271–4279. doi:10.1158/1078-0432.CCR-18-3065
25. Zhang B, Tian J, Dong D, et al. Radiomics features of multiparametric MRI as novel prognostic factors in advanced nasopharyngeal carcinoma. Clin Cancer Res. 2017;23(15):4259–4269. doi:10.1158/1078-0432.CCR-16-2910
26. Zhang B, He X, Ouyang F, et al. Radiomic machine-learning classifiers for prognostic biomarkers of advanced nasopharyngeal carcinoma. Cancer Lett. 2017;403:21–27. doi:10.1016/j.canlet.2017.06.004
27. Bodelon C, Polley MY, Kemp TJ, et al. Circulating levels of immune and inflammatory markers and long versus short survival in early-stage lung cancer. Ann Oncol. 2013;24(8):2073–2079. doi:10.1093/annonc/mdt175
28. Proctor MJ, Morrison DS, Talwar D, et al. An inflammation-based prognostic score (mGPS) predicts cancer survival independent of tumour site: a Glasgow Inflammation Outcome Study. Br J Cancer. 2011;104(4):726–734. doi:10.1038/sj.bjc.6606087
29. Kobayashi S, Matsumura Y, Karube Y, et al. Inflammation-based prognostic score predicts postoperative survival of patients with interstitial pneumonia after undergoing lung cancer resection. World J Surg. 2018;42(7):2143–2152. doi:10.1007/s00268-017-4426-4
30. Wang Y, Qu X, Kam N-W, et al. An inflammation-related nomogram for predicting the survival of patients with non-small cell lung cancer after pulmonary lobectomy. BMC Cancer. 2018;18(1):692. doi:10.1186/s12885-018-4513-4
31. Z H, Ueland PM, U A, et al. Plasma biomarkers of inflammation, the kynurenine pathway, and risks of all-cause, cancer, and cardiovascular disease mortality: the Hordaland Health Study. Am J Epidemiol. 2016;183(4):249–258. doi:10.1093/aje/kwv242
32. Li W, Liu G, Lin L, et al. MRI-detected residual retropharyngeal lymph node after intensity-modulated radiotherapy in nasopharyngeal carcinoma: prognostic value and a nomogram for the pretherapy prediction of it. Radiother Oncol. 2020;145:101–108. doi:10.1016/j.radonc.2019.12.018
33. Vickers AJ, Elkin EB. Decision curve analysis: a novel method for evaluating prediction models. Med Decis Making. 2006;26(6):565–574. doi:10.1177/0272989X06295361
34. Lam WKJ, Chan KCA, Lo YMD. Plasma Epstein-Barr virus DNA as an archetypal circulating tumour DNA marker. J Pathol. 2019;247(5):641–649. doi:10.1002/path.5249
35. Liu L, Chen Q-Y, Tang L-Q, et al. Neoadjuvant or adjuvant chemotherapy plus concurrent CRT versus concurrent CRT alone in the treatment of nasopharyngeal carcinoma: a study based on EBV DNA. J Natl Compr Canc Netw. 2019;17(6):703–710. doi:10.6004/jnccn.2018.7270
36. Chan C, Ma BBY, Lo YMD, et al. Phase II study of neoadjuvant carboplatin and paclitaxel followed by radiotherapy and concurrent cisplatin in patients with locoregionally advanced nasopharyngeal carcinoma: therapeutic monitoring with plasma Epstein-Barr virus DNA. J Clin Oncol. 2004;22(15):3053–3060. doi:10.1200/JCO.2004.05.178
37. Liu L, Tang L-Q, Chen Q-Y, et al. The prognostic value of plasma epstein-barr viral dna and tumor response to neoadjuvant chemotherapy in advanced-stage nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2015;93(4):862–869. doi:10.1016/j.ijrobp.2015.08.003
38. Zhang J, Peng H, Li W-F, et al. Individualized induction chemotherapy by pre-treatment plasma Epstein-Barr viral DNA in advanced nasopharyngeal carcinoma. BMC Cancer. 2018;18(1):1276. doi:10.1186/s12885-018-5177-9
39. Xu C, Zhang S, Li W-F, et al. Selection and validation of induction chemotherapy beneficiaries among patients with T3N0, T3N1, T4N0 nasopharyngeal carcinoma using epstein-barr virus DNA: a joint analysis of real-world and clinical trial Data. Front Oncol. 2019;9:1343. doi:10.3389/fonc.2019.01343
40. Li JY, Huang CL, Luo WJ, et al. An integrated model of the gross tumor volume of cervical lymph nodes and pre-treatment plasma Epstein-Barr virus DNA predicts survival of nasopharyngeal carcinoma in the intensity-modulated radiotherapy era: a big-data intelligence platform-based analysis. Ther Adv Med Oncol. 2019;11:1758835919877729.
41. Kim K, Le Q-T, Yom SS, et al. Clinical utility of epstein-barr virus DNA testing in the treatment of nasopharyngeal carcinoma patients. Int J Radiat Oncol Biol Phys. 2017;98(5):996–1001. doi:10.1016/j.ijrobp.2017.03.018
42. Nicholls JM, Lee VH-F, Chan S-K, et al. Negative plasma Epstein-Barr virus DNA nasopharyngeal carcinoma in an endemic region and its influence on liquid biopsy screening programmes. Br J Cancer. 2019;121(8):690–698. doi:10.1038/s41416-019-0575-6
43. Le Q-T, Zhang Q, Cao H, et al. An international collaboration to harmonize the quantitative plasma Epstein-Barr virus DNA assay for future biomarker-guided trials in nasopharyngeal carcinoma. Clin Cancer Res. 2013;19(8):2208–2215. doi:10.1158/1078-0432.CCR-12-3702
44. Ma brigette BY, K A, Lo YMD, et al. Relationship between pre-treatment level of plasma Epstein-Barr virus DNA, tumor burden, and metabolic activity in advanced nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2006;66(3):714–720. doi:10.1016/j.ijrobp.2006.05.064
45. L J, C Y, Z G, et al. Liquid biopsy tracking during sequential chemoradiotherapy identifies distinct prognostic phenotypes in nasopharyngeal carcinoma. Nat Commun. 2019;10(1):3941.
46. Tang L-Q, Li C-F, Chen Q-Y, et al. High-sensitivity C-reactive protein complements plasma Epstein-Barr virus deoxyribonucleic acid prognostication in nasopharyngeal carcinoma: a large-scale retrospective and prospective cohort study. Int J Radiat Oncol Biol Phys. 2015;91(2):325–336. doi:10.1016/j.ijrobp.2014.10.005
47. Chua melvin LK, Tan SH, K G, et al. Neutrophil-to-lymphocyte ratio as a prognostic marker in locally advanced nasopharyngeal carcinoma: a pooled analysis of two randomised controlled trials. Eur J Cancer. 2016;67:119–129. doi:10.1016/j.ejca.2016.08.006
48. Wang Y-Q, Zhang Y, Jiang W, et al. Development and validation of an immune checkpoint-based signature to predict prognosis in nasopharyngeal carcinoma using computational pathology analysis. J Immunother Cancer. 2019;7(1):298. doi:10.1186/s40425-019-0752-4
49. Yp C, Yq W, Jw L, et al. Identification and validation of novel microenvironment-based immune molecular subgroups of head and neck squamous cell carcinoma: implications for immunotherapy. Ann Oncol. 2019;30(1):68–75.
50. Tang X-R, Li Y-Q, Liang S-B, et al. Development and validation of a gene expression-based signature to predict distant metastasis in locoregionally advanced nasopharyngeal carcinoma: a retrospective, multicentre, cohort study. Lancet Oncol. 2018;19(3):382–393. doi:10.1016/S1470-2045(18)30080-9
51. L N, Chen N-Y, Cui R-X, et al. Prognostic value of a microRNA signature in nasopharyngeal carcinoma: a microRNA expression analysis. Lancet Oncol. 2012;13(6):633–641. doi:10.1016/S1470-2045(12)70102-X
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



