Back to Journals » Cancer Management and Research » Volume 9
Prognostic and clinicopathological value of Ki-67/MIB-1 expression in renal cell carcinoma: a meta-analysis based on 4579 individuals
Authors Wang Z, Xie H, Guo LP, Cai QL, Shang ZQ, Jiang N, Niu YJ
Received 12 May 2017
Accepted for publication 9 October 2017
Published 21 November 2017 Volume 2017:9 Pages 679—689
DOI https://doi.org/10.2147/CMAR.S141670
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Kenan Onel
Zhun Wang, Hui Xie, Linpei Guo, Qiliang Cai, Zhiqun Shang, Ning Jiang, Yuanjie Niu
Department of Urology, Tianjin Institute of Urology, The Second Hospital of Tianjin Medical University, Tianjin, China
Background: Previous studies have investigated the prognostic significance of Ki-67/MIB-1 expression in renal cell carcinoma (RCC), however, the reports are controversial and inconsistent. This study aimed to investigate Ki-67/MIB-1 expression in RCC and its correlation with prognosis and clinicopathological features.
Methods: We searched relevant studies that reported associations between Ki-67/MIB-1 expression and prognosis in RCC from PubMed, Embase, Web of Science, and Cochrane Library studies published until April 14, 2017. Hazard ratios (HRs) and 95% confidence intervals (CIs) were extracted from eligible studies. Fixed and random effects models were used to calculate pooled HRs and 95% CIs according to heterogeneity.
Results: A total of 4579 participants from 23 eligible studies were included in this analysis. The results showed that Ki-67/MIB-1 expression was associated with poor overall survival (HR=2.06, 95% CI: 1.64–2.57) and cancer specific survival (HR=2.01, 95% CI: 1.66–2.44). In addition, Ki-67/MIB-1 expression was also correlated with TNM stage (III/IV vs I/II: OR=1.92, 95% CI: 1.61–2.28), pathological T stage (pT3/pT4 vs pT1/pT2: OR=1.56, 95% CI: 1.21–2.02), distant metastasis (M1 vs M0: OR=1.81, 95% CI: 1.34–2.43), and Fuhrman grade (III/IV vs I/II: OR=1.94, 95% CI: 1.21–3.10).
Conclusion: Our study demonstrates that the presence of high Ki-67/MIB-1 expression and advanced clinicopathological features were correlated with poor prognosis in RCC patients.
Keywords: Ki-67/MIB-1, renal cell carcinoma, prognosis, meta-analysis
Introduction
Renal cell carcinoma (RCC) ranks the seventh most prevalent cancer type in men and ninth in women1. Each year, about three hundred thousand cases of RCC are diagnosed, and about 134 thousand deaths are reported worldwide.2,3 There are multiple treatment methods that could be applied to treat localized RCC; surgery treatment is the most effective, followed by chemotherapy and radiotherapy. Patients with RCC at an early stage may receive complete surgical resection to achieve the purpose of cure; about half of the patients experience disease recurrence after curative resection, and about 30% of RCC patients have metastases at the time of the initial diagnosis.4 Metastatic RCC is a treatment-resistant malignant tumor, which is usually treated with targeted drugs or immunosuppressive points for systemic therapy;5 however, it has limited effect. Therefore, reliable prognostic biomarkers are needed to distinguish high-risk patients with RCC and improve clinical outcomes of RCC.
MIB-1, also known as Ki-67, is a marker for cell proliferation and tumor growth, which is present during all active phases of the cell cycle, ie, G1, S, G2, and mitosis, but is absent in resting cells (G0 phase).6 High Ki-67/MIB-1 expression is often correlated with the clinical course of the disease, and its coexpression with other well-known markers of proliferation indicates a pivotal role in cell division. It is reported that Ki-67/MIB-1 expression predicts poor prognosis in various multiple solid tumor types, including breast cancer,7 prostate cancer,8 cervical cancer,9 gliomas,10 and hepatocellular carcinoma.11 Many studies have reported the prognostic value of p53 expression in RCC, but the results were conflicting.12–34 Therefore, it is necessary to conduct a comprehensive meta-analysis to evaluate the prognostic and clinicopathological value of Ki-67/MIB-1 expression in patients with RCC.
We retrieved relevant literature and extracted data from eligible studies to perform a meta-analysis. We aim to reveal the association between Ki-67/MIB-1 expression and prognosis and clinicopathological features in patients with RCC.
Materials and methods
Search strategy
We did this meta-analysis using a predefined protocol in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).35 We searched PubMed, Embase, Web of Science, and the Cochrane electronic databases for studies published before April 14, 2017. The keywords were searched as follows: “renal cell carcinoma” or “renal cell cancer” or “renal cell adenocarcinoma” or “kidney tumor” and “Ki-67” or “MIB-1” and “prognosis” or “survival” or “outcome” in humans, and the language of publications was restricted to English.
Two reviewers (ZW and HX) independently screened the titles and abstracts of all initially identified studies according to the selection criteria. Full-text articles of studies that met all selection criteria were retrieved.
Inclusion and exclusion criteria
To be eligible for inclusion in this meta-analysis, a study must meet the following criteria: 1) the prognostic value of Ki-67/MIB-1 expression for overall survival (OS) and/or cancer-specific survival (CSS) were reported; 2) all patients were diagnosed with histologically confirmed RCC; 3) hazard ratios (HRs) and their 95% CIs for survival analysis were reported in text or could be computed from given data; and 4) the expression of Ki-67 was measured by immunohistochemistry (IHC).
The exclusion criteria were as follows: 1) non-human studies, non-English articles; 2) abstract, case reports, review articles, or comment letters; 3) duplicate publications; 4) with insufficient data to calculate the HR and its 95% CIs, or the Kaplan–Meier curve in the article could not be extracted; and 5) with no >30 eligible RCC patients.
Data extraction and quality
Data was independently extracted by ZW and Shuanghe Peng (The Second Hospital of Tianjin Medical University), and in case of any inconsistency, a consensus was reached with the involvement of QLC. The quality of the selected articles was assessed according to the Newcastle–Ottawa Scale.36 Study with a score of 6 or higher was considered as a high quality study. We used a predesigned data extraction form to obtain relevant information. The data extracted from the eligible studies including the following items: first author, year of publication, country of origin, the number of patients, histopathological stage, detection method, cut-off value, antibody staining for Ki-67/MIB-1, the number of patients with positive Ki-67/MIB-1 expression, HR for survival (OS and/or CSS), and follow-up time. For articles that only provided survival data in a Kaplan–Meier curve, software designed by Tierney et al was used to digitize and extract the relative risk and its 95% CI.37
Statistical analysis
Data were analyzed by using Stata SE12.0 (Stata Corp LP, College Station, TX, USA). According to the Meta-analysis Of Observational Studies in Epidemiology guidelines,38 the associations between clinical factors and Ki-67/MIB-1 expression were presented by odds ratio (OR) and 95% CI. HR with a 95% CI was computed to reveal the correlation between Ki-67/MIB-1 expression and prognosis (OS and CSS). Inter-study heterogeneity was evaluated using the chi-square test and I2 statistic (100% × [(Q-df)/Q]),39,40 the value of Pheterogeneity <0.1 and I2>50% represents the existence of significant heterogeneity. A fixed effects model was used when the value of Pheterogeneity was >0.05 and I2<50%; otherwise, a random effects model was applied. Subgroup analysis was performed for OS and CSS analysis. Begg’s funnel plot and Egger’s linear regression test evaluated the potential for publication bias. Two-tailed value of P<0.05 was considered statistically significant.
Results
Features of included studies
The work flowchart for this study is shown in Figure 1. Three hundred and one potentially relevant citations were initially retrieved through initial search of relevant databases. After title and abstract screening, 38 articles remained for full-text assessment. Then 15 articles were excluded (2 articles were duplicate studies, 12 lacked key information, and 1 did not measure Ki-67 expression by IHC). At last, 23 studies12–34 published from 2000 to 2016 with 4579 patients met our inclusion criteria and were included in the meta-analysis.
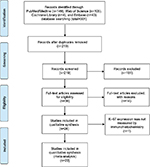  | Figure 1 Flow diagram of the study selection process. |
Summary of major characteristics of these studies are shown in Table 1. All the studies were of retrospective study design and detected Ki-67/MIB-1 expression using IHC. The sample size ranged from 43 to 741. Nineteen studies were conducted in non-Asian countries, including France,12 Finland,22,28,33,34 Germany,18,29,30 Italy,25 Sweden,27 and USA.13,15–17,20,21,24,26,31 Four studies were conducted in Asian countries, including China,32 Turkey,19 and Japan.14,23 For the prognostic indicator of Ki-67/MIB-1 expression in RCC, 1 article reported both OS and CSS, 6 articles only reported OS, and 16 articles only reported CSS.
Prognostic value of Ki-67/MIB-1 expression for OS and CSS
The association between Ki-67/MIB-1 expression and prognosis for OS and CSS in RCC patients were estimated; pooled HRs and 95% CIs are shown in Table 2 and Figure 2.
OS values were available from 7 studies.13,21,22,25,28,33,34 The Ki-67/MIB-1 expression had a significant association with poor OS (HR=2.06, 95% CI: 1.64–2.57, P<0.001; I2=0.0%, Pheterogeneity =0.4.73, Table 2, Figure 2A). Seventeen studies12,14–21,23,24,26,27,29–32 evaluated CSS outcome. The pooled results indicated that Ki-67/MIB-1 expression was significantly related to poor CSS (HR=2.01, 95% CI: 1.66–2.44, P<0.001; I2=41%, Pheterogeneity = 0.04, Table 2, Figure 2B).
Subgroup analysis
Subgroup analyses were stratified by nation, HR estimate, and pathological types (Table 2). Subgroup analysis according to nation showed that Ki-67/MIB-1 expression predicted worse CSS (n=4, HR=3.13, 95% CI: 1.60–6.11, P=0.001; I2=0.0%, pheterogeneity =0.67) in Asian studies. In subgroup analysis according to HR estimate, all the 3 HR estimate methods suggested that Ki-67/MIB-1 expression was significantly associated with poor OS and CSS (Table 2). With regard to histology, Ki-67/MIB-1 expression was significantly correlated with poor CSS (n=13, HR=2.08, 95% CI: 1.67–2.59, P<0.001; I2=45%, Pheterogeneity =0041) and poor OS (n=2, HR=3.86, 95% CI: 0.49–30.66, P<0.001; I2=73%, Pheterogeneity =0053) in clear cell renal cell carcinoma patients, although a significant heterogeneity exists.
Evaluation of Ki-67/MIB-1 expression and clinicopathological characteristics
We also estimated the association between Ki-67/MIB-1 expression and clinicopathological characteristics in RCC patients. Ki-67/MIB-1 expression was significantly associated with TNM (III/IV vs I/II, OR=1.92, 95% CI: 1.61–2.28), grade (3/4 vs 1/2, OR=1.94, 95% CI: 1.21–3.10), M (M1 vs M0, OR=1.81, 95% CI: 1.34–2.43), N (N1 vs N0, OR=1.67, 95% CI: 1.33–2.12), and tumor stage (pT3/4 vs pT1/2, OR=1.56, 95% CI: 1.21–2.02) (Figure 3 and Table 3).
Publication bias
Funnel plots for meta-analysis of Ki-67/MIB-1 expression, OS, and CSS are shown in Figure 4. Both the Begg’s funnel plot test (OS: P=1.000, CSS: P=0.149; Figure 4) and the Egger’s (OS: P=0.494, CSS: P=0.010) test verified that there was no publication bias within the included cohorts. The funnel plots for clinical features also indicated no obvious publication bias (Figure 4, Table 3).
Sensitivity analysis
Sensitivity analysis was performed to examine the stability of the current meta-analysis. The selected studies were sequentially omitted to investigate whether any single study could have an influence on the pooled OS or CSS. As shown in Figure 5, the stable overall HR was found to be not dominantly influenced by each individual study.
Discussion
MIB-1, a nuclear protein, is famous as a marker of cell proliferation and tumor growth. Since Gerdes et al42 first suggested that Ki-67 labeling index predicted poor prognosis in non-Hodgkin’s lymphomas, a number of studies have examined the usefulness of Ki-67 expression in various tumor types. In recent years, several reports suggested that high Ki-67 expression can serve as a promising biomarker for prognostication in various tumors.7–11 Many studies have also reported the prognostic value of Ki-67 expression in RCC, but the results were still conflicting.12–34,41 Therefore, we performed this meta-analysis to explore the association between Ki-67/MIB-1 expression and prognostic value and clinicopathological features in patients with RCC.
Our analysis mainly reports the prognostic role of Ki-67/MIB-1 expression in RCC. Studies from different countries are included in the meta-analysis. Fixed effects model and random effects model were used for the meta-analysis. In this study, we focused on validating Ki-67/MIB-1 expression and evaluated the prognostic values of Ki-67/MIB-1 expression in RCC. Based on results from 24 studies with 4579 participants, we concluded that Ki-67/MIB-1 expression predicted poor prognostic value for patients with RCC. RCC patients with Ki-67/MIB-1 expression exhibited poor OS and CSS. Subgroup analysis results revealed that the pooled HRs obtained from Kaplan–Meier curves and those directly extracted from studies both demonstrated that Ki-67/MIB-1 expression was significantly associated with poor OS and CSS. Our results showed that Ki-67/MIB-1 expression was an unfavorable predictor for prognosis in RCC, which were in accordance with conclusions from other solid cancer types, such as breast cancer,7 prostate cancer,8 cervical cancer,9 gliomas,10 and hepatocellular carcinoma.11 In addition, Ki-67/MIB-1 expression was also associated with clinical factors in RCC; Ki-67/MIB-1 expression had positive relationship with higher tumor stage and grade, as well as lymph node involvement and distant metastases, which suggested that Ki-67/MIB-1 had potential to be used as a dichotomous biomarker.
The relationship between Ki-67/MIB-1 expression and clinicopathological features was also evaluated. The result suggested that RCC patients with Ki-67/MIB-1 expression were significantly associated with primary tumor stage, regional lymph node involvement, distant metastases, nuclear grade, and TNM stage. High Ki-67/MIB-1 expression was likely to have a higher primary tumor stage, TNM stage, positive regional lymph node involvement and distant metastasis, and a higher nuclear grade.
There are several limitations in this study that should be acknowledged. First, all included studies in this meta-analysis measured Ki-67/MIB-1 expression via IHC, but the cut-off criteria to determine the positive or negative expression of Ki-67/MIB-1 were inconsistent in different studies, which may potentially contribute to heterogeneity. Therefore, a more unified standard should be defined in the future. Second, the number of patients included in the most eligible studies was relatively small. Therefore, large-scale studies are needed to conceive more reliable results. Third, relatively few studies were extracted in some subgroup analyses, which might render premature results. Finally, research with positive results is potentially more likely to be submitted and published than work with negative results, which could cause publication bias, although this bias was not detected in the present analysis.43
Conclusion
Our meta-analysis suggests that Ki-67/MIB-1 expression predicted a poor OS and CSS in patients with RCC. The results also indicate that Ki-67/MIB-1 expression was associated with more aggressive clinical features in patients with RCC. Hence, the detection of Ki-67/MIB-1 in clinic will be beneficial to the treatment and prognostic evaluation for RCC patients. More prospective and large-scale studies are needed to clarify our results.
Acknowledgments
This meta-analysis has been financially supported by the National Natural Science Foundation of China (No 81472682 and No 81572538). The authors thank Shuanghe Peng for their assistance with data extraction.
Disclosure
The authors report no conflicts of interest in this work.
References
Rini BI, Campbell SC, Escudier B. Renal cell carcinoma. Lancet. 2009;373(9669):1119–1132. | ||
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. | ||
Fitzmaurice C, Dicker D, Pain A, et al. The Global Burden of Cancer 2013. JAMA Oncol. 2015;1(4):505–527. | ||
Janzen NK, Kim HL, Figlin RA, Belldegrun AS. Surveillance after radical or partial nephrectomy for localized renal cell carcinoma and management of recurrent disease. Urol Clin North Am. 2003;30(4):843–852. | ||
Meskawi M, Sun M, Trinh QD, et al. A review of integrated staging systems for renal cell carcinoma. Eur Urol. 2012;62(2):303–314. | ||
Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol. 2000;182(3):311–322. | ||
Liedtke C, Packeisen J, Hess KR, et al. Systematic analysis of in vitro chemosensitivity and mib-1 expression in molecular breast cancer subtypes. Eur J Cancer. 2012;48(13):2066–2074. | ||
Luczynska E, Gasinska A, Wilk W. Expression of Ki-67 (MIB-1) and GLUT-1 proteins in non-advanced prostatic cancer. Pol J Pathol. 2012;63(4):272–277. | ||
Pan D, Wei K, Ling Y, Su S, Zhu M, Chen G. The prognostic role of Ki-67/MIB-1 in cervical cancer: a systematic review with meta-analysis. Med Sci Monit. 2015;21:882–889. | ||
Skjulsvik AJ, Mork JN, Torp MO, Torp SH. Ki-67/MIB-1 immunostaining in a cohort of human gliomas. Int J Clin Exp Pathol. 2014;7(12):8905–8910. | ||
Shi W, Hu J, Zhu S, et al. Expression of MTA2 and Ki-67 in hepatocellular carcinoma and their correlation with prognosis. Int J Clin Exp Pathol. 2015;8(10):13083–13089. | ||
Rioux-Leclercq N, Turlin B, Bansard J, et al. Value of immunohistochemical Ki-67 and p53 determinations as predictive factors of outcome in renal cell carcinoma. Urology. 2000;55(4):501–505. | ||
Olumi AF, Weidner N, Presti JC. p53 immunoreactivity correlates with Ki-67 and bcl-2 expression in renal cell carcinoma. Urol Oncol. 2001;6(2):63–67. | ||
Yuba H, Okamura K, Ono Y, Ohshima S. Growth fractions of human renal cell carcinoma defined by monoclonal antibody Ki-67. Predictive values for prognosis. Inte J Urol. 2001;8(11):609–614. | ||
Cheville JC, Zincke H, Lohse CM, et al. pT1 clear cell renal cell carcinoma: a study of the association between MIB-1 proliferative activity and pathologic features and cancer specific survival. Cancer. 2002;94(8):2180–2184. | ||
Bui MH, Visapaa H, Seligson D, et al. Prognostic value of carbonic anhydrase IX and KI67 as predictors of survival for renal clear cell carcinoma. J Urol. 2004;171(6 Pt 1):2461–2466. | ||
Kim HL, Seligson D, Liu X, et al. Using protein expressions to predict survival in clear cell renal carcinoma. Clin Cancer Res. 2004;10(16):5464–5471. | ||
Lehmann J, Retz M, Nurnberg N, et al. The superior prognostic value of humoral factors compared with molecular proliferation markers in renal cell carcinoma. Cancer. 2004;101(7):1552–1562. | ||
Yildiz E, Gokce G, Kilicarslan H, Ayan S, Goze OF, Gultekin EY. Prognostic value of the expression of Ki-67, CD44 and vascular endothelial growth factor, and microvessel invasion, in renal cell carcinoma. BJU Int. 2004;93(7):1087–1093. | ||
Kim HL, Seligson D, Liu X, et al. Using tumor markers to predict the survival of patients with metastatic renal cell carcinoma. J Urol. 2005;173(5):1496–1501. | ||
Kramer BA, Gao X, Davis M, Hall M, Holzbeierlein J, Tawfik O. Prognostic significance of ploidy, MIB-1 proliferation marker, and p53 in renal cell carcinoma. J Am Coll Surg. 2005;201(4):565–570. | ||
Kankuri M, Soderstrom KO, Pelliniemi TT, Vahlberg T, Pyrhonen S, Salminen E. The association of immunoreactive p53 and Ki-67 with T-stage, grade, occurrence of metastases and survival in renal cell carcinoma. Anticancer Res. 2006;26(5B):3825–3833. | ||
Phuoc NB, Ehara H, Gotoh T, et al. Immunohistochemical analysis with multiple antibodies in search of prognostic markers for clear cell renal cell carcinoma. Urology. 2007;69(5):843–848. | ||
Tollefson MK, Thompson RH, Sheinin Y, et al. Ki-67 and coagulative tumor necrosis are independent predictors of poor outcome for patients with clear cell renal cell carcinoma and not surrogates for each other. Cancer. 2007;110(4):783–790. | ||
Gontero P, Ceratti G, Guglielmetti S, et al. Prognostic factors in a prospective series of papillary renal cell carcinoma. BJU Int. 2008;102(6):697–702. | ||
Parker AS, Leibovich BC, Lohse CM, et al. Development and evaluation of BioScore: a biomarker panel to enhance prognostic algorithms for clear cell renal cell carcinoma. Cancer. 2009;115(10):2092–2103. | ||
Zubac DP, Bostad L, Kihl B, Seidal T, Wentzel-Larsen T, Haukaas SA. The expression of thrombospondin-1 and p53 in clear cell renal cell carcinoma: its relationship to angiogenesis, cell proliferation and cancer specific survival. J Urol. 2009;182(5):2144–2149. | ||
Kankuri-Tammilehto MK, Soderstrom KO, Pelliniemi TT, Vahlberg T, Pyrhonen SO, Salminen EK. Prognostic evaluation of COX-2 expression in renal cell carcinoma. Anticancer Res. 2010;30(7):3023–3030. | ||
Toma MI, Weber T, Meinhardt M, et al. Expression of the Forkhead transcription factor FOXP1 is associated with tumor grade and Ki67 expression in clear cell renal cell carcinoma. Cancer Invest. 2011;29(2):123–129. | ||
Weber T, Meinhardt M, Zastrow S, Wienke A, Fuessel S, Wirth MP. Immunohistochemical analysis of prognostic protein markers for primary localized clear cell renal cell carcinoma. Cancer Invest. 2013;31(1):51–59. | ||
Gayed BA, Youssef RF, Bagrodia A, et al. Ki67 is an independent predictor of oncological outcomes in patients with localized clear-cell renal cell carcinoma. BJU Int. 2014;113(4):668–673. | ||
Teng J, Gao Y, Chen M, et al. Prognostic value of clinical and pathological factors for surgically treated localized clear cell renal cell carcinoma. Chin Med J. 2014;127(9):1640–1644. | ||
Rautiola J, Lampinen A, Mirtti T, et al. Association of angiopoietin-2 and Ki-67 expression with vascular density and sunitinib response in metastatic renal cell Carcinoma. PLoS One. 2016;11(4):e0153745. | ||
Virman JP, Bono P, Luukkaala TH, Sunela KL, Kujala PM, Kellokumpu-Lehtinen PL. Combined angiogenesis and proliferation markers’ expressions as long-term prognostic factors in renal cell cancer. Clin Genitourin Cancer. 2016;14(4):e283–e289. | ||
Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100. | ||
Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605. | ||
Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. | ||
Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–2012. | ||
Handoll HH. Systematic reviews on rehabilitation interventions. Arch Phys Med Rehabil. 2006;87(6):875. | ||
Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. | ||
Visapaa H, Bui M, Huang Y, et al. Correlation of Ki-67 and gelsolin expression to clinical outcome in renal clear cell carcinoma. Urology. 2003;61(4):845–850. | ||
Gerdes J, Schwab U, Lemke H, Stein H. Production of a mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int J Cancer. 1983;31(1):13–20. | ||
Sutton AJ, Song F, Gilbody SM, Abrams KR. Modelling publication bias in meta-analysis: a review. Stat Methods Med Res. 2000;9(5):421–445. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.







