Back to Journals » OncoTargets and Therapy » Volume 9
Prognostic and clinicopathological significance of Gankyrin overexpression in cancers: evidence from a meta-analysis
Authors Zhao X, Liu F, Zhang Y, Li P
Received 2 December 2015
Accepted for publication 18 February 2016
Published 4 April 2016 Volume 2016:9 Pages 1961—1968
DOI https://doi.org/10.2147/OTT.S101687
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Min Li
Xiaotong Zhao,1,* Fangzhou Liu,2,* Yuan Zhang,2 Peihua Li1
1Department of Otolaryngology, Affiliated Hospital of XuZhou Medical College, Xuzhou, 2Department of Otolaryngology, The Affiliated Cancer Hospital, Nanjing Medical University, Nanjing, Jiangsu, People’s Republic of China
*These authors contributed equally to this work
Abstract: Many studies have indicated that Gankyrin is a promising and novel prognostic tumor biomarker. However, the results of different studies remained controversial. Hence, a meta-analysis was undertaken to investigate the association between Gankyrin expression and cancer prognosis. Eligible studies were identified by searching the electronic databases PubMed, Embase, and Cochrane Library up to November 2015. Prognostic value of Gankyrin expression was evaluated by hazard ratio with 95% confidence interval (CI). Meanwhile, relative risk (RR) with 95% CI was used to assess the effects of Gankyrin expression on clinicopathological parameters. In total, ten studies with 1,326 patients were included for final analysis. A significant association was found between Gankyrin overexpression and poorer overall survival in patients with cancer (hazard ratio =1.73, 95% CI: 1.29–2.31, P=0.000). In the subgroup analysis, the association was also detected in Chinese patients and patients with cancers of the digestive system. The pooled RR indicated that Gankyrin overexpression was related to advanced tumor–node–metastasis stage (RR =0.72, 95% CI: 0.60–0.86, P=0.000), positive lymph node metastasis (RR =1.66, 95% CI: 1.41–1.94, P=0.000), and distant metastasis (RR =1.43, 95% CI: 1.20–1.70, P<0.000). The meta-analysis demonstrated that Gankyrin is a novel biomarker for predicting cancers, especially digestive system cancers, and is more suitable for predicting cancer prognoses in Asians.
Keywords: Gankyrin, cancer, prognosis, meta-analysis
Introduction
Gankyrin protein (also named as PSMD10, p28[Gank], or p28) is a small protein (25 kDa). It was initially identified and characterized as the p28 component of a regulatory subunit of the 26S proteasome in 1998.1 Gankyrin is highly conserved in all mammals.2,3 “Gann” means cancer in Japanese, and ankyrin is functional domain that is involved in protein–protein interactions. Gankyrin protein contains two typical domains including seven ankyrin repeats and Rb-recognition motif (LXCXE), and the name of Gankyrin was derived from these factors.4–6 Previous studies showed that Gankyrin enhances cancer cell proliferation through interaction with p53 and Rb,7–9 and the aberrant expression of Gankyrin is associated with cancer progression and poor prognoses. Although many clinical studies suggested that the overexpression of Gankyrin was a predictor for worse outcome in cancers,10–17 some other conflicting conclusions were arrived.18,19 Therefore, the prognostic value of Gankyrin overexpression remains controversial. To overcome the limitation of the single study, this meta-analysis was conducted to investigate the relationship between Gankyrin expression and its prognostic value in cancers.
Materials and methods
Search strategy
A systematic literature search of the databases PubMed, Embase, and Cochrane Library was performed with the following strategy: “Gankyrin” OR “p28” OR “p28Gank” OR “PSMD10” AND “prognosis” OR “prognostic” OR “survival” OR “outcome” AND “cancer” OR “carcinoma” OR “neoplasm” OR “tumor” OR “malignant” up to November 2015. To explore additional studies, we also inspected the references of the included studies. The eligible reports were identified independently by two investigators (Zhao and Liu).
Inclusion criteria
Eligible studies have to meet the following inclusion criteria: 1) had to evaluate the Gankyrin expression in human cancer tissues; 2) had to evaluate the association between Gankyrin expression and cancer prognosis; and 3) had to have sufficient information to estimate the hazard ratios (HRs) with 95% confidence intervals (CIs). The exclusion criteria were as follows: 1) reviews, case reports, letters, and conference abstracts and 2) overlapping data. The flow diagram of study selection is presented in Figure 1.
  | Figure 1 The flow diagram of study selection. |
Data extraction
Data from eligible studies were extracted independently by two investigators (Zhao and Liu) according to the inclusion and exclusion criteria mentioned earlier. The following items were collected: first author’s name, year of publication, nationality, number of cases, cancer type, detecting method (immunohistochemistry [IHC] or real-time polymerase chain reaction), cutoff value, HRs with corresponding 95% CIs for overall survivals (OSs), and clinicopathological parameters. Any disagreements were settled by a third investigator (Li).
Quality assessment
The quality of included studies was assessed by two independent investigators (Zhao and Liu) on the basis of the Newcastle–Ottawa scale (NOS) system.20 NOS is a 9-point scoring system, and studies with an NOS score ≥6 were regarded as high-quality studies.
Statistical analysis
HRs with their 95% CIs were calculated to evaluate the association between Gankyrin expression levels and the cancer prognosis (OS). If available, we obtained data from article directly. If not, we calculated data from Kaplan–Meier curves.21 Meanwhile, the impact of Gankyrin expression on clinicopathological parameters was performed by relative risks (RRs) with their 95% CIs. The heterogeneity among the included studies was checked by the chi-squared Q test.22 If the heterogeneity exists (P<0.10 or I2>50%), the random-effects model was used; otherwise, the fixed-effects model was used.23 Subgroup analyses were conducted to explore the source of heterogeneity. Moreover, a sensitivity analysis was carried out to evaluate whether any single study could have effects on the pooled HRs by sequentially discarding individual studies. Egger’s test and Begg’s funnel plots were applied to assess publication biases.24 All statistical analyses were performed using Stata 12.0 (StataCorp LP, College Station, TX, USA). P-value <0.05 was considered statistically significant.
Results
Study characteristics
According to the search strategy, a total of 51 studies were selected after the initial search. All the studies were identified by the inclusion and exclusion criteria mentioned earlier. Finally, ten eligible studies, published from 2008 to 2015, were included in this meta-analysis. The main characteristics of the eligible studies are summarized in Table 1. A total of 1,326 patients from the People’s Republic of China,10,11,13–16,19 Japan,17,18 and Korea12 were involved. Of all the eligible studies, nine were in English language,10,12–19 whereas one was in Chinese11 (seven were about digestive system cancers [hepatocellular cancer, colorectal cancer, esophageal cancer, gastric cancer, and cholangiocarcinoma cancer] and three were about other cancer types [non-small-cell lung cancer, glioma cancer, and liposarcoma]). IHC was the main method used to detect the Gankyrin expression in these studies, but in one study, it was detected by real-time polymerase chain reaction.
Meta-analysis results
Gankyrin expression and OS
As shown in Figure 2, Gankyrin overexpression was significantly associated with poor OS in cancers (HR =1.73, 95% CI: 1.29–2.31, P=0.000). Owing to the heterogeneity detected in these studies (P=0.019, I2=54.6%), the random-effects model was applied.
To explore the source of heterogeneity, subgroup analyses were performed by nationality, cancer type, detecting method, and cutoff value. In our subgroup analysis, a significant association between Gankyrin overexpression and poor OS was observed in the “People’s Republic of China” subgroup (HR =1.72, 95% CI: 1.40–2.11, P=0.000), “digestive system cancers” subgroup (HR =1.62, 95% CI: 1.05–2.49, P=0.028), “IHC” subgroup (HR =1.66, 95% CI: 1.20–2.29, P=0.002), and “cutoff value median” subgroup (HR =1.71, 95% CI: 1.29–2.27, P=0.000; Table 2).
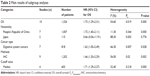  | Table 2 Main results of subgroup analyses |
Gankyrin expression and clinicopathological parameters
As shown in Figure 3 and Table 3, increased Gankyrin was significantly associated with poorer tumor–node–metastasis (TNM) stage (RR =0.72, 95% CI: 0.60–0.86, P=0.000), positive lymph node metastasis (RR =1.66, 95% CI: 1.41–1.94, P=0.000), and positive distant metastasis (RR =1.43, 95% CI: 1.20–1.70, P=0.000), but not with sex (P=0.271) and histological grade (P=0.070). Owing to insufficient data, we did not detect the relationship between Gankyrin overexpression and other clinicopathological parameters.
  | Table 3 Meta-analysis: RR value and 95% CI of clinicopathological parameters |
Sensitivity analysis
Sensitivity analysis showed no significant changes in HRs when any individual study was discarded (Figure 4).
  | Figure 4 Sensitivity analysis for overall survival. |
Publication bias
Egger’s test and Begg’s funnel plots were applied to assess publication biases. The P-value of Egger’s test for OS (P=0.946) indicated no publication bias among these studies. The symmetric funnel plots also suggested no evidence of publication bias in this meta-analysis (Figure 5). Because of the limited number of included studies (n<10), the publication bias for clinicopathological parameters was not assessed.
  | Figure 5 Begg’s funnel plot of Gankyrin expression and OS in patients with cancer. |
Discussion
The Gankyrin gene is located on human chromosome Xq22.3 and encodes a protein with seven ankyrin repeats, which is required for protein–protein interactions.1,25 Gankyrin was considered to enhance tumor onset and progression by modulating the phosphorylation of Rb and the ubiquitylation of p53.8 In 2000, Higashitsuji et al identified the Gankyrin as a gene that was consistently overexpressed in hepatocellular cancer and then opened the investigative prolog in Gankyrin expression in malignant tumor. In the following years, Gankyrin have attracted much attention, especially in Asia. It was reported that Gankyrin was negatively or weakly expressed in normal tissues, but was highly expressed in various cancers.10–19,26–30 Although the focus area of the research is in digestive system cancers, the role of Gankyrin in other malignant tumors has received increasing attention in recent years. Several studies have found that Gankyrin might be an important prognostic marker for malignant cancers.10–17 However, some conflicting conclusions have been reported. Umemura et al18 came to the opposite conclusion that positive expression of Gankyrin might predict a better prognosis in hepatocellular cancer. Moreover, an insignificant result was obtained by Jing et al.19 Since the prognostic value of Gankyrin expression remained inconclusive, a meta-analysis was carried out to address the controversial issue.
To the best of our knowledge, the present analysis is the first meta-analysis to evaluate the role of Gankyrin as a cancer prognostic. Here, we identified ten studies including 1,326 cases. We observed that Gankyrin overexpression was associated with a poor prognosis in patients with cancer (HR =1.73, 95% CI: 1.29–2.31, P=0.000). Additionally, the results revealed that elevated Gankyrin expression was related to TNM stage, lymph node metastasis, and distant metastasis. Based on the results, Gankyrin might act as a reliable biomarker for cancers and could help us to predict clinical outcomes of patients with cancer.
To explain the heterogeneity in OS, subgroup analyses were performed. It revealed that a high Gankyrin expression was related to poor prognosis in Chinese patients (HR =1.72, 95% CI: 1.40–2.11, P=0.000), but was not statistically significant in Japanese patients (HR =0.66, 95% CI: 0.04–11.91, P=0.776). Because most of patients enrolled in this meta-analysis were Chinese, it demonstrated that Gankyrin may play a greater role in Chinese patients. We deduced that the discrepancy might be caused by genotype difference and the small sample size (only two studies with 115 Japanese patients were included). The results might not be representative, and more large size studies are necessary to enhance the reliability of the conclusion. The results also indicated that elevated Gankyrin expression was significantly associated with poor OS in digestive system cancers (HR =1.62, 95% CI: 1.05–2.49, P=0.028). In terms of methods for detection, we found that IHC was widely applied and considered as an effective method (HR =1.66, 95% CI: 1.20–2.29, P=0.002). In addition, we found that the heterogeneity of OS reduced in the “median” subgroup. This may be because different standards of cutoff values caused the heterogeneity. Significant heterogeneity was also found for the association between evaluated Gankyrin with histological grade, TNM stage, and distant metastasis. The random-effects model was applied. In view of the limited number of studies (n=5, 8, 6, respectively), the subgroup analyses were not performed. The heterogeneity could be explained by the differences in characteristics of patients, detecting methods, cutoff values, antibodies, and so on. Therefore, to increase the reliability of the conclusions, more homogeneous samples are needed.
Although efforts were made to conduct this meta-analysis, some limitations should be acknowledged. First, most of the included studies focused on the digestive system cancers, and the small number of the studies focusing on each type of cancer might weaken our conclusions; thus, more efforts are needed in the future. Second, all the studies included were from Asian countries; hence, our conclusion might be more applicable to the Asian populations. It is difficult to make a reliable conclusion for other regions. Third, if the study did not provide HRs with 95% CIs, we had to extract data from Kaplan–Meier curves, and the calculated data might be less reliable. Fourth, although most of the studies detected the Gankyrin expression by IHC, the different antibody concentrations and the variable cutoff value might influence the results.
Conclusion
In conclusion, the meta-analysis indicated that Gankyrin overexpression was significantly associated with poor prognosis and might be a promising biomarker in predicting clinical outcomes for cancer patients, especially patients with cancers of the digestive system. Further clinical researches are needed to strengthen our conclusions and confirm the precise prognostic value of Gankyrin in cancers.
Disclosure
The authors report no conflicts of interest in this work.
References
Hori T, Kato S, Saeki M, et al. cDNA cloning and functional analysis of p28 (Nas6p) and p40.5 (Nas7p), two novel regulatory subunits of the 26S proteasome. Gene. 1998;216(1):113–122. | ||
Yuan C, Li J, Mahajan A, Poi MJ, Byeon IJ, Tsai MD. Solution structure of the human oncogenic protein gankyrin containing seven ankyrin repeats and analysis of its structure–function relationship. Biochemistry. 2004;43(38):12152–12161. | ||
Manjasetty BA, Quedenau C, Sievert V, et al. X-ray structure of human gankyrin, the product of a gene linked to hepatocellular carcinoma. Proteins. 2004;55(1):214–217. | ||
Chen Y, Li HH, Fu J, et al. Oncoprotein p28 GANK binds to RelA and retains NF-kappaB in the cytoplasm through nuclear export. Cell Res. 2007;17(12):1020–1029. | ||
Li J, Tsai MD. Novel insights into the INK4-CDK4/6-Rb pathway: counter action of gankyrin against INK4 proteins regulates the CDK4-mediated phosphorylation of Rb. Biochemistry. 2002;41(12):3977–3983. | ||
Higashitsuji H, Itoh K, Nagao T, et al. Reduced stability of retinoblastoma protein by gankyrin, an oncogenic ankyrin-repeat protein overexpressed in hepatomas. Nat Med. 2000;6(1):96–99. | ||
Higashitsuji H, Higashitsuji H, Itoh K, et al. The oncoprotein gankyrin binds to MDM2/HDM2, enhancing ubiquitylation and degradation of p53. Cancer Cell. 2005;8(1):75–87. | ||
Dawson S, Higashitsuji H, Wilkinson AJ, Fujita J, Mayer RJ. Gankyrin: a new oncoprotein and regulator of pRb and p53. Trends Cell Biol. 2006;16(5):229–233. | ||
Iwai A, Marusawa H, Kiuchi T, et al. Role of a novel oncogenic protein, gankyrin, in hepatocyte proliferation. J Gastroenterol. 2003;38(8):751–758. | ||
Wang WP, Yan XL, Li WM, et al. Clinicopathologic features and prognostic implications of Gankyrin protein expression in non-small cell lung cancer. Pathol Res Pract. 2015;211(12):939–947. | ||
Wu Q, He F, Yang P, et al. Association of gankyrin protein expression in human colorectal cancer with postoperative prognosis. Chin J Gastrointest Surg. 2015;18(6):611–615. | ||
Hwang JA, Yang HM, Hong DP, et al. Gankyrin is a predictive and oncogenic factor in well-differentiated and dedifferentiated liposarcoma. Oncotarget. 2014;5(19):9065–9078. | ||
Zheng T, Hong X, Wang J, et al. Gankyrin promotes tumor growth and metastasis through activation of IL-6/STAT3 signaling in human cholangiocarcinoma. Hepatology. 2014;59(3):935–946. | ||
Zheng JY, Hu H, Du JJ, Li XH, Zhao QC. p28GANK is a novel marker for prognosis and therapeutic target in gastric cancer. Mol Biol. 2014;48(1):84–90. | ||
Yang Y, Zhang C, Li L, et al. Up-regulated oncoprotein P28GANK correlates with proliferation and poor prognosis of human glioma. World J Surg Oncol. 2012;10:169. | ||
Fu J, Chen Y, Cao J, et al. p28GANK overexpression accelerates hepatocellular carcinoma invasiveness and metastasis via phosphoinositol 3-kinase/AKT/hypoxia-inducible factor-1alpha pathways. Hepatology. 2011;53(1):181–192. | ||
Ortiz CM, Ito T, Tanaka E, et al. Gankyrin oncoprotein overexpression as a critical factor for tumor growth in human esophageal squamous cell carcinoma and its clinical significance. Int J Cancer. 2008;122(2):325–332. | ||
Umemura A, Itoh Y, Itoh K, et al. Association of gankyrin protein expression with early clinical stages and insulin-like growth factor-binding protein 5 expression in human hepatocellular carcinoma. Hepatology. 2008;47(2):493–502. | ||
Jing H, Zhang G, Meng L, Meng Q, Mo H, Tai Y. Gradually elevated expression of Gankyrin during human hepatocarcinogenesis and its clinicopathological significance. Sci Rep. 2014;4:5503. | ||
Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605. | ||
Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. | ||
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. | ||
Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med. 1997;127(9):820–826. | ||
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. | ||
Krzywda S, Brzozowski AM, Higashitsuji H, et al. The crystal structure of gankyrin, an oncoprotein found in complexes with cyclin-dependent kinase 4, a 19 S proteasomal ATPase regulator, and the tumor suppressors Rb and p53. J Biol Chem. 2004;279(2):1541–1545. | ||
Bai Z, Tai Y, Li W, et al. Gankyrin activates IL-8 to promote hepatic metastasis of colorectal cancer. Cancer Res. 2013;73(14):4548–4558. | ||
Liu Y, Zhang J, Qian W, et al. Gankyrin is frequently overexpressed in cervical high grade disease and is associated with cervical carcinogenesis and metastasis. PLoS One. 2014;9(4):e95043. | ||
Kim YH, Kim JH, Choi YW, et al. Gankyrin is frequently overexpressed in breast cancer and is associated with ErbB2 expression. Exp Mol Pathol. 2013;94(2):360–365. | ||
Zhang J, Yang Y, Zhang Z, et al. Gankyrin plays an essential role in estrogen-driven and GPR30-mediated endometrial carcinoma cell proliferation via the PTEN/PI3K/AKT signaling pathway. Cancer Lett. 2013;339(2):279–287. | ||
Meng Y, He L, Guo X, et al. Gankyrin promotes the proliferation of human pancreatic cancer. Cancer Lett. 2010;297(1):9–17. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



