Back to Journals » Neuropsychiatric Disease and Treatment » Volume 16
Prognostic Analysis of Thymoma-Associated Myasthenia Gravis (MG) in Chinese Patients and Its Implication of MG Management: Experiences from a Tertiary Hospital
Authors Chen D , Peng Y, Li Z, Jin W, Zhou R, Li Y, Xu Q, Yang H
Received 23 December 2019
Accepted for publication 28 March 2020
Published 14 April 2020 Volume 2020:16 Pages 959—967
DOI https://doi.org/10.2147/NDT.S243519
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jun Chen
Di Chen, Yuyao Peng, Zhibin Li, Wanlin Jin, Ran Zhou, Yi Li, Qiushuang Xu, Huan Yang
Department of Neurology, Xiangya Hospital, Central South University, Changsha 410008, People’s Republic of China
Correspondence: Huan Yang
Department of Neurology, Xiangya Hospital, Central South University, Changsha 410008, People’s Republic of China
Email [email protected]
Background: Myasthenia gravis (MG) is an autoantibody-mediated neuromuscular disorder. Approximately 10– 20% of all MG patients experience thymoma (benign tumor arising from thymus tissue). Thymectomy has been the standard of care for thymomatous myasthenia gravis (TMG). However, the clinical outcome of TMG after thymectomy has not been sufficiently studied, especially the long-term prognosis. Therefore, the aim of this study was to analyze the clinical characteristics contributing to the prognostic factors of TMG.
Methods: We reviewed 70 TMG patients in the Xiangya Hospital and classified them into the minimal manifestation (MM) group and No MM group, according to the long-term treatment outcome. MM-or-better status was defined as the goal treatment for TMG patients. We collected and analyzed the demographic data, the WHO classification of thymoma, MG-associated antibody levels, disease severity, treatment-related data as well as clinical outcome at six months. Variables selected by univariate analysis were used in the multivariate logistic regression model to identify the prognostic factors.
Results: The differences in clinical outcome at six months and worst QMGS were significant, while the differences in other factors were insignificant between groups. Clinical outcome at six months (OR=23.5 95% CI 2.4– 231.5, P=0.007) and dyspnea before thymectomy (OR=0.2, 95% CI 0.03– 0.75, P=0.021) were identified as the prognostic factors of long-term treatment.
Conclusion: Demographic and clinical features were similar in TMG patients treated at our hospital. The early achievement of MM-or-better status may indicate a good outcome in the long term. Dyspnea before thymectomy appears to associate with a poor prognosis.
Keywords: myasthenia gravis, thymoma, the WHO classification, clinical presentation, prognostic factors
Introduction
Myasthenia gravis (MG) is an autoimmune neuromuscular disorder predominantly mediated by antibodies against the acetylcholine receptor (AChR).1 Approximately 10–20% of MG patients have thymoma and 30% of thymoma patients have a secondary MG.2,3 Thymoma originates from thymic epithelial cells within the thymic gland and can be categorized by the World Health Organization (WHO) classification based on histological findings. The WHO classification categorizes thymoma into five types, type A, AB, B1, B2, B3, and C.4,5 Thymectomy has been recommended as the first-line treatment of thymoma. Attenuation in MG symptoms has been reported after surgical resection.6 Post-operative radiotherapy was once considered necessary for increasing survival rate and preventing local recurrence of thymoma, typically for patients with advanced stages of the disease. However, a few studies demonstrated that adjuvant radiation after complete tumor resection does not reduce recurrent rates nor improve the survival rate of Masaoka stage 2 thymoma.7,8 Furthermore, irradiation-related exacerbation of MG in TMG patients has been observed. A long duration of MG as well as severe MG before radiotherapy were indicated as the risk factors for these exacerbations.9,10 In the present study, we aimed to analyze and summarize the demographic and clinical characteristics of our TMG patients to determine the prognostic factors of long-term MG treatment outcomes. The outcome of this study may be helpful for clinical management of TMG patients in the future.
Methods
Patients and Data Collection
Records from MG patients who were treated at Xiangya Hospital from July 2013 to July 2019 in the Clinical Neurology department were retrospectively reviewed. The study was approved by the Ethics Committee of Xiangya Hospital and conducted in accordance with the ethical standards put forth in the 1964 Declaration of Helsinki and its later amendments. The certificate number is 201703107. Due to the retrospective design and lack of study-related interventions, no consent to participate was obtained. This manuscript does not contain patient data.
The inclusion criteria are: (1) confirmed diagnosis of MG and thymoma; (2) follow up at least 12 months after diagnosis; and (3) comprehensive demographic and clinical data. The diagnosis of MG was confirmed through typical clinical manifestations, positive edrophonium-testing, electrophysiological recordings consistent with impaired neuromuscular transmission on repetitive nerve stimulation and/or increased jitter single-fiber electromyography (sfEMG) and positive test for specific autoantibodies, the neurologist would be consulted if necessary. Thymoma was confirmed by post-operative histopathological diagnosis. Patients with post-thymectomy MG, severe cardiovascular diseases, other malignant tumors, and type C thymoma (thymic carcinoma) were excluded. We included a total of 70 TMG patients in this study.
The following demographic data were collected: sex, age at disease onset, age at thymectomy, time between MG onset and thymectomy, disease duration, E-L classification (early-onset MG with onset age ≤ 50 or late-onset MG with onset age > 50). We also included the status of MG-associated antibodies, anti-AChR Ab, anti-muscle-specific tyrosine kinase antibody (anti-MuSK) Ab, anti-ryanodine receptor antibody (anti-RyR Ab), and anti-Titin Ab. In our center, patient serum samples were routinely sent to the DAAN Clinical Laboratory Central in Guangzhou, China to detect antibody levels. Due to the high cost of testing, 41 patient samples were tested in the DAAN central, and some of the patients had their antibody levels tested in other institutions before coming to our center. Thus, we only analyzed the antibody results of patients from the DAAN central. According to the center, antibodies against AChR, RyR, and titin were tested with ELISA, while the anti-MuSK Ab was tested by ELISA before October 2017 and with radioimmunoassay after. The antibodies were regarded as positive if anti-AChR Ab > 0.45 nmol/L, anti-Musk Ab > 9.5 pmol/L before October 2017 or >0.05 nmol/L after, anti-RyR Ab > 900 ng/mL, and anti-titin Ab > 187 pg/mL.
Clinical data included: the WHO classification of thymoma, treatment of MG before and after thymectomy, time between onset and treatment of MG, time between surgery and MG treatment, post-operative irradiation as an adjuvant therapy, Osserman’s classification and MG symptoms before and after surgery, worst quantitative myasthenia gravis score (QMGS) after thymectomy, clinical outcome at six months, and treatment outcome by the last visit. In this study, minimal manifestation (MM) status-or-better was defined as the treatment goal. The myasthenia gravis foundation of America (MGFA) classified post-intervention status (PIS) as complete stable remission (CSR), pharmacologic remission (PR), minimal manifestation (MM), improved (I), unchanged (U), worse (W), exacerbation (E) and died of MG (D of MG).11 Patients were separated into the “MM group” or “No MM group” according to the treatment outcome at the last visit. Osserman’s classification, MG symptoms, and worst QMGSs were the indexes of MG severity.
Statistical Analysis
We compared demographic and therapeutic parameters between groups with Student’s t test when the data were normally distributed or the Mann–Whitney test when the data were not normally distributed and Chi-square test or Fisher’s exact test for categorical variable as appropriate. Univariate analysis was used to select the potential prognostic factors of treatment outcome. The factors with a P-value of <0.05 in the univariate analysis were then used in a multivariate logistic regression model to estimate the odds ratios (ORs) and 95% confidence intervals (CIs). A P-value of <0.05 was regarded as significant. We also calibrated the model by comparing the predicted and observed risk and calculating the Hosmer–Lemeshow and C statistic.12,13
All continuous data were reported as mean ± SD (standard deviation) or median (range), and categorical variables were expressed as counts and proportions. A two-tailed P-value < 0.05 was considered statistically significant. Data analysis was carried out using SPSS version 21.0 software (IBM, Armonk, New York).
Results
Demographic Characteristics
A flowchart of patient inclusion is presented in Figure 1. In total, 70 TMG patients with 31 women and 39 men were included (Table 1). Of these, 57 patients reached the long-term treatment goal and 13 failed. The mean age at MG onset was 45.3 ± 9.6 years and the mean age at thymectomy was 45.9 ± 9.4 years. Forty-eight patients (68.6%) were early-onset, while 22 patients (31.4%) were late-onset. The median duration from MG onset to thymectomy was four (1–60) months. The median disease duration was 48 (19–130) months. Differences in sex, age at onset, age at thymectomy, time between MG onset and thymectomy, disease duration, and E-L classification between the groups were insignificant.
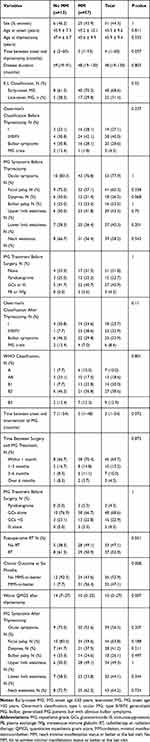 |
Table 1 Demographic and Clinical Characteristics of TMG Patients |
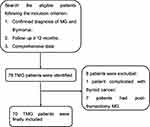 |
Figure 1 The flowchart of the process of patient inclusion. |
MG-Associated Antibody Status
MG-associated antibody levels are shown in Table 2. As mentioned earlier in the methods section, the antibody results of 41 patients were obtained. All patient samples were tested for anti-AChR Ab, and the positive rate was 100%. Antibodies against RyR, MuSK, and titin were tested in 33 patients. Positive anti-MuSK Ab was only found in 1 late-onset female patient with type B3 thymoma. We did not analyze anti-MuSK Ab levels in these patients due to the change of measurement method. Anti-RyR Ab and anti-titin Ab were positive only in two male patients. One was early-onset generalized MG with B1 type thymoma, and the other was early-onset generalized MG with B3 type thymoma. The average titer of the antibodies against AChR, RyR, and titin was 31.5 ± 13.1 (9.3–58.2) nmol/L, 424.7 ± 363.2 (139.7–2123.4) ng/mL, and 115.2 ± 70.4 (37.2–390.6) pg/mL, respectively. The levels of anti-AChR Ab and anti-RyR Ab were higher in the No MM group (anti-AChR Ab 35.8 ± 9.3 (19.4–54.3) nmol/L; anti-RyR Ab 483.1 ± 195.9 (169.44–647.0) ng/mL) than those in the MM group (anti-AChR Ab 30.3 ± 13.9 (9.3–58.2) nmol/L; anti-RyR Ab 411.7 ± 392.4 (139.7–2123.4) ng/mL) even though the differences in the levels of anti-AChR Ab and anti-RyR Ab were insignificant.
 |
Table 2 MG-Associated Antibody Level |
Clinical Features of the Total TMG Patients
Clinical features are shown in Table 1. The numbers of patients with type A, AB, B1, B2, and B3 thymoma were 7 (10.0%), 13 (18.6%), 14 (20.0%), 27 (38.6%), and 9 (12.9%), respectively. There was no significant difference in the WHO classification of thymoma between the groups. Before thymectomy, 19 patients (27.1%) were Osserman stage I, 20 patients (28.6%) had obvious bulbar symptoms, 3 patients (4.3%) suffered from MG crisis and 28 patients (40.0%) were Osserman stage II–IV. After thymectomy, 18 patients (25.7%) were Osserman stage I, 23 patients (32.9%) belonged to Osserman stage II–IV, 23 (32.9%) presented obvious bulbar symptoms and 6 patients (8.6%) had MG crisis. After thymectomy, MG symptoms (ocular symptoms, facial palsy, dyspnea, bulbar palsy, upper and lower limb weakness, and neck weakness) changed only slightly with the exception of less patients presenting with ocular symptoms after the surgery. However, the worst QMGSs were significantly different between the groups (No MM 14 (7–27) vs MM 10 (0–25), P=0.007).
Before the surgery, 21 patients received no anti-myasthenic treatment, 15 used pyridostigmine, 27 received GCs or other immunosuppressants (IS) like tacrolimus, mycophenolate mofetil (MMF), or azathioprine, and 3 patients with severe symptoms accepted plasma exchange (PE) or IVIg to attenuate the symptoms and prepare for thymectomy. The pre-treatment of four patients was unknown. The time between onset and MG intervention of No MM (7 (1–54) months) was insignificantly longer than MM (3 (1–48) months). After thymectomy, most patients started anti-myasthenic treatment within six months; 3 patients (4.3%) only used pyridostigmine, 48 patients (68.6%) initiated with GCs alone, 16 (22.9%) begun with GCs plus an IS (tacrolimus, MMF, or azathioprine), and 3 patients (4.3%) used IS alone. Post-operative irradiation was conducted in 37 TMG patients, and most of them were type B2 (24 patients) and B3 thymoma (7 patients). At six months, 32 patients achieved MM-or-better status, and almost all of them maintained this status by the last visit. The differences in anti-myasthenic treatment before and after thymectomy and post-operative radiotherapy were insignificant.
Prognostic Factors of TMG
To investigate the prognostic factors for the long-term clinical outcome, time between onset of MG and thymectomy, time between onset and intervention of MG, time between surgery and MG treatment, disease duration, post-operative radiation, post-operative MG treatment, the WHO histological classification of thymoma, severity of MG before and after surgery, clinical outcome at six months, and worst QMGS were first analyzed by univariate analysis. Eligible factors were tested by the multivariate logistic regression model. Worst QMGS after thymectomy, dyspnea before thymectomy, and clinical outcome at six months were selected to enter the multivariate model (Table 3). Clinical outcome at six months and dyspnea before thymectomy were identified as independent prognostic factors (Table 4). Clinical outcome at six months was a positive predictor (OR=23.5, 95% CI 2.4–231.5, P=0.007), while dyspnea before thymectomy was negatively associated with the outcome of long-term treatment (OR=0.2, 95% CI 0.03–0.75, P=0.021).
 |
Table 3 Univariate Analysis of Prognostic Factors |
 |
Table 4 Prognostic Factors Identified by Multivariate Regression Model |
Discussion
In this study, we summarized the demographic, MG-associated antibody levels, and clinical characteristics of Chinese TMG patients in our hospital and explored the prognostic factors of the long-term outcome of MG.
Demographic Characteristics
Previous studies have demonstrated that the occurrence of MG is influenced by sex and age: women are affected nearly three times more often than men during early adulthood (aged < 40 years), whereas the incidence is roughly equal during puberty and after 40. After 50 years of age, the incidence is higher in men.14 In our TMG patients, the mean onset age was approximately 45. Most of the patients were late-onset MG. There were only slightly more males than females; this finding is similar to previous reports.15 The disease duration and age at thymectomy were close between the groups. Patients in the No MM group had their thymoma removed after a longer duration than those in the MM group. While this difference was insignificant (P=0.057), early removal of thymoma may still benefit the long-term outcome of MG and the difference may become significant with increased sample size.
MG-Associated Antibody
MG-associated antibodies are the most important biomarkers in guiding MG diagnosis and helping to define MG subtypes. Antibodies against acetylcholine receptors can be detected in 70% of all patients with MG.16 Antibodies against titin are mostly detected in patients with TMG or late-onset MG, while anti-RyR Abs are presented in 70% of anti-AChR Ab positive MG (AChR-MG) and in 14% of patients with late-onset AChR-MG.17,18 In our study, anti-AChR Ab was positive in all patients and present at high concentrations. The high level of anti-AChR Ab might be caused by autoreactive T cells that emerge from defective negative selection in the thymoma.19 These helper T cells activate B cells to produce anti-AChR Ab in the periphery.20 The patients with type B1 thymoma had the lowest anti-AChR Ab titer possibly due to the excision of thymoma. In seven out of the eight patients with type B1 thymoma, we tested for antibodies after surgical removal of the thymoma. Downregulated antibody levels were thought to be due to termination of autoreactive T cell production. However, the positive rates of anti-titin Ab and anti-RyR Ab in our TMG patients were much lower than previous studies.21–23 The difference in these antibodies might be caused by the varying methods of measurement. For example, Yamamoto et al tested anti-titin Ab levels with a radioligand assay while anti-titin Ab in our study was measured by ELISA.23 Previous studies also indicated that anti-RyR Ab and anti-titin Ab may positively correlate with the severity of MG.18,22 Nevertheless, we only found a positive correlation between anti-AChR Ab level and worst QMGS (r=0.511, P=0.015). Ethnic heterogeneity may contribute to the difference. A Norwegian study showed that patients with high anti-titin Ab levels had a more severe course of MG, but a study on Japanese patients showed the opposite result when controlled for onset age.17,23 Other factors like medication regimen and the scale of disease severity at the time of serum sample collection could lead to inconsistent results. In this study, our results indicated that MG-associated antibodies were not associated with the prognosis of MG.
Clinical Manifestations
The incidence of thymoma in our MG patients was B2 > B1 > AB > B3 > A. Most patients had generalized MG with the Osserman stage II–IV and bulbar symptoms regardless of thymectomy. The patients with type A thymoma mainly complained of pure ocular symptoms, while type B thymoma patients presented weakness in other skeletal muscles. Ocular symptoms were the most common symptoms, but there was an obvious drop in the number of patients with ocular symptoms after thymectomy. We assumed that ocular symptoms might be attenuated after thymectomy. The number of patients with bulbar palsy and dyspnea was similar before thymectomy, while more patients presented dyspnea after thymectomy. The severity of MG could be a risk factor for the clinical outcome of MG.13 Our analysis identified dyspnea before thymectomy as a prognostic factor of the long-term treatment outcome. No other evidence in our study supported that WHO classification of thymoma was associated with the long-term treatment of MG. In this study, we excluded patients with type C thymoma or thymic carcinoma based on its extremely low incidence in MG and its carcinoma resembling cytoarchitecture, which is different from other types of thymoma.24 We believe the bias caused by excluding type C thymoma was limited.
MG symptoms barely changed in most patients after thymectomy, but six patients had myasthenic crisis (MC), a life-threatening medical emergency that requires ventilatory support. The incidence of post-operative MC in our patients was much lower than previous reports.12,25 This may result from the relatively mild symptoms and well-controlled disease severity by the intensive peri-operative management from the multiple disciplinary cooperation. Before surgery, the neurologist in our hospital would diagnose, assess, and stage the TMG patients to offer them a tailored anti-myasthenic treatment. The pre-operative treatment usually includes anti-cholinesterase agents and/or a medium dose of GCs. For patients who had contraindications for GCs, proper IS would be prescribed as an alternative. When the symptoms were severe and needed to be attenuated quickly, PE and IVIg were used.
Our analysis showed that the pre-operative MG treatments in the MM and No MM groups were similar and indicated that the pre-operative therapy of MG may not be associated with the long-term treatment outcome. We also investigated the time between onset and intervention of MG of the two groups. Although the duration between onset and MG treatment of the No MM group was longer than the MM group with insignificant difference, we still think it is crucial to initiate intervention of MG as soon as possible. When MG symptoms were controlled, patients would be referred to thoracic surgeons for thymectomy, and after operation, they were asked to visit a neurologist for the evaluation of disease severity and anti-myasthenic therapy within one month. A majority of the patients started MG treatment shortly after the surgery, and only a few maintained it over three months due to the mild symptoms after thymectomy. No difference in the time between thymectomy and MG treatment was found in this study. GCs (prednisone or prednisolone) alone or the combination of GCs with azathioprine is the first-line immunosuppressive therapy for all MG subgroups and for generalized MG patients; prednisolone combined with other immunosuppressants is recommended to obtain maximum effect with minimal adverse effects for long-term treatment.26 Our results showed that the rates of achieving long-term treatment goals were close in the patients who received the GCs alone, GCs plus IS, and IS alone. Thus, for the treatment of MG after thymectomy, GCs plus other IS might be no better than GCs alone or IS alone.
Additionally, patients were recommended to visit the radiation oncologist for a radiotherapy consultation after discharge. Generally, total thymectomy is enough for type A thymoma. Post-operative radiation as an adjuvant treatment was preferred in incomplete excision and in type B thymoma at an advanced stage. Most patients started radiotherapy within one month after thymectomy unless their MG symptoms were severe or the physical conditions were too poor to tolerate radiotherapy. Li’s study found that adjuvant radiation could induce the exacerbation of MG, and longer disease duration as well as more severe MG symptoms before irradiation were the risk factors of the worsening.9 Only two out of 30 patients who received radiotherapy showed a deterioration in their MG symptoms. The short duration between MG onset and thymectomy as well as a lower severity of MG in our patients might explain the low incidence of deterioration during irradiation. Furthermore, our analysis indicated that post-operative irradiation may have no effect on the long-term treatment outcome of MG. Therefore, we suggest that TMG patients should have their thymomas removed in a timely manner followed by aggressive peri-operative management and monitoring by multiple disciplines. This will aid in avoiding MC and irradiation-induced worsening after thymectomy.
QMGS was tested periodically to monitor the disease severity and to instruct the modification of clinical management. We used the worst QMGS as an index of maximum severity during the follow-up after thymectomy and our results indicate that the maximum severity of the No MM group was worse than the MM group. However, the drawback is that the worst QMGS does not necessarily reflect the severity at the beginning of the disease. The time-weighed QMGS can offer a comprehensive view of QMGS change through time and should be applied in future studies.
Previously, some researchers used CSR as the treatment goal, yet it may not be an appropriate method since less than 10% of MG patients achieve this status.27 MM-or-better status might be a more suitable goal for MG treatment.1 Therefore, we applied the MM-or-better status and investigated the clinical outcome at six months and the last visit. We found that there were more patients in the No MM group than the MM group failed to reach the treatment goal at six months. The multiple regression analysis also identified the clinical treatment at six months as a protective factor of the long-term treatment outcome. It may suggest that patients who achieved the treatment goal within first six months would be more likely to achieve the long-term treatment goal.
Limitations
It should be noted that this study has several limitations. The most significant is that it is a retrospective study in a single center with the possibility of selection bias. The antibody tests were performed in just a fraction of patients and not on all patients either before or after the surgery or immunosuppressive therapy. There is no appropriate way to compare the change of antibody levels resulting from thymectomy or immunosuppressive treatment. Masaoka staging of thymoma is a predictive factor of thymoma prognosis; however, the staging of thymoma was not provided in the record system in our hospital. In addition, the sample size was relatively small, especially the No MM group, which could increase the risk of overestimating the predictive performance of the model.28 A cohort study with larger sample size in multiple centers would be required in the future.
Conclusions
The demographic and clinical characteristics were similar between our TMG patients. Early achievement of MM-or-better status may indicate a good treatment outcome in the long run. Dyspnea symptoms before thymectomy may be associated with a poor prognosis. Thus, we recommend aggressive anti-myasthenic treatment for TMG patients, especially those with dyspnea symptoms, to reach MM-or-better status as soon as possible.
Acknowledgment
This work is supported by the National Natural Science Foundation of China [grant number: 1571173] and we sincerely thanked Dr. Asheebo Rojas (Emory University, GA, USA) for his generous help with the language.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sanders DB, Wolfe GI, Benatar M, et al. International consensus guidance for management of myasthenia gravis: executive summary. Neurology. 2016;87(4):419–425. doi:10.1212/WNL.0000000000002790
2. Maggi L, Andreetta F, Antozzi C, et al. Thymoma-associated myasthenia gravis: outcome, clinical and pathological correlations in 197 patients on a 20-year experience. J Neuroimmunol. 2008;201:237–244. doi:10.1016/j.jneuroim.2008.07.012
3. Marx A, Pfister F, Schalke B, Saruhan-Direskeneli G, Melms A, Ströbel P. The different roles of the thymus in the pathogenesis of the various myasthenia gravis subtypes. Autoimmun Rev. 2013;12(9):875–884. doi:10.1016/j.autrev.2013.03.007
4. Okumura M, Ohta M, Tateyama H, et al. The World Health Organization histologic classification system reflects the oncologic behavior of thymoma: a clinical study of 273 patients. Cancer. 2002;94(3):624–632. doi:10.1002/cncr.10226
5. Kondo K, Yoshizawa K, Tsuyuguchi M, et al. WHO histologic classification is a prognostic indicator in thymoma. Ann Thorac Surg. 2004;77(4):1183–1188. doi:10.1016/j.athoracsur.2003.07.042
6. Lanska DJ. Indications for thymectomy in myasthenia gravis. Neurology. 1990;40(12):1828–1829. doi:10.1212/WNL.40.12.1828
7. Chen YD, Feng QF, Lu HZ, et al. Role of adjuvant radiotherapy for stage II thymoma after complete tumor resection. Int J Rad Oncol Biol Phys. 2010;78(5):1400–1406. doi:10.1016/j.ijrobp.2009.09.066
8. Berman AT, Litzky L, Livolsi V, et al. Adjuvant radiotherapy for completely resected stage 2 thymoma. Cancer. 2011;117(15):3502–3508. doi:10.1002/cncr.25851
9. Li Y, Chen P, Ding L, et al. Clinical outcome and predictive factors of irradiation-associated myasthenia gravis exacerbation in thymomatous patients. Neurol Sci. 2015;36(11):2121–2127. doi:10.1007/s10072-015-2325-8
10. Lysandropoulos AP, Mavroudakis N. Exacerbation of myasthenia gravis after postoperative radiotherapy for a thymoma. Acta Neurol Belg. 2012;112(1):65–66. doi:10.1007/s13760-012-0004-6
11. Jaretzki A, Barohn RJ, Ernstoff RM, et al. Myasthenia gravis: recommendations for clinical research standards. Neurology. 2000;55(1):16–23. doi:10.1212/WNL.55.1.16
12. Xue L, Wang L, Dong J, et al. Risk factors of myasthenic crisis after thymectomy for thymoma patients with myasthenia gravis. Eur J Cardiothorac Surg. 2017;52:692–697. doi:10.1093/ejcts/ezx163
13. Kanai T, Uzawa A, Kawaguchi N, et al. Predictive score for oral corticosteroid-induced initial worsening of seropositive generalized myasthenia gravis. J Neurol Sci. 2019;396(2019):8–11. doi:10.1016/j.jns.2018.10.018
14. Grob D, Brunner N, Namba T, Pagala M. Lifetime course of myasthenia gravis. Muscle Nerve. 2008;37(2):141–149. doi:10.1002/mus.20950
15. Zekeridou A, McKeon A, Lennon VA. Frequency of synaptic autoantibody accompaniments and neurological manifestations of thymoma. JAMA Neurol. 2016;73(7):853–859. doi:10.1001/jamaneurol.2016.0603
16. Gilhus NE. Myasthenia gravis. N Engl J Med. 2016;375(26):2570–2581. doi:10.1056/NEJMra1602678
17. Romi F, Skeie GO, Gilhus NE, Aarli JA. Striational antibodies in myasthenia gravis: reactivity and possible clinical significance. Arch Neurol. 2005;62(3):442–446. doi:10.1001/archneur.62.3.442
18. Romi F, Skeie GO, Aarli JA, Gilhus NE. The severity of myasthenia gravis correlates with the serum concentration of titin and ryanodine receptor antibodies. Arch Neurol. 2000;57(11):1596–1600. doi:10.1001/archneur.57.11.1596
19. Marx A, Willcox N, Leite MI, et al. Thymoma and paraneoplastic myasthenia gravis. Autoimmunity. 2010;43(5–6):413–427. doi:10.3109/08916930903555935
20. Buckley C, Douek D, Newsom‐Davis J, Vincent A, Willcox N. Mature, long‐lived CD4+ and CD8+ T cells are generated by the thymoma in myasthenia gravis. Ann Neurol. 2001;50(1):64–72. doi:10.1002/ana.1017
21. Chen XJ, Qiao J, Xiao BG, Lu CZ. The significance of titin antibodies in myasthenia gravis. J Neurol. 2004;251(8):1006–1011. doi:10.1007/s00415-004-0479-z
22. Mygland A, Aarli JA, Matre R, Gilhus NE. Ryanodine receptor antibodies related to severity of thymoma associated myasthenia gravis. J Neurol Neurosurg Psychiatry. 1994;57(7):843–846. doi:10.1136/jnnp.57.7.843
23. Yamamoto AM, Gajdos P, Eymard B, et al. Anti-titin antibodies in myasthenia gravis: tight association with thymoma and heterogeneity of nonthymoma patients. Arch Neurol. 2001;58(6):885–890. doi:10.1001/archneur.58.6.885
24. Detterbeck FC. Clinical value of the WHO classification system of thymoma. Ann Thorac Surg. 2006;81:2328–2334. doi:10.1016/j.athoracsur.2005.11.067
25. Li Y, Wang H, Chen P, et al. Clinical outcome and predictive factors of postoperative myasthenic crisis in 173 thymomatous myasthenia gravis patients myasthenic crisis in thymomatous patients. Int J Neurosci. 2017:01–26. doi:10.1080/00207454.2017.1366905.
26. Gilhus NE, Skeie GO, Romi F, Lazaridis K, Zisimopoulou P, Tzartos S. Myasthenia gravis-autoantibody characteristics and their implications for therapy. Nat Rev Neurol. 2016;12(5):259. doi:10.1038/nrneurol.2016.44
27. Imai T, Utsugisawa K, Murai H, et al. Oral corticosteroid dosing regimen and long-term prognosis in generalised myasthenia gravis: a multicentre cross-sectional study in Japan. J Neurol Neurosurg Psychiatry. 2018;89(5):513–517. doi:10.1136/jnnp-2017-316625
28. Moons KG, Royston P, Vergouwe Y, Grobbee DE, Altman DG. Prognosis and prognostic research: what, why, and how? BMJ. 2009;338:b375. doi:10.1136/bmj.b375
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
