Back to Journals » Cancer Management and Research » Volume 10
Prognosis of rare pathological primary urethral carcinoma
Authors Abudurexiti M, Wang J, Shao N, Wan FN, Zhu Y, Dai B, Ye D
Received 16 August 2018
Accepted for publication 13 November 2018
Published 7 December 2018 Volume 2018:10 Pages 6815—6822
DOI https://doi.org/10.2147/CMAR.S184197
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Kenan Onel
Mierxiati Abudurexiti,1,2,* Jun Wang,1,2,* Ning Shao,1,2 Fang-Ning Wan,1,2 Yao Zhu,1,2 Bo Dai,1,2 Ding-Wei Ye1,2
1Department of Urology, Fudan University Shanghai Cancer Center, Shanghai, China; 2Department of Oncology, Shanghai Medical College, Fudan University, Shanghai, China
*These authors contributed equally to this work
Purpose: Urethral carcinoma (UC), as a rare tumor, is not widely studied. There have been no systematic studies of rare pathological types of UC. We conducted this study to further investigate rare pathological types of primary urethral carcinoma (PUC).
Materials and methods: We used the population-based Surveillance, Epidemiology, and End Results (SEER) database to evaluate prognostic factors in rare pathological types of PUC. From 1978 to 2015, 2,651 and 257 cases were identified in the SEER database as common and rare pathological types of PUC, respectively. Overall and cancer-specific survival (CSS) times were computed using the Kaplan–Meier method, and the Cox proportional hazards analysis was used to evaluate patient age at diagnosis, gender, race, and TNM stage.
Results: The median overall survival (OS) rates were 36 and 59 months for rare and common pathological groups, respectively, and their respective 10-year OS rates were 31.9% and 42.4%, respectively. The median CSS rate was 61 months for the rare pathological group. Through multivariate analysis, it was found that age, race, T stage, and M stage were independent prognostic risk factors for rare pathological type of urethral cancer. In the age group, the HR ratio of patients aged older than 60 years and younger or equal to 60 years was 2.778 (P<0.001). The HR ratio of other races to Whites was 1.444 (P=0.040). In TNM staging, the HR ratio between T3–T4 and Ta–T2 was 2.386 (P=0.046), and the HR value of M1 and M0 was 5.847 (P<0.001).
Conclusion: Age, race, T stage, and M stage were predictive of OS and CSS in rare pathological PUC.
Keywords: urethral cancer, SEER, age, race, TNM
Introduction
When talking about urethral carcinoma (UC), what we tend to think of is its rare nature, accounting for less than 1% of malignancy.1 Owing to its rare nature, clinicians have failed to notice the occurrence and pathophysiology of UC. What is more, no one has mentioned the effect of rare pathological types on individuals.
Cancer statistics of 2017 revealed the data of ureter and other urinary organs, including an estimated 3,630 new cases and 920 estimated deaths.2 The annual incidence of primary urethral carcinoma (PUC) is estimated to be 1.6/million in men and 0.6/million in women with an age-standardized ratio in Europe, while in the USA, the annual incidence is 4.3/million in men and 1.5/million in women, as analyzed by the Surveillance, Epidemiology, and End Results (SEER) registry.3,4
Predominant histological types of UC are composed of urothelial carcinoma of the urethral (54%–65%), squamous cell carcinoma (SCC; 16%–22%), and adenocarcinoma (AC; 10%–16%), as recorded in the European Association of Urology guidelines of 2018.5 If the above three pathological types are proposed as common pathological types, then what we are mainly discussing in this paper is the collection of all the pathological types except the above three, which we will call rare pathological types. The relation between survival time and gender, age, race, and TNM stage of common pathological types of PUC has been demonstrated by many researchers.6–10 Until now, rare pathological types of PUC have not been mentioned in any paper. In this study, we discuss rare pathological types of PUC, analyzing the relationship between survival time and the gender, age, race, and TNM stage.
Materials and methods
We collected PUC data from the SEER registry from the years 1978–2015, where the sequence number – central code number “0, 1” is defined as PUC. T0 stage was also excluded as no evidence of primary tumor. According to the pathological type, the data were divided into two groups, the common pathological type group and the rare pathological type group. A total of 2,908 PUC cases were collected, of which 257 cases were of a rare pathological type and 2,651 cases were of a common type. We excluded urothelial carcinoma (8120, 8122, 8130, 8131), SCC (8070–8076, 8050–8052), and AC (8140, 8144, 8200, 8240, 8260–8262, 8310, 8323, 8380, 8460, 8480, 8481, 8490, 8500, 8542, 8560, 8570). Data with specific survival times were included in this study (Figure S1). In order to study the 10-year survival time, any time periods of more than 10 years were adjusted to 10 years, and the corresponding survival status was also adjusted. “Dead” was changed into “alive” in the survival status where survival times were more than 10 years. The ages were divided into old and young groups according to the WHO age classification standard, with the cutoff point at 60-years old.11 Race was marked as “White” and “Others”. In T staging, Ta–T2 were combined in one group, T3–T4 were combined in another group, and not mentioned and undocumented in SEER registries was the third group. The presence of lymph node metastasis is the criteria for N staging, and the metastatic criterion was also used in M staging.
All statistical analyses were performed using SPSS 22.0 (IBM Corporation, Armonk, NY, USA). All tumor characteristics were compared using chi-square analyses. Survival curves were generated by the Kaplan–Meier method, and the differences between curves were analyzed using log-rank tests. Univariate and multivariate Cox hazard analyses were used to determine factors correlated with survival. Two-sided P<0.05 was considered to indicate statistical significance.
Ethical approval
SEER data are deidentified before release and do not contain any personally identifying information. As the data are publicly available, no ethical approval is required.
Results
The number of male and female patients in the common pathological type group was 1,761 and 890, respectively, while the number of patients in the rare pathological type group was 117 and 140, respectively, which were statistically significant (P<0.001). In the TNM stage, there was also statistical significance between the two groups of data (P<0.001; Table 1). The survival analysis between the common and rare pathologic groups showed that there was a statistical significance between the 10-year overall survival (OS) and the cancer-specific survival (CSS; P<0.001; Figure 1A and B). The median OS time was 36 and 59 months for rare and common pathological groups, respectively, and their respective 10-year OS rates were 31.9% and 42.4%, respectively. The median CSS time was 61 months for the rare pathological group, while it was not reached in the common pathological group, and their respective 10-year CSS rates were 49.8% and 61%, respectively. It can be seen from the univariate analysis results of rare pathological types that there is statistical significance in age groups (P<0.001), T stages (P=0.018; P=0.010; P=0.032), and M stages (P<0.001; P<0.001; P=0.074; Table 2). It can be also seen from the survival curve that there are differences in age, T stage, and M stage in survival (Figures 2C and D and 3A–B and E–F) and no differences in race, gender, and N stage (Figures 2A, B, E, and F and 3C–D). However, in multivariate analysis, it was found that age, race, T stage, and M stage were independent prognostic risk factors for rare pathological types of urethral cancer (Table 3). The HR ratio of patients aged older than 60 years and younger or equal to 60 years was 2.778 (P<0.001). The HR ratio of other races to Whites was 1.444 (P=0.040). In TNM staging, the HR ratio between T3–T4 and Ta–T2 was 2.386 (P=0.046), and the HR value of M1 and M0 was 5.847 (P<0.001).
  | Table 1 Basic information |
  | Figure 1 Survival analysis between the common and rare pathologic groups: (A) OS and (B) CSS. Abbreviations: OS, overall survival; CSS, cancer-specific survival. |
  | Table 2 Univariate analysis of OS in common pathological type Abbreviations: OS, overall survival; Ref, reference. |
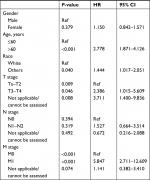  | Table 3 Multivariate analysis of OS in common pathological type Abbreviations: OS, overall survival; Ref, reference. |
Discussion
This study used the latest SEER data to analyze the gender, age, ethnicity, and pathological TNM staging of patients with rare UCs. When we compared rare types with common pathologic types, we found that the prognosis of rare pathological types was relatively poor and the median difference in survival time between the two groups was almost 2 years, which was indeed surprising. Our study is the first to study pathologic types in two large groups (common and rare). In univariate analysis, age and T and M staging were statistically significant for OS, and when we put these data into multivariate analysis, we found that race was an independent prognostic factor in addition to the abovementioned three. This may be the reason that confounders cannot be excluded in univariate analysis, so we considered age, ethnicity, and T and M staging to be independent prognostic factors for rare types of urethral cancer.
In previous studies, the 10-year survival rate of urethral cancer was 29.3%.12 These results were obtained from data of all pathological types of UC patients and were not able to distinguish the pathological types. However, in our study, the 10-year survival rate of rare and common pathological types of urethral cancer was 31.9% and 42.4%, respectively, while the 10-year CSS rate was 49.8% and 61%, respectively. In particular, there was no further understanding of the rare pathological types of PUC. Through our analysis, we found that the prognosis of UC with rare pathologic types was indeed poor compared with the prognosis of common pathological types (urothelial carcinoma, SCC, AC).
In this study, there was no difference in prognosis between men and women, which has also been confirmed in other studies.13,14 However, although SCC is most common among women,15 studies have reported that women’s prognosis is poorer than that of men in AC.13 There have also been reports of higher clinical stages of AC in Black women than men.14 In the USA, the onset time of male and female urethral cancer is approximately 60 years.15,16 In this study, we studied the age group with 60-years old as the dividing line, and the prognosis of patients older than 60 years with rare pathological cases was worse than that of patients younger or equal to 60 years. Sui et al13 reported that the prognosis for Blacks is worse than that for non-Blacks. In our study, Whites had a better prognosis than other races. The study of Rabbani17 showed that the higher the T stage, the worse the prognosis of the disease; in the N stage, the positive lymph node patients had a worse prognosis than the negative patients; in the M stage, distant metastasis had a relatively poor prognosis. Gakis et al18 evaluated survival factors in a large international cohort study, which showed that recurrence-free survival of PUC was significantly associated with clinical nodal stage, tumor location, and age; however, clinical nodal stage was the only independent predictor for OS of PUC. In our rare pathological types of UC, lymph node positivity was not statistically significant, while T staging and M staging were consistent with Rabbani’s results. This may be relevant to fewer cases in our rare group. Cahn et al19 demonstrated that definitive multimodal therapy (definitive surgery [cystectomy]+systemic therapy± radiotherapy) for PUC of urothelial histology had OS benefits; however, this survival benefit was not demonstrated with squamous or AC histologies. For the rare types, it is not clear whether the multimodal therapy is beneficial, and it requires more clinical research. We hope to bring more attention to the treatment of rare pathological types of PUC through our presentation on the prognosis.
There are corresponding limitations in this study, which are summarized as follows: First, studies have reported that distal urethral tumors have a better prognosis than proximal urethral tumors.20 There are no data on the specific cancer location in the SEER database, which is why we failed to classify the cancer in this study. Second, there is no record of chemotherapy in the SEER database, so we cannot know whether chemotherapy affects prognosis at the time of this study. Third, this study did not classify the treatment process, such as surgery or local radiotherapy, which may also have had an impact on the results. Fourth, due to the limited number of cases, the rare pathological types cannot be further subdivided and discussed.
Conclusion
After screening PUC in the SEER database between 1978 and 2015, data were divided into two groups according to common or uncommon pathological types. We found that age, race, T stage, and M stage were independent prognostic factors of rare UCs. This may have a predictive prognostic effect for our future clinical work. As a rare disease, UC requires further studies with gene or molecular typing to better diagnose and treat UC patients.
Acknowledgment
This study was sponsored by the National Natural Science Foundation of China (No. 81472377) and the Natural Science Foundation of Shanghai (No. 16ZR1406500).
Disclosure
The authors report no conflicts of interest in this work.
References
Gatta G, van der Zwan JM, Casali PG, et al. Rare cancers are not so rare: the rare cancer burden in Europe. Eur J Cancer. 2011;47(17):2493–2511. | ||
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. | ||
Visser O, Adolfsson J, Rossi S, et al. Incidence and survival of rare urogenital cancers in Europe. Eur J Cancer. 2012;48(4):456–464. | ||
Swartz MA, Porter MP, Lin DW, Weiss NS. Incidence of primary urethral carcinoma in the United States. Urology. 2006;68(6):1164–1168. | ||
N’Dow J. Primary urethral carcinoma - limited update March 2018. Eur Urol. 2018;6. | ||
Champ CE, Hegarty SE, Shen X, et al. Prognostic factors and outcomes after definitive treatment of female urethral cancer: a population-based analysis. Urology. 2012;80(2):374–382. | ||
Dalbagni G, Zhang ZF, Lacombe L, Herr HW. Male urethral carcinoma: analysis of treatment outcome. Urology. 1999;53(6):1126–1132. | ||
Dimarco DS, Dimarco CS, Zincke H, et al. Surgical treatment for local control of female urethral carcinoma. Urol Oncol. 2004;22(5):404–409. | ||
Dimarco DS, Dimarco CS, Zincke H, et al. Outcome of surgical treatment for primary malignant melanoma of the female urethra. J Urol. 2004;171(2 Pt 1):765–767. | ||
Ouzaid I, Hermieu JF, Dominique S, Fernandez P, Choudat L, Ravery V. Management of adenocarcinoma of the female urethra: case report and brief review. Can J Urol. 2010;17(5):5404–5407. | ||
WiKiPEDIA [homepage on the Internet]. Old age. Available from: https://en.wikipedia.org/wiki/Old_age. Accessed November 15, 2018. | ||
Zhang M, Adeniran AJ, Vikram R, et al. Carcinoma of the urethra. Hum Pathol. 2018;72:35–44. | ||
Sui W, Roychoudhury A, Wenske S. Outcomes and prognostic factors of primary urethral cancer. Urology. 2017;100:180–186. | ||
Wei Y, Wu YP, Xu N, et al. Sex-related differences in clinicopathological features and survival of patients with primary urethral carcinoma: a population-based study. Onco Targets Ther. 2017;10:3381–3389. | ||
Dalbagni G, Zhang ZF, Lacombe L, Herr HW. Female urethral carcinoma: an analysis of treatment outcome and a plea for a standardized management strategy. Br J Urol. 1998;82(6):835–841. | ||
Dalbagni G, Zhang ZF, Lacombe L, Herr HW. Male urethral carcinoma: analysis of treatment outcome. Urology. 1999;53(6):1126–1132. | ||
Rabbani F. Prognostic factors in male urethral cancer. Cancer. 2011;117(11):2426–2434. | ||
Gakis G, Morgan TM, Efstathiou JA, et al. Prognostic factors and outcomes in primary urethral cancer: results from the international collaboration on primary urethral carcinoma. World J Urol. 2016;34(1):97–103. | ||
Cahn DB, Handorf E, Ristau BT, et al. Contemporary practice patterns and survival outcomes for locally advanced urethral malignancies: A national cancer database analysis. Urol Oncol. 2017;35(12):670.e15–670. | ||
Dayyani F, Hoffman K, Eifel P, et al. Management of advanced primary urethral carcinomas. BJU Int. 2014;114(1):25–31. |
Supplementary material
  | Figure S1 Flow chart on inclusion and exclusion of patients. Abbreviation: SEER, Surveillance, Epidemiology, and End Results. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


