Back to Journals » OncoTargets and Therapy » Volume 7
Prognosis of patients with hepatocellular carcinoma and hypersplenism after surgery: a single-center experience from the People's Republic of China
Authors Li C, Zhao H, Zhao J, Li Z, Huang Z, Zhang Y, Bi X, Cai J
Received 26 March 2014
Accepted for publication 17 April 2014
Published 9 June 2014 Volume 2014:7 Pages 957—964
DOI https://doi.org/10.2147/OTT.S64921
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Cong Li, Hong Zhao, Jianjun Zhao, Zhiyu Li, Zhen Huang, Yefan Zhang, Xinyu Bi, Jianqiang Cai
Department of Abdominal Surgery, Cancer Institute and Hospital, Peking Union Medical College and Chinese Academy of Medical Sciences, Beijing, People's Republic of China
Purpose: As prognosis of patients with hepatocellular carcinoma (HCC) and hypersplenism is rarely reported, this study examined prognostic factors for patients who underwent surgery for this condition.
Patients and methods: This study retrospectively analyzed prognostic factors in 181 consecutive HCC patients using univariate and multivariate analyses, as well as subgroup analyses for disease-free survival (DFS) and overall survival (OS) of two groups: one group who received splenectomies (Sp) and one group who did not (non-Sp).
Results: 1, 3, and 5 year OS rates were 88.4%, 67.1%, and 52.8%, respectively; corresponding DFS rates were 67.0%, 43.8%, and 31.6%, respectively. Age ≥55 years old, cigarette smoking, tumor size ≥5 cm, microvascular invasion, and Child-Pugh grade B (versus A) correlated significantly with OS (P<0.05). Interestingly, in patients with tumor lymph node metastasis (TNM) stage I disease, DFS of the Sp-group (median DFS, 24.1 months; n=34) was significantly lower than that of the non-Sp group (median DFS, 62.1 months; n=74), P=0.034; whereas at TNM stage II, OS of the Sp-group (median OS, 79.1 months; n=21) was significantly better than that of the non-Sp group (median OS, 23.3 months; n=30), P=0.018.
Conclusion: Hepatectomy without concomitant splenectomy can contribute to improved DFS of TNM stage I HCC patients with hypersplenism, whereas simultaneous hepatectomy and splenectomy can prolong OS for patients at TNM stage II.
Keywords: hepatectomy, splenectomy, overall survival, disease-free survival
Introduction
Hepatocellular carcinoma (HCC) is a very common malignancy worldwide, and approximately half of the new cases and deaths reported yearly are believed to occur in the People’s Republic of China.1 The main causes of HCC in the People’s Republic of China are chronic hepatitis B and C virus infections, that result in hepatic cirrhosis, often accompanied by portal hypertension and secondary hypersplenism.2 Hypersplenism has often been considered a contraindication to liver resection in HCC patients due to anemia, leucopenia, and thrombocytopenia.3 Although liver transplantation (LT) is an ideal treatment for these patients and can lead to acceptable long-term outcomes, especially when patients meet Milan criteria,4 the shortage of available organs, tumor progression in candidates on waiting lists, high costs, and graft rejection have resulted in limited LT availability for patients with HCC and hypersplenism (HCC-HSP) for whom surgical resection is an optional treatment. Recent surgical approaches and technologies have reduced complications from liver resection. Additionally, splenectomy has been shown to improve liver function5,6 and increase the safety of liver resection in certain HCC-HSP patients by reducing the possibility of bleeding complications and bilirubin overload.7,8 Therefore, simultaneous and staged hepatectomy and splenectomy are commonly performed in certain HCC-HSP patients. However, the prognostic impacts of surgical treatment for HCC-HSP patients have rarely been studied. To identify post-surgical outcomes and risk factors that can influence patient survival and tumor recurrence in HCC-HSP patients, the current authors reviewed their experiences in treating patients with HCC-HSP over the past 10 years.
Patients and methods
Patients
We established a database of clinicopathologic information obtained from medical records of patients who received surgery for HCC-HSP at the Department of Abdominal Surgical Oncology at the Cancer Institute and Hospital, Chinese Academy of Medical Sciences, Beijing, People’s Republic of China. Hypersplenism was defined as splenomegaly with a peripheral white blood cell (WBC) count of >4 × 109/L or platelet count (PLT) of >100 × 109/L. From January 1999 to February 2013, 181 consecutive patients with HCC-HSP underwent curative liver resections, defined as complete removal of all gross lesions of tumor-free margins. A pathological diagnosis of HCC was confirmed by a senior pathologist. Patients were divided into two groups: a splenectomy group (Sp group, n=60) and a non-splenectomy group (non-Sp group, n=121); that is to say, patients who had a liver resection with or without splenectomy.
Preoperative assessments
Prior to surgery, several routine tests were performed, including but not limited to: complete blood count; liver function panel including alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin (TBIL), albumin (ALB); coagulation tests including prothrombin time (PT) and activated partial thromboplastin time (APTT); hepatitis virus infection index; and α-fetoprotein (AFP). Chest radiography images were examined to exclude pulmonary metastasis. Abdominal ultrasonography (US), contrast-enhanced computed tomography (CT) scans, and magnetic resonance imaging (MRI) were used to assess tumor resectability and extent of splenomegaly. Mild splenomegaly was defined as the largest dimension of spleen >11 cm in size; moderate splenomegaly was defined as the largest dimension of spleen 11–20 cm in size. Child-Pugh score was used to assess liver function reserve. Patients with Child-Pugh grade A, or those with grade B who were expected to recover to grade A after short-term pharmacotherapy, were eligible for surgery.
Surgical procedures
All patients underwent conventional open surgery, including curative wedge hepatectomy, subsegmentectomy, segmentectomy, and hemihepatectomy with minimum resection margins >1 cm. Segmentectomy and hemihepatectomy were classified as major hepatectomy; subsegmentectomy and wedge hepatectomy were classified as minor hepatectomy. In the Sp group, splenectomy was performed first, followed by hepatectomy. During liver resection, cut-ultrasound aspiration (CUSA) was used to avoid intraoperative bleeding. Hepatic inflow occlusion was used only when intrahepatic bleeding was not controllable. To prevent portal and splenic vein thrombosis (PSVT), prophylactic anticoagulant therapy, including daily injection of low molecular weight heparin over the first week after surgery and oral aspirin for 4 weeks, was regularly administered to Sp group patients.
Follow-up evaluations
After discharge from hospital, all patients were followed every 3 months for the first 2 years, and every 6 months thereafter or when clinically indicated. Follow-up data were obtained by mail and telephone correspondence and outpatient department visits. Routine follow-up visits consisted of physical examination, routine blood tests, liver function tests, AFP levels, chest radiography, abdominal US, contrast-enhanced CT scans, and liver MRI. A diagnosis of PSVT was determined by US and CT scan results. Postoperative mortality was defined as death within 30 days of surgery. No follow-up evaluations were performed after November 30, 2013 or following death.
Statistical analyses
Continuous variables are presented as mean ± standard deviations and compared using Mann–Whitney U-test or Fisher’s exact test. The paired t-test was used to compare laboratory data. Categorical results were compared using chi-square. The Kaplan–Meier method was used to determine overall survival (OS) rates and disease-free survival (DFS). Differences in survival outcomes between the two groups were compared by log-rank test. The Cox proportional hazards model was used for multivariate analysis. P<0.05 derived from two-tailed tests was considered significant. All data were analyzed using IBM SPSS version 20.0 statistics software (IBM Corporation, Armonk, NY, USA).
Results
Baseline characteristics
We investigated 181 patients (Table 1) that included 146 males and 35 females, with an average age of 55.6±10.6 years old (median: 55 years old; range: 32–83 years old); 88.4% had hepatitis B virus infection and 87.8% had Child-Pugh grade A liver function.
Clinical characteristics
Clinical pathological characteristics of patients from both groups were compared (Table 2). Except for WBC count, they did not significantly differ in age, sex, Child-Pugh grade, TNM stage, extent of splenomegaly, hepatitis type, tumor location, and pre-surgery AFP levels. Although the Sp group had significantly more patients with larger tumors that required longer surgeries (P<0.05), operative data, including hepatectomy type, amount of blood loss, and need for intraoperative transfusions did not significantly differ between the two groups.
Postoperative complications
Overall incidence of postoperative complications was 22.1%. These included wound infection, pulmonary infection, pleural effusion, ascites, gastrointestinal bleeding, intra-abdominal bleeding, and acute liver failure. Incidence of severe complications such as gastrointestinal bleeding, intra-abdominal bleeding, and acute liver failure did not significantly differ between the two groups (P>0.05). Postoperative mortality was 3.9% overall and did not significantly differ between Sp (5.0%) and non-Sp groups (3.3%) (P>0.05). Seven patients (11.7%) in the Sp group were diagnosed with PSVT by US or CT during routine follow-up visits but had no typical symptoms. They received anticoagulation therapy and achieved complete dissolution, as confirmed by follow-up CT (Table 3).
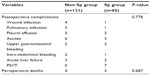  | Table 3 Postoperative complications of patients with HCC and hypersplenism |
Postoperative hematological variables
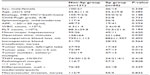
Patient liver function hematological variables recovered well during the study. We compared changes in liver function and HSP-related variables between the two groups. Levels of ALT, AST, and TBIL returned to normal by postoperative day (POD) 90, and there were no significant differences between the two groups (P>0.05). PLT in the Sp group increased rapidly to normal levels by POD 7, and was significantly higher than the non-Sp group at PODs 7, 30, and 90 (P<0.05). No patients required PLT transfusion after surgery. Although pre-surgery WBC counts were significantly lower in the Sp group compared to the non-Sp group, WBC count in the Sp group increased to normal ranges by POD 30 and was significantly higher than in the non-Sp group by POD 90 (P<0.05) (Figure 1).
Long-term outcomes
Median follow-up duration was 25 months (range: 1–169 months). The 1, 3 and 5 year DFS rates were 67.0%, 43.8%, and 31.6%, respectively. Among the 181 patients, 97 experienced recurrence or metastasis and 60 died. Intrahepatic recurrence and metastasis was the main type of disease progression (74.2%, n=72). The most common locations of extrahepatic metastasis were lung (10.3%, n=10), bone (6.2%, n=6), and retroperitoneal lymph nodes (5.2%, n=5). The 1, 3 and 5 year OS rates were 88.4%, 67.1%, and 52.8%, respectively.
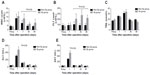
We performed subgroup analyses for survival for both groups. Among patients with stage I disease, DFS in the Sp-group (median DFS, 24.1 months; n=34) was significantly shorter than that of the non-Sp group (median DFS, 62.1 months; n=74), P=0.034. However, among patients with stage II disease, OS in the Sp-group (median OS, 79.1 months; n=21) was significantly better than that of the non-Sp group (median OS, 23.3 months; n=30), P=0.018 (Figure 2). Subgroup analyses found no treatment benefits in any other subgroups, including age, sex, smoking status, alcohol consumption, Child-Pugh grade, degree of splenomegaly, AFP level, and tumor location, size, or number (data not shown).
Univariate analysis
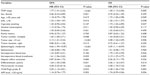
In patients with HCC-HSP, univariate analysis showed that TNM stage, female sex, Karnofsky performance score (KPS) ≤70, multiple tumors, tumor size ≥5 cm, moderate versus (vs) mild splenomegaly, intraoperative blood transfusion, poor cell differentiation, microvascular invasion, and Child-Pugh grade B (vs A) significantly influenced DFS (P<0.05); univariate analysis also showed that TNM stage, age ≥55 years old, cigarette use, tumor size ≥5 cm, moderate vs mild splenomegaly, intraoperative KPS score ≤70, blood loss, intraoperative blood transfusion, poor cell differentiation, microvascular invasion, and Child-Pugh grade B (vs A) significantly influenced OS (P<0.05; Table 4).
Multivariate analyses
Factors found to be significant by univariate analysis were subjected to multivariate analysis to determine adjusted odds ratios (Table 5). Results showed Child-Pugh grade B (vs A) was the only independent predictor of poor DFS (P=0.045), whereas independent factors for OS for patients with HCC with cirrhotic hypersplenism were age ≥55 years old, cigarette use, tumor size ≥5 cm, microvascular invasion, and Child-Pugh grade B (vs A).
Discussion
In the People’s Republic of China, about 85%–90% of HCC patients have liver cirrhosis, which generally results in cirrhotic hypersplenism. Incidence of hypersplenism has been found in 11% to 55% of patient deaths due to cirrhosis and portal hypertension.9 As surgical techniques and perioperative management approaches to preserve liver function improve, liver resection has become safer for patients with HCC and cirrhosis.10,11 Postoperative mortality and morbidity outcomes, which were acceptable in the current cohort, corresponded well with recent studies on surgical treatment strategies in HCC patients with cirrhotic hypersplenism.7,12–14
After surgery, liver function and blood cell counts recovered significantly compared to preoperative levels. Moreover, severe complications and postoperative deaths between Sp and non-Sp groups did not significantly differ. One life-threatening complication after splenectomy was overwhelming post-splenectomy infection (OPSI). Although relatively rare, OPSI has a high mortality rate.15 Risk factors for OPSI include splenectomy in the last three years, patients <5 years old, and patients who have undergone splenectomy for trauma or hematologic disease.16–18 We found no OPSI in the Sp group, probably because all SP patients were adults who had undergone splenectomy for cirrhotic hypersplenism, which is not a risk factor for OPSI. Another common complication after splenectomy was PSVT, with a reported incidence of 6%–55%.19–22 We saw seven cases of PSVT in the Sp group (11.7%), none of them fatal. Reportedly, PSVT incidence after open splenectomy was significantly less than after laparoscopic splenectomy.23 In the current study, all patients underwent open surgery and received regular anticoagulant therapy afterwards, which was thought to reduce PSVT incidence.20,22
Earlier studies have shown prognosis for HCC patients to be mainly determined by tumor burden, liver function, and performance status.24,25 Nevertheless, postoperative outcomes for HCC patients with cirrhotic hypersplenism are seldom reported. In the present study, age ≥55 years old, cigarette use, tumor size ≥5 cm, microvascular invasion, and Child-Pugh grade B (vs A) were identified as independent prognostic factors of OS by multivariate analysis, consistent with previous reports. This indicated that despite association with hypersplenism, risk predictors for HCC patient prognosis after surgery were comparable.
Among these factors, Child-Pugh grade was significantly associated with recurrence rates and long-term survival outcomes. Therefore, prior to planning surgery for HCC patients with cirrhotic hypersplenism, evaluation of liver function reserve and preoperative improvements from Child-Pugh grade B to A was critical. Moreover, patients who require Child-Pugh grade improvements should be followed-up more frequently after surgery to identify possible early recurrence.
Interestingly, cigarette smoking affected long-term outcomes in HCC patients. Studies have associated smoking with HCC development,26,27 and have attributed smoking to three major adverse effects on the liver: direct and indirect toxic effects, immunological effects, and oncogenic effects.28 However, confounding factors such as alcohol, genetics, the environment, and other less clearly defined factors have also been found to affect HCC development. The association between cigarette smoking and HCC-HSP calls for more clinical and basic research.
Similarly to previous studies,12,14,29 results from our multivariate analysis found no correlation between concomitant splenectomy and improved long-term survival in the study population. However, subgroup analysis found that DFS of the non-Sp group was longer than that of the Sp group for stage I disease patients; in contrast, concomitant splenectomy improved long-term survival of patients with stage II disease and, this finding might be explained by the anti-tumor immunological effects of the spleen. The spleen has been reported to prevent carcinogenesis in early-stage tumors, and to inhibit the tumor-induced immune response for late-stage tumors even after tumor resection.13 Concomitant splenectomy for patients with HCC-HSP could improve anti-tumor immune function by promoting balance in T lymphocyte subsets and TH1/TH2 cytokines.30,31 Furthermore, experiments in rat models have confirmed that positive effects of splenectomy on liver regeneration were greater in patients with larger liver resections.32,33 Therefore, it is vital to consider TNM stage when planning surgery in patients with HCC-HSP, especially for patients with stage I or II disease.
Our study was limited by its retrospective nature and sample size. Larger studies are necessary to confirm these results and explore the pathogenic mechanisms of HCC-HSP.
Conclusion
Age ≥55 years old, cigarette smoking, tumor size ≥5 cm, microvascular invasion, and Child-Pugh grade B were independent negative predictors of OS in patients with HCC-HSP. Spleen-preserving strategies could improve DFS of patients with stage I HCC-HSP, whereas concomitant splenectomy (vs hepatectomy only) could prolong survival for patients with stage II disease.
Acknowledgments
This study was supported by grants from the National Natural Science Foundation of China (81201967), the Beijing Natural Science Foundation (7132193), Beijing Nova Program (No. 2009A69), and the State Key Project on Infectious Diseases of China (China National Science and Technology Major Project Grant during the Twelfth Five-year Plan Period No.2012ZX10002–016).
Disclosure
The authors report no conflicts of interest in this work.
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. | |
Schuppan D, Afdhal NH. Liver cirrhosis. Lancet. 2008;371:838–851. | |
Capussotti L, Ferrero A, Vigano L, Muratore A, Polastri R, Bouzari H. Portal hypertension: contraindication to liver surgery? World J Surg. 2006;30(6):992–999. | |
Stanca CM, Fiel MI, Aledort L, Cohen E, del Rio Martin J, Schiano TD. Factors associated with persistent thrombocytopenia after liver transplantation. Transplant Proc. 2010;42:1769–1773. | |
Ikegami T, Shimada M, Imura S. Recent role of splenectomy in chronic hepatic disorders. Hepatol Res. 2008;38:1159–1171. | |
Imura S, Shimada M, Utsunomiya T, et al. Impact of splenectomy in patients with liver cirrhosis: Results from 18 patients in a single center experience. Hepatol Res. 2010;40:894–900. | |
Oh JW, Ahn SM, Kim KS, Choi JS, Lee WJ, Kim BR. The role of splenectomy in patients with hepatocellular carcinoma and secondary hypersplenism. Yonsei Med J. 2003;44(6):1053–1058. | |
Sugawara Y, Yamamoto J, Shimada K, et al. Splenectomy in patients with hepatocellular carcinoma and hypersplenism. J Am Coll Surg. 2000;190(4):446–450. | |
Henderson JM. Surgical treatment of portal hypertension. Baillieres Best Pract Res Clin Gastroenterol. 2000;14(6):911–925. | |
Fan ST, Lo CM, Liu CL, et al. Hepatectomy for hepatocellular carcinoma: toward zero hospital deaths. Ann Surg. 1999;229(3):322–330. | |
Jaeck D, Bachellier P, Oussoultzoglou E, Eber JC, Wolf P. Surgical resection of hepatocellular carcinoma. Post-operative outcome and long-term results in Europe: an overview. Liver Transpl. 2004;10:S58–S63. | |
Kim SH, Kim DY, Lim JH, et al. Role of splenectomy in patients with hepatocellular carcinoma and hypersplenism. ANZ J Surg. 2013; 83(11):865–870. | |
Nomura Y, Kage M, Ogata T, et al. Influence of splenectomy in patients with liver cirrhosis and hypersplenism. Hepatol Res. Epub September 3, 2013. | |
Chen XP, Wu ZD, Huang ZY, Qiu FZ. Use of hepatectomy and splenectomy to treat hepatocellular carcinoma with cirrhotic hypersplenism. Br J Surg. 2005;92(3):334–339. | |
Lynch AM, Kapila R. Overwhelming postsplenectomy infection. Infect Dis Clin North Am. 1996;10(4):693–707. | |
Di Cataldo A, Puleo S, Li Destri G, et al. Splenic trauma and overwhelming postsplenectomy infection. Br J Surg. 1987;74(5):343–345. | |
Holdsworth RJ, Irving AD, Cuschieri A. Postsplenectomy sepsis and its mortality rate: actual versus perceived risks. Br J Surg. 1991;78(9):1031–1038. | |
Fuchs C, Scheer C, Schulz K, Dombrowski F, Brückmann S, Kuhn SO. Overwhelming postsplenectomy infection syndrome. Anaesthesist. 2014;63(3):225–230. | |
Atweh N, Kavic SM, Dudrick SJ. Portal vein thrombosis after splenectomy. J Am Coll Surg. 2001;192(4):551–552. | |
Lai W, Lu SC, Li GY, et al. Anticoagulation therapy prevents portal-splenic vein thrombosis after splenectomy with gastroesophageal devascularization. World J Gastroenterol. 2012;18(26):3443–3450. | |
Francoz C, Belghiti J, Vilgrain V, et al. Splanchnic vein thrombosis in candidates for liver transplantation: usefulness of screening and anticoagulation. Gut. 2005;54(5):691–697. | |
Pan C, Shi Y, Zhang JJ, et al. Single-center experience of 253 portal vein thrombosis patients undergoing liver transplantation in China. Transplant Proc. 2009;41(9):3761–3765. | |
Chaffanjon PC, Brichon PY, Ranchoup Y, Gressin R, Sotto JJ. Portal vein thrombosis following splenectomy for hematologic disease: prospective study with Doppler color flow imaging. World J Surg. 1998;22:1082–1086. | |
Marrero JA, Fontana RJ, Barrat A, et al. Prognosis of hepatocellular carcinoma: comparison of 7 staging systems in an American cohort. Hepatology. 2005;41(4):707–716. | |
Gomaa AI, Hashim MS, Waked I. Comparing staging systems for predicting prognosis and survival in patients with hepatocellular carcinoma in Egypt. PLoS One. 6, 2014;9(3):e90929. | |
Koh WP, Robien K, Wang R, Govindarajan S, Yuan JM, Yu MC. Smoking as an independent risk factor for hepatocellular carcinoma: the Singapore Chinese Health Study. Br J Cancer. 2011;105(9):1430–1435. | |
Marrero JA, Fontana RJ, Fu S, Conjeevaram HS, Su GL, Lok AS. Alcohol, tobacco and obesity are synergistic risk factors for hepatocellular carcinoma. J Hepatol. 2005;42(2):218–224. | |
El-Zayadi AR. Heavy smoking and liver. World J Gastroenterol. 2006;12(38):6098–6101. | |
Ogata T, Okuda K, Sato T, et al. Long-term outcome of splenectomy in advanced cirrhotic patients with hepatocellular carcinoma and thrombocytopenia. Kurume Med J. 2013;14;60(2):37–45. | |
Cao ZX, Chen XP, Wu ZD. Changes of immune function in patients with liver cirrhosis after splenectomy combined with resection of hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2003;2(4):562–565. | |
Wang Q, Sun K, Li XH, Peng BG, Liang LJ. Surgical treatment for hepatocellular carcinoma and secondary hypersplenism. Hepatobiliary Pancreat Dis Int. 2006;5(3):396–400. | |
Kim J, Kim CJ, Ko IG, Joo SH, Ahn HJ. Splenectomy affects the balance between hepatic growth factor and transforming growth factor-beta and its effect on liver regeneration is dependent on the amount of liver resection in rats. J Korean Surg Soc. 2012;82(4):238–245. | |
Ren YS, Qian NS, Tang Y, et al. Beneficial effects of splenectomy on liver regeneration in a rat model of massive hepatectomy. Hepatobiliary Pancreat Dis Int. 2012;11(1):60–65. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.