Back to Journals » Cancer Management and Research » Volume 12
Progastrin-Releasing Peptide Precursor and Neuron-Specific Enolase Predict the Efficacy of First-Line Treatment with Epidermal Growth Factor Receptor (EGFR) Tyrosine Kinase Inhibitors Among Non-Small-Cell Lung Cancer Patients Harboring EGFR Mutations
Authors Dong J , Tong S, Shi X, Wang C, Xiao X, Ji W, Sun Y
Received 6 October 2020
Accepted for publication 21 December 2020
Published 5 January 2021 Volume 2020:12 Pages 13607—13616
DOI https://doi.org/10.2147/CMAR.S285121
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Chien-Feng Li
Juanjuan Dong,1 Sihao Tong,1 Xianfeng Shi,1 Chao Wang,1 Xin Xiao,1 Wenping Ji,2 Yimian Sun3
1Department of Oncology, Anhui Medical University-Affiliated Chaohu Hospital, Hefei 238000, Anhui, People’s Republic of China; 2Department of Education, Anhui Medical University-Affiliated Chaohu Hospital, Hefei 238000, Anhui, People’s Republic of China; 3Department of Gynecology and Obstetrics, Huaian City Second People’s Hospital, Huaian 223000, Jiangsu, People’s Republic of China
Correspondence: Yimian Sun
Department of Gynecology and Obstetrics, Huaian City Second People’s Hospital, Huaian 223000, Jiangsu, People’s Republic of China
Tel +86 15955175587
Fax +86 55182324656
Email [email protected]
Purpose: Lung cancer is the leading cause of cancer-related mortality and non-small-cell lung cancer (NSCLC) accounts for 80– 90% of all lung cancers. However, biomarkers to predict the prognosis of NSCLC patients upon treatment with tyrosine kinase inhibitors remain unreliable. Different types of EGFR mutations can help predict the efficacy of tyrosine kinase inhibitor (TKI) treatment among advanced NSCLC patients harboring them. However, survival varies among individuals harboring the same mutation after targeted therapy. This study aimed to investigate the value of serum tumor markers (STMs) and EGFR mutations in the prognostic assessment of progression-free survival (PFS) in advanced-stage EGFR-mutated NSCLC.
Patients and Methods: A retrospective clinical review was performed on 81 NSCLC patients harboring EGFR mutations and for whom STM data, measured before commencement of first‐line treatment with tyrosine kinase inhibitors, were available. Associations among EGFR mutations, STMs, baseline clinical features, and PFS were analyzed. Kaplan−Meier method was used to plot survival curves, and Cox logistic regression models were used to identify independent prognostic factors.
Results: Exon 19 deletion (19-del) in EGFR, negative neuron-specific enolase (NSE), negative pro-gastrin-releasing peptide precursor (ProGRP) value, and “never smoking” status were significantly associated with improved PFS (P=0.007, P=0.001, P< 0.001, and P< 0.001, respectively). Multivariate Cox analysis revealed that 19-del in EGFR, never smoking, negative ProGRP value, and negative NSE were independent predictors of PFS.
Conclusion: This study demonstrated that 19-del in EGFR may predict longer PFS in advanced-stage EGFR-mutated NSCLC treated with TKIs. Additionally, longer PFS can be predicted by serum tumor markers with negative ProGRP value, negative NSE value before initial treatment, and “never smoking.” Therefore, in addition to the EGFR mutation type and smoking status, physicians can also prognosticate the PFS of tyrosine kinase inhibitors treatment according to the values of ProGRP and NSE before treatment.
Keywords: non-small-cell lung cancer, serum tumor markers, prognosis, progression-free survival
Introduction
Lung cancer ranks first among cancer-related deaths worldwide. About 1.7 million people die of lung cancer every year, posing a major threat to public health.1 Based on its biological characteristics, lung cancer is classified into non-small-cell lung cancer (NSCLC) and small-cell lung cancer (SCLC). NSCLC accounts for almost 80–90% of all lung cancers. Most NSCLC patients present with advanced disease.2 Approximately 40% of Asian NSCLC patients harbor epidermal growth factor receptor (EGFR) mutations; this prevalence is >20% for non-Asian patients. Furthermore, the mutation rate is alarmingly high among women (61.1%) and never-smokers (60.7%).3,4 In the past few decades, treatment modalities for inoperable metastatic NSCLC patients have developed from traditional therapy, such as cytotoxic chemotherapy and radiotherapy, to precision therapy. Particularly, tyrosine kinase inhibitors (TKIs) have emerged as first-line treatment agents for patients harboring exon 19 deletions (19-del) or exon 21 substitution (Leu858Arg) and diagnosed as advanced inoperable NSCLC.5 TKI treatment has not only markedly improved the objective response rates (ORR), progression-free survival (PFS), and overall survival (OS), but also improved the quality of life of patients with advanced lung adenocarcinoma, with fewer side effects.6–8
Lung cancer patients harboring EGFR mutations treated with TKIs report different clinical characteristics and different prognoses. With the increasing application of targeted therapy for treating NSCLC patients harboring EGFR mutations, evidence indicates that sex, performance status, ethnicity, brain metastasis, and EGFR mutation type may have independent prognostic values in EGFR-mutant NSCLC.6,9 However, better biomarkers are warranted to predict the prognosis of NSCLC patients treated with TKIs. Serum tumor markers (STMs) are not only helpful for the diagnosis of lung cancer but are also of great significance to the prognosis of cancer and follow-up surveillance.10,11 Clinical studies have reported that pro-gastrin-releasing peptide precursor (ProGRP), carcinoembryonic antigen (CEA), neuron-specific enolase (NSE), the soluble fragment of cytokeratin 19 (CYFRA 21–1), and squamous cell carcinoma (SCC) antigen are optimal markers for managing lung cancer.4 ProGRP and NSE are usually considered diagnostic and prognostic tumor markers for SCLC.12,13 However, elevated ProGRP and NSE levels have also been reported in some NSCLC patients.14,15 However, the prognosis of STMs for targeted therapy remains controversial. In particular, it has not been reported whether baseline serum ProGRP can be used as a predictive marker for first-line TKIs therapy for EGFR mutated NSCLC. Therefore, in this study, we aimed to evaluate the predictive and prognostic values of plasma ProGRP and NSE before treatment for NSCLC-positive first-line EGFR-TKIs.
Patients and Methods
Patients
Eighty-one advanced NSCLC patients receiving first‐line TKI (erlotinib 150 mg per day or gefitinib 250 mg per day) treatment at the Anhui Medical University-affiliated Chaohu Hospital between June 2016 and November 2019 were enrolled. A total of 271 patients with advanced NSCLC were recruited in this study, of which 118 cases were found with EGFR mutations; patients without treatment or treated with chemotherapy were excluded. Eighty-six patients were treated with TKIs as first-line treatment, of which five cases were not included in the statistics due to incomplete follow-up data. Most patients had adenocarcinoma (73/81); only two had SCC, five had adenosquamous carcinoma, and one had NSCLC but not otherwise specified (NOS).
Inclusion criteria: (I) histologically confirmed stage IV NSCLC according to the 7th American Joint Committee on Cancer (AJCC) staging manual; (II) harboring either the exon 19-del or/and 21-L858R mutations in EGFR, detected through polymerase chain reaction (PCR); (III) receiving first-line EGFR-TKI treatment; (IV) subjected to STM measurement, including ProGRP, CEA, NSE, CYFRA 21–1, CA72-4, and SCC, in plasma samples collected before treatment with first-generation EGFR‐TKIs. All the patients in the group with NSCLC were diagnosed by experienced clinicians based on their histopathology results, imaging, and other examinations, in accordance with international guidelines.
Exclusion criteria: Severe liver and kidney damage or autoimmune diseases, cardiovascular diseases, thrombus and hemorrhagic diseases, uncontrolled infectious diseases in the past 2 weeks, recent anti-tumor therapy such as chemotherapy or radiotherapy, no blood routine liver and renal function tests before EGFR‐TKI therapy, and life expectancy <3 months.
The primary study endpoint was PFS, determined from the first day when patients start receiving EGFR-TKI treatment to the day the tumor progressed or the last follow-up date. The tumor response after EGFR-TKI treatment was evaluated according to RECIST v1.1 criterion. ORR is defined as the total number of patients in partial and complete remission divided by the total number of patients receiving TKI treatment. The Ethics Committee of the Anhui Medical University-affiliated Chaohu Hospital approved the study (201,601-kyxm-01). Further, the study was conducted in accordance with the principles of the Declaration of Helsinki. We obtained informed consent from all patients before participating in the study.
Methods
STM Measurement
We obtained the following data from medical records: age, sex, type of EGFR mutation, smoking status, pathological type, Cooperative Oncology Group Performance Status (ECOG PS) of 0, 1, or 2, and STMs before treatment. Blood samples of tumor markers were obtained by peripheral venipuncture before TKI treatment. We define the following threshold as the upper limit of the reference range: ProGRP, 85.7 pg/mL; CEA, 5 ng/mL; NSE, 24.0 ng/mL; CYFRA 21–1, 3.3 ng/mL; CA72-4, 5.6 U/mL; and SCC, 2.5 ng/mL. Therefore, when the value of the tumor marker exceeds these thresholds, it is considered to be positive; when the contrary is the case, it is negative. STMs were measured using a commercial electrochemiluminescence immunoassay on the Roche Modular E601 system (Roche Diagnostics, Shanghai, China). The reference threshold for STM elevation was provided by the manufacturer.
Analyses for Immunohistochemistry and EGFR Gene Mutations
The histopathological types and malignant degrees of NSCLCs were judged by experienced pathologists according to the immunohistochemical markers. During immunohistochemical analysis, pathologists judged the pathological types according to the following immune markers: TTF-1, NAP-A, CK, CK, Ki67 (Maixin, Fuzhou, China), which were used to classify the tumors into adenocarcinoma, SCC, adenosquamous carcinoma, large cell carcinoma, and other rare types.
Tumor EGFR mutations were detected by fiberoptic bronchoscopy, percutaneous lung puncture, or metastatic lymph node biopsy. In the absence of the above tissues, EGFR mutations can also be detected using the cytological samples of serous effusion. PCR (Next Seq CN500, Illumina, USA) was used to analyze the existence/absence of EGFR gene mutation. If no exon mutation was detected, the mutation was identified as “EGFR wild type,” whereas if any exon mutation is detected, it was identified as “EGFR mutation.”
Statistical Analysis
We used SPSS version 22.0 software (SPSS Inc., Chicago, IL, USA) to analyze the P values of the tumor markers studied. The Kaplan–Meier method was used to estimate the PFS distribution, and Cox proportional hazard regression analyses were performed for univariate and multivariate survival analyses. Variables with P < 0.1 in the univariate analysis were incorporated into the multivariate analysis. P < 0.05 was considered statistically significant.
Results
Patient Characteristics
The clinical characteristics of the 81 patients diagnosed with advanced NSCLC are listed in Table 1. The median age of the cohort was 65 years (range, 39–85 years), and 44 (54.3%) patients were females. Fifty-nine (72.8%) patients were never-smokers. Most of the patients were diagnosed with adenocarcinoma (73/81), and just eight had other types of NSCLC. The median PFS of the cohort was 12.9 months. Forty-four patients (54.3%) harbored a del-19 and 37 (45.7%) harbored an L858R mutation. Patients with elevated ProGRP (40.7% vs. 59.3%), CEA (74.1% vs. 25.9%), NSE (38.3% vs. 61.7%), CYFRA 21–1 (65.4% vs. 34.6%), CA 72–4 (19.8% vs. 80.2%), and SCC (8.6% vs. 91.4%) showed no correlation with EGFR mutation status (data not shown).
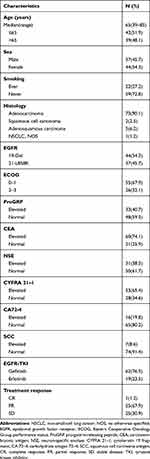 |
Table 1 Clinical Characteristics of the 81 Patients |
Association Between Baseline STMs and EGFR-TKI Treatment Outcomes
The response of patients with different baseline STMs and their characteristics are presented in Table 2. There was no significant correlation between age, sex, smoking status, or ECOG score and treatment response. Furthermore, the baseline ProGRP, NSE, CYFRA 21–1, CA 72–4, and SCC were not significantly associated with treatment outcomes. However, patients with elevated CEA levels and EGFR 19-del mutation presented better responses than those with normal CEA levels and EGFR 21- L858R. The ORR for patients with elevated CEA levels and normal CEA levels were 81.7 vs. 33.3% (P<0.001) after first-line EGFR‐TKI treatment. Furthermore, after the first-line EGFR‐TKI treatment, the ORR for patients with EGFR 19-del mutation and EGFR 21- L858R were 80.4 vs. 54.3% (P=0.033), respectively.
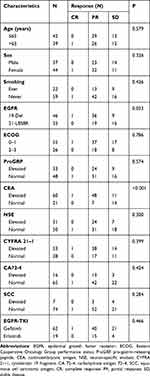 |
Table 2 Disease Response Associated with Clinical Characteristics |
Survival and Prognostic Factors
Univariate and multivariate analyses were used to identify the predictive and prognostic value of clinical features and the pre-therapeutic STMs in the EGFR-mutant NSCLC patients treated with EGFR-TKIs as first-line therapy. Univariate logistic regression analysis indicated that patients in the EGFR 19-del mutation, normal NSE, normal ProGRP, and never smoking groups had significantly longer PFS (Figures 1–4). The median PFS of patients with exon 19-del mutation was significantly longer than that of patients with exon 21-L858R mutation after first-line TKI treatment (16.1 vs. 7.5 months; P=0.007, Figure 1). The median PFS of patients with normal NSE value was significantly longer than that of patients with elevated NSE levels (16.1 vs. 7.0 months; P=0.001, Figure 2). The median PFS of patients with normal ProGRP value was significantly longer than that of patients with elevated ProGRP levels (16.1 vs. 7.1 months; P<0.001, Figure 3). The median PFS of never smoking patients were significantly longer than that of ever smoking ones (14.7 vs. 6.9 months; P<0.001, Figure 4). However, other STMs, such as age, sex, and ECOG score, were not associated with PFS (Table 3).
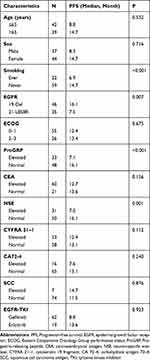 |
Table 3 Univariate Analysis of PFS |
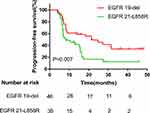 |
Figure 1 Kaplan–Meier curves for each patient group classified according to EGFR mutation. |
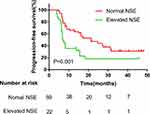 |
Figure 2 Kaplan–Meier curves for each patient group classified according to NSE levels. |
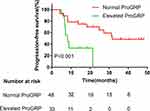 |
Figure 3 Kaplan–Meier curves for each patient group classified according to Pro‐GRP levels. |
 |
Figure 4 Kaplan–Meier curves for each patient group classified according to smoking status. |
On multivariate Cox regression analysis, EGFR mutations remained an independent predictor of PFS among patients treated with first-line EGFR-TKIs (Hazard ratio [HR], 1.832; 95% CI, 1.061–3.160; P =0.03), along with baseline ProGRP (HR, 2.462; 95% CI, 1.338–4.531; P =0.004), baseline NSE (HR, 2.169; 95% CI, 1.215–3.873; P=0.009), and smoking status (Table 4, HR, 0.351; 95% CI, 0.191–0.643; P=0.001).
 |
Table 4 Univariate and Multivariate Analysis of PFS |
Discussion
In this study, we evaluated the predictive and prognostic values of ProGRP and NSE in plasma before treatment of NSCLC with first-line EGFR-TKIs. We found that 19-del in EGFR, never smoking, negative ProGRP value, and negative NSE were significantly associated with better PFS among NSCLC patients with EGFR driving gene positive mutations treated with first-line TKIs.
Personalized therapy for NSCLC, guided by targeted driver genes, has already been initiated, especially among individuals with molecular subtypes, including EGFR mutations and ALK mutations. In-frame 19-del and point mutations of exon 21 (Leu858Arg) are the two most common activating mutations, together accounting for >90% of known activating EGFR mutations.16 Concurrent with our results, most previous studies have reported that NSCLC patients harboring 19-del have a more favorable response rate and survival than those with exon 21 L858R mutations.5,17,18 Furthermore, a previous meta-analysis revealed that smokers, male patients, and patients with EGFR exon 21 L858R mutations are potentially associated with poor PFS compared to non-smokers, female patients, and patients harboring EGFR 19-del upon treatment with EGFR-TKIs.9
Adverse treatment results associated with exon 21 mutations may be attributed to or confused by the higher incidence of concomitant mutations. A previous study showed that 69% and 41% of patients harbored an EGFR exon 21 mutation and 19-del mutation, respectively (P = 0.04).19 Conversely, a retrospective clinical study by Xue et al17 reported no significant differences in response rate and PFS between patients with 19-del and those with an exon 21 L858R mutation after icotinib treatment. Therefore, prognostic evaluation based only on gene mutation types is not adequate. Further studies are required to evaluate more biomarkers for comprehensive prognostic evaluation of NSCLC.
Plasma ProGRP and NSE levels have long been considered for the diagnosis of SCLC, with 60–70% sensitivity and 96–97% specificity.13,20,21 However, high serum ProGRP and NSE levels were also observed among NSCLC patients, especially those with large cell neuroendocrine carcinoma and NSCLC with neuroendocrine manifestations.13,21 Although the present data are controversial, most studies have reported that serum NSE is an independent prognostic factor in advanced NSCLC, concurrent with our findings. In the era of chemotherapy as first-line treatment, elevated serum NSE levels may serve as an independent factor for the poor prognosis of NSCLC, being potentially associated with greater aggressiveness of NSCLC.10,22,23 Suh reported that baseline serum NSE levels are an independent predictive marker among EGFR-mutant NSCLC patients treated with first-line EGFR-TKIs; however, ProGRP was not discussed in their research.24 ProGRP is a signal peptide produced by SCLC cells. A previous meta-analysis reported that ProGRP has a higher specificity and similar differentiation potential in diagnosing SCLC compared to NSE.25 However, only a few studies have investigated whether baseline serum ProGRP serves as a predictive marker for EGFR-mutant NSCLC treated with TKIs. This study reported that positive ProGRP and NSE values measured before initial treatment may predict poor PFS, both being independent prognostic factors of PFS. A previous study reported no significant association between STMs at baseline and OS;26 however, these results are different from the present endpoint PFS only associated with the duration of prognosis, whereas OS was associated with not only initial treatment but also advanced treatment. Because of the great difference in the mode of second-line treatment, OS was not considered the focus of this study. Furthermore, the treatment modality included both chemotherapy and EGFR-TKIs as first-line treatment, which is different from that in this study wherein patients were treated with only EGFR-TKIs. Inomata reported that higher NSE levels are associated with poor prognosis; however, association between serum ProGRP levels and survival was not observed.27 Their selection criteria were both treatment with TKIs as first-line therapy among 34 (82.9%) patients and as second-line therapy among seven (17.1%) patients, which were different from those in this study. Herein, no significant association was noted between other STMs at baseline and PFS. Although a meta-analysis reported the powerful predictive value of CYFRA 21–1 in NSCLC, it includes different treatment modes, different stages, and even different pathological types.28
The prognostic and predictive values of pre-treatment CEA and CYFRA 21–1 serum levels were assessed among advanced NSCLC patients treated with gefitinib or erlotinib. Furthermore, Jung et al29 reported that pre-treatment CEA and CYFRA 21–1 serum levels could predict survival among advanced NSCLC patients with unknown EGFR mutation status treated with TKIs, while EGFR mutation status was subsequently identified as a strong predictor for PFS and OS when treated with TKIs.
The major reason underlying the difference between the survival rates of the elevated ProGRP and NSE groups may be tumor heterogeneity. Previous studies reported that 2–10% of patients harbored a mixture of small-cell and non-small-cell tumors, as revealed through the histological features of all pulmonary biopsies and pulmonary resections.30,31 Although NSCLC cases with elevated serum NSE levels had higher chemotherapeutic ORR, a higher ORR to cytotoxic drugs did not improve patient survival.24,32 Lung adenocarcinoma and SCLC cells might harbor a common precursor for alveolar type II cells.33,34 In one case, the SCLC component was dominant among NSCLC patients after EGFR-TKI treatment, displaying an elevation in pre-treatment plasma ProGRP levels.20 In another case, small-cell transformation and metastasis to the breast occurred in a lung adenocarcinoma patient after EGFR-TKI treatment, accompanied by an elevation in serum NSE level.35 In both cases mentioned above, the patients were diagnosed with adenocarcinoma after initial biopsy and did not present a neuroendocrine component. Oya36 reported that ProGRP and NSE can predict SCLC transformation after observing chemotherapeutic resistance to ALK-TKIs even when the serum NES and ProGRP levels were within reference limits upon diagnosis. Notwithstanding extensive tumor heterogeneity, an initial diagnosis is inadequate. Previous studies have reported some patients being initially diagnosed with small-cell carcinoma but subsequently presenting a mixed tumor cell population upon surgery or autopsy,30 probably owing to the small size of the biopsy samples. For most patients with stage IV cancer, surgical gross specimens are lacking, and multiple biopsies are performed to obtain a sufficient amount of tissue. The present results potentially help define NSCLC with positive ProGRP and NSE values as prone to TKI resistance and SCLC transformation. Assessment of NSCLC heterogeneity potentially requires biopsy from multiple sites. However, considering the poor performance of patients with advanced lung cancer, this is frequently unrealistic.37
This study has some limitations. First, this study had a relatively small cohort size, was single-centered, and had a retrospective design. Furthermore, we did not investigate the prognostic value of STMs for OS because patients received different modes of treatment after disease progression. Also, this study does not account for all potential prognostic factors such as ctDNA and brain metastases because the record was not complete. In addition, there was no stratified analysis of the rising degree of STMs in this study. Hence, these results should be interpreted carefully and larger sample based prospective studies are warranted for further exploration.
Conclusions
This study showed that EGFR mutation type, baseline serum NSE and ProGRP levels, and smoking status are independent predictive markers of first-line EGFR-TKI treatment among EGFR-mutant NSCLC patients. Measurement of serum NSE and ProGRP levels before EGFR-TKI treatment could help clinicians predict the PFS upon administration of first-line EGFR-TKI therapy. Further studies are required to indicate whether new treatment strategies are needed for patients with EGFR mutations associated with elevated baseline NSE and ProGRP levels.
Abbreviations
SCLC, small-cell lung cancer; NSCLC, non-small-cell lung cancer; EGFR, epidermal growth factor receptor; ECOG, Eastern Cooperative Oncology Group performance status; TKI, tyrosine kinase inhibitor. CR, complete response; PR, partial response; SD, stable disease; ORR, objective response rate; PFS, progression-free survival; OS, overall survival; STM, serum tumor marker; ProGRP, progastrin-releasing peptide precursor; CEA, carcinoembryonic antigen; NSE, neuron-specific enolase; CYFRA 21-1, soluble fragment of cytokeratin 19; SCC, squamous cell carcinoma antigen; ctDNA, circulating tumor DNA.
Data Sharing Statement
The data and materials that support the findings of this study are available from the corresponding author upon reasonable request.
Ethics Approval
This study was approved by the Ethics Committee of the Anhui Medical University-affiliated Chaohu Hospital (201,601-kyxm-01) and conducted in accordance with the principles of the Declaration of Helsinki. All the patients signed informed consent. The authors are accountable for all aspects of the work and for ensuring that questions related to the accuracy or integrity of all parts of the work are appropriately investigated and resolved.
Acknowledgments
We thank Yaqi Wang of the Fudan University Shanghai Cancer Center for editing our manuscript.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
No funding was received.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Gelatti ACZ, Drilon A, Santini FC. Optimizing the sequencing of tyrosine kinase inhibitors (TKIs) in epidermal growth factor receptor (EGFR) mutation-positive non-small cell lung cancer (NSCLC). Lung Cancer. 2019;137:113–122. doi:10.1016/j.lungcan.2019.09.017
2. D’Addario G, Früh M, Reck M, et al. Metastatic non-small-cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(Suppl 5):v116–v119. doi:10.1093/annonc/mdq189
3. Shi Y, Li J, Zhang S, et al. Molecular epidemiology of EGFR mutations in asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology – mainland china subset analysis of the PIONEER study. PLoS One. 2015;10(11):e0143515. doi:10.1371/journal.pone.0143515
4. Wang S, Ma P, Ma G, et al. Value of serum tumor markers for predicting EGFR mutations and positive ALK expression in 1089 Chinese non-small-cell lung cancer patients: a retrospective analysis. Eur J Cancer. 2020;124:1–14. doi:10.1016/j.ejca.2019.10.005
5. Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised Phase 3 trial. Lancet Oncol. 2012;13(3):239–246. doi:10.1016/S1470-2045(11)70393-X
6. Hong W, Wu Q, Zhang J, et al. Prognostic value of EGFR 19-del and 21-L858R mutations in patients with non-small cell lung cancer. Oncol Lett. 2019;18(4):3887–3895. doi:10.3892/ol.2019.10715
7. Lara-Guerra H, Waddell TK, Salvarrey MA, et al. Phase II study of preoperative gefitinib in clinical stage I non–small-cell lung cancer. J Clin Oncol. 2009;27(36):6229–6236. doi:10.1200/JCO.2009.22.3370
8. Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66(4):271–289. doi:10.3322/caac.21349
9. Buonerba C, Iaccarino S, Dolce P, et al. Predictors of Outcomes In Patients with EGFR-mutated non-small cell lung cancer receiving EGFR tyrosine kinase inhibitors: a systematic review and meta-analysis. Cancers (Basel). 2019;11(9):1259. doi:10.3390/cancers11091259
10. Maeda T, Ueoka H, Tabata M, et al. Prognostic factors in advanced non-small cell lung cancer: elevated serum levels of neuron specific enolase indicate poor prognosis. Jpn J Clin Oncol. 2000;30(12):534–541. doi:10.1093/jjco/hyd139
11. Nakamura H, Nishimura T. History, molecular features, and clinical importance of conventional serum biomarkers in lung cancer. Surg Today. 2017;47(9):1037–1059.
12. Zhou M, Wang Z, Yao Y, et al. Neuron-specific enolase and response to initial therapy are important prognostic factors in patients with small cell lung cancer. Clin Transl Oncol. 2017;19(7):865–873. doi:10.1007/s12094-017-1617-2
13. Miyake Y, Kodama T, Yamaguchi K. Pro-gastrin-releasing peptide(31–98) is a specific tumor marker in patients with small cell lung carcinoma. Cancer Res. 1994;54(8):2136–2140.
14. Molina R, Auge JM, Alicarte J, Filella X, Viñolas N, Ballesta AM. Pro-gastrin-releasing peptide in patients with benign and malignant diseases. Tumour Biol. 2004;25(1–2):56–61. doi:10.1159/000077724
15. Nisman B, Heching N, Biran H, et al. The prognostic significance of circulating neuroendocrine markers chromogranin a, pro-gastrin-releasing peptide and neuron-specific enolase in patients with advanced non-small-cell lung cancer. Tumour Biol. 2006;27(1):8–16. doi:10.1159/000090151
16. Tan C-S, Gilligan D, Pacey S. Treatment approaches for EGFR-inhibitor-resistant patients with non-small-cell lung cancer. Lancet Oncol. 2015;16(9):e447–e459. doi:10.1016/S1470-2045(15)00246-6
17. Xue ZX, Wen WX, Zhuang Y, et al. Comparison of the efficacy of icotinib in patients with non-small-cell lung cancer according to the type of epidermal growth factor receptor mutation. Mol Clin Oncol. 2016;5(3):265–268. doi:10.3892/mco.2016.956
18. Carey KD, Garton AJ, Romero MS, et al. Kinetic analysis of epidermal growth factor receptor somatic mutant proteins shows increased sensitivity to the epidermal growth factor receptor tyrosine kinase inhibitor, erlotinib. Cancer Res. 2006;66(16):8163–8171. doi:10.1158/0008-5472.CAN-06-0453
19. Hong S, Gao F, Fu S, et al. Concomitant genetic alterations with response to treatment and epidermal growth factor receptor tyrosine kinase inhibitors in patients with EGFR -mutant advanced non–small cell lung cancer. JAMA Oncol. 2018;4(5):739–742. doi:10.1001/jamaoncol.2018.0049
20. Kato Y, Tanaka Y, Hino M, et al. ProGRP as early predictive marker of non-small-cell lung cancer to small-cell lung cancer transformation after EGFR-TKI treatment. Respir Med Case Rep. 2019;27:100837. doi:10.1016/j.rmcr.2019.100837
21. Kudo K, Ohyanagi F, Horiike A, et al. Clinicopathological findings of non-small-cell lung cancer with high serum progastrin-releasing peptide concentrations. Lung Cancer. 2011;74(3):401–404. doi:10.1016/j.lungcan.2011.03.019
22. van Zandwijk N, Jassem E, Bonfrer JM, et al. Serum neuron-specific enolase and lactate dehydrogenase as predictors of response to chemotherapy and survival in non-small cell lung cancer. Semin Oncol. 1992;19(1 Suppl 2):37–43.
23. Pujol J-L, Boher J-M, Grenier J, et al. Cyfra 21-1, neuron specific enolase and prognosis of non-small cell lung cancer: prospective study in 621 patients. Lung Cancer. 2001;31(2–3):221–231. doi:10.1016/S0169-5002(00)00186-0
24. Suh KJ, Keam B, Kim M, et al. Serum neuron-specific enolase levels predict the efficacy of first-line Epidermal Growth Factor Receptor (EGFR) tyrosine kinase inhibitors in patients with non-small cell lung cancer harboring EGFR mutations. Clin Lung Cancer. 2016;17(4):245–252 e1. doi:10.1016/j.cllc.2015.11.012
25. Wang J, Gao J, He J. Diagnostic value of ProGRP and NSE for small cell lung cancer: a meta-analysis. Zhongguo Fei Ai Za Zhi. 2010;13(12):1094–1100. doi:10.3779/j.issn.1009-3419.2010.12.03
26. Yoshimura A, Uchino J, Hasegawa K, et al. Carcinoembryonic antigen and CYFRA 21-1 responses as prognostic factors in advanced non-small cell lung cancer. Transl Lung Cancer Res. 2019;8(3):227–234. doi:10.21037/tlcr.2019.06.08
27. Inomata M, Hayashi R, Yamamoto A, et al. Plasma neuron-specific enolase level as a prognostic marker in patients with non-small cell lung cancer receiving gefitinib. Mol Clin Oncol. 2015;3(4):802–806. doi:10.3892/mco.2015.568
28. Xu Y, Xu L, Qiu M, et al. Prognostic value of serum cytokeratin 19 fragments (Cyfra 21-1) in patients with non-small cell lung cancer. Sci Rep. 2015;5(1):9444. doi:10.1038/srep09444
29. Jung M, Kim SH, Lee YJ, et al. Prognostic and predictive value of CEA and CYFRA 21-1 levels in advanced non-small cell lung cancer patients treated with gefitinib or erlotinib. Exp Ther Med. 2011;2(4):685–693. doi:10.3892/etm.2011.273
30. Adelstein DJ, Tomashefski JF, Snow NJ, et al. Mixed small cell and non-small cell lung cancer. Chest. 1986;89(5):699–704. doi:10.1378/chest.89.5.699
31. Mangum MD, Greco FA, Hainsworth JD, et al. Combined small-cell and non-small-cell lung cancer. J Clin Oncol. 1989;7(5):607–612. doi:10.1200/JCO.1989.7.5.607
32. Petrovic M, Baskić D, Banković D, et al. Neuroendocrine differentiation as an indicator of chemosensitivity and prognosis in nonsmall cell lung cancer. Biomarkers. 2011;16(4):311–320. doi:10.3109/1354750X.2011.560281
33. Sutherland KD, Song J-Y, Kwon MC, et al. Multiple cells-of-origin of mutant K-Ras–induced mouse lung adenocarcinoma. Proc Natl Acad Sci U S A. 2014;111(13):4952–4957. doi:10.1073/pnas.1319963111
34. Mainardi S, Mijimolle N, Francoz S, et al. Identification of cancer initiating cells in K-Ras driven lung adenocarcinoma. Proc Natl Acad Sci U S A. 2014;111(1):255–260. doi:10.1073/pnas.1320383110
35. Lin Q, Cai G-P, Yang K-Y, et al. Case report: small cell transformation and metastasis to the breast in a patient with lung adenocarcinoma following maintenance treatment with epidermal growth factor receptor tyrosine kinase inhibitors. BMC Cancer. 2016;16(1):593. doi:10.1186/s12885-016-2623-4
36. Oya Y, Yoshida T, Uemura T, et al. Serum ProGRP and NSE levels predicting small cell lung cancer transformation in a patient with ALK rearrangement-positive non-small cell lung cancer: a case report. Oncol Lett. 2018. doi:10.3892/ol.2018.9158
37. Lee Y, Park S, Kim WS, et al. Correlation between progression-free survival, tumor burden, and circulating tumor DNA in the initial diagnosis of advanced-stage EGFR -mutated non-small cell lung cancer. Thorac Cancer. 2018;9(9):1104–1110. doi:10.1111/1759-7714.12793
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
