Back to Journals » Neuropsychiatric Disease and Treatment » Volume 16
Profiles of Vertebral Artery Dissection with Congenital Craniovertebral Junction Malformation: Four New Cases and a Literature Review
Authors Xue S, Yang Y, Li P, Liu P, Du X, Ma X
Received 21 May 2020
Accepted for publication 3 August 2020
Published 22 October 2020 Volume 2020:16 Pages 2429—2447
DOI https://doi.org/10.2147/NDT.S262078
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Yuping Ning
Sufang Xue,1,* Yi Yang,1,* Pengyu Li,2 Ping Liu,3 Xiangying Du,2 Xin Ma1
1Neurology Department of Xuanwu Hospital, Capital Medical University, Beijing, People’s Republic of China; 2Radiology Department of Xuanwu Hospital, Capital Medical University, Beijing, People’s Republic of China; 3Neurology Department of Hejian People’s Hospital, Cangzhou, Hebei, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xin Ma
Neurology Department of Xuanwu Hospital, Capital Medical University, Beijing, People’s Republic of China
, Tel +86-135-0139-0691
Email [email protected]
Objective: Vertebral artery dissection (VAD) combined with congenital craniovertebral junction malformation (CVJM) is rare. This study aimed to analyze the etiology, clinical and imaging features, treatment, and prognosis of VAD with CVJM.
Methods: Four new cases of VAD with congenital CVJM and 28 similar cases found in the literature were included. Detailed clinical data from all cases were retrospectively analyzed.
Results: A total of 32 patients (28 men, four women; mean age 19.01± 12.53 years) were included. Seventeen of 32 cases (53.1%) had had multiple ischemic episodes. The most common neurological symptoms were limb numbness/weakness (20/32), ataxia (15/32), and dizziness/vertigo (12/32). In sum, 31 of 32 cases had multiple infarcts scattered throughout the posterior circulation area on cranial computed tomography or resonance imaging. Dissection had occurred in the V3 segment of the VA in 29/31 cases (93.5%). The most common congenital CVJMs were atlantoaxial dislocation and atlantoaxial subluxation (found in 20/32 cases [62.5%]), while 27/32 cases (84.3%) had multiple combined abnormalities. Seven of eleven cases (63.6%) with initial antiplatelet treatment and one of eleven (9.1%) with initial anticoagulation treatment experienced stoke recurrence. Fusion or vertebral fixation was performed in 16 patients and aneurysm resection in one patient. There was no reported recurrence after surgery in 13 patients with follow-up data.
Conclusion: Underlying CVJM is a rare but overlooked etiology in VAD, and is prone to induce recurrent ischemic stroke. Patients with VAD, especially that localized in the V3 segment, should be examined for CVJM. Timely assessment is critical for determining the specific cause and to provide targeted intervention.
Keywords: vertebral artery dissection, craniovertebral junction malformation, clinical and imaging features, treatment, prognosis
Introduction
Vertebral artery dissection (VAD) is an important cause of posterior-circulation ischemic stroke (PCIS) in young patients, with an estimated incidence of 1.87 per 100,000 people. VAD is usually spontaneous or caused by chiropractic neck manipulations, trauma, nose-blowing, turning the head, some connective-tissue disorders,1 or rheumatoid arthritis.2 The VA has four parts. It shares a close association with cervical vertebrae in its second part (intraforaminal), which is more or less fixed. It is mobile, with loops in its third part.3 Very rarely, VAD can also derive from a congenital craniovertebral junction malformation (CVJM), involving anomalies of the bones and soft tissue surrounding the foramen magnum, which often leads to instability of this mobile area.4–29 Clinically, congenital CVJM underlying VAD is easy to overlook, and treatment is often delayed, especially in the early stage. Further, the clinical profiles of VAD with congenital CVJM may differ from those of patients with general VAD. However, to our knowledge there have been no systematic, comprehensive reports of such cases. Herein, we report four cases with a definite diagnosis of VAD with congenital CVJM, and present a literature review of similar cases.4–26 This systematic summary and analysis of the etiology, clinical and imaging characteristics, treatment, and prognosis of VAD with CVJM aims to improve the understanding of this rare pathology and provide clues for early diagnosis and effective treatment.
Methods
In addition to our case reports of four patients diagnosed with VAD and congenital CVJM in our hospital, the details of 28 similar reported cases were also examined using the following search protocol. Studies written in English were searched in PubMed between January 1, 1966 and February 1, 2020 using the terms “vertebral artery dissection” or “vertebral artery dissecting aneurysm” or “posterior circulation strokes” and “craniovertebral junction malformation” or “craniovertebral junction anomaly” or “craniovertebral anomaly” or “occipitocervical anomaly” or “atlantooccipital anomaly” or “atlantoaxial anomaly” or “atlantoaxial subluxation” or “atlantoaxial instability” or “atlantoaxial dislocation” or “basilar invagination” or “basilar impression” or “platybasia” or “assimilation of atlas” or “atlantooccipital fusion” or “occipitalization of the atlas” or “Klippel–Feil syndrome” or “Chiari malformation”. A neurologist and radiologist then carefully reviewed the studies, and only cases with a diagnosis of VAD with congenital CVJM and complete clinical data were included. A final 32 patients were included in the present study. Detailed clinical data are shown in Tables 1 and 2.
 |  |  |  |
Table 1 Demographics, symptoms, treatment, and prognosis in patients |
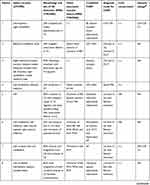 | 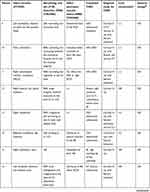 | 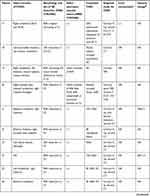 |  |
Table 2 Multimodal imaging features and possible ischemic mechanisms in patients |
Case Reports
Case 1
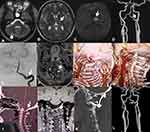
A 31-year-old woman was admitted to our hospital with a complaint of sluggishness, memory decline, and weakness in the right limbs for >20 days. Three months prior, she had experienced sudden numbness in the right limbs and was diagnosed with acute infarction in the left thalamus. Since then, she had received oral daily treatment of 100 mg aspirin plus 75 mg clopidogrel as antithrombotic therapy. She had no medical history of hypertension, diabetes, heart disease, smoking, or alcohol use. After hospitalization, neurological physical examination showed slight speechlessness, decline in recent memory and calculation, tongue deviation to the right when extended, and slight numbness in her right limbs. Routine laboratory examination showed no significant abnormalities, while cranial magnetic resonance imaging (MRI) showed a new infarct in the anterior portion of the left thalamus, with old infarcts in the left thalamus and right cerebellar hemisphere (Figure 1A–C). Head computed-tomography angiography (CTA) and digital subtraction angiography (DSA) showed a localized, small protuberance at the V3–V4 junction area of the left VA (Figure 1D and E), which was thought to be a dissection on high-resolution (HR) MRI (Figure 1F). Carotid ultrasonography and CTA both showed that the left VA was normal when the neck was in the neutral position (Figure 1G), but was occluded when the neck was rotated to the right (Figure 1H). Atlanto-occipital fusion and basilar invagination were also observed on CTA (Figure 1I), and MRI showed the left atlas lateral mass was small with dysplasia (Figure 1J). The patient was given anticoagulant therapy (150 mg twice daily) with dabigatran and a neck brace to prevent excessive neck activity. At the 3-month follow-up, the left VA was still occluded during neck rotation, although the patient had not had stroke recurrence. She then underwent atlantoaxial fusion (Figure 1K), and at 6 months after surgery repeated CTA showed disappearance of the small protuberance at the V3–V4 junction of the left VA (Figure 1L).
Case 2
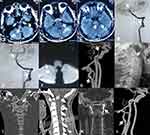
A 16-year-old male sports student who had started shot-put training 8 months prior was admitted to our hospital after a sudden headache and limb weakness for 8 days. Head MRI in a local hospital showed multiple infarcts in the bilateral cerebellar hemisphere and the pons (Figure 2A–C). He had no anomalies on neurological physical examination, and was administered aspirin, clopidogrel, and statins orally once hospitalized. Laboratory examination showed no significant abnormalities on routine blood tests. Head CTA showed severe distal stenosis or occlusion of the basilar artery (BA) and irregular aneurysmal dilation at the site where the left VA exited the axial foramen. The following morning, he suffered weakness in the left limbs and aphasia at rest, and immediate DSA plus mechanical thrombectomy was performed. The arterial abnormalities observed during surgery were similar to those on head CTA (Figure 2D), and complete recanalization of the BA was achieved after mechanical thrombectomy (Figure 2E). At discharge, he had recovered entirely, and was given aspirin plus clopidogrel as antithrombotic therapy. However, at 3 weeks after discharge from hospital, he was readmitted with dizziness, weakness in the left limbs, and pain in the posterior occipital area after sneezing. Cranial MRI showed a new infarct in the right cerebellar hemisphere (Figure 2F). CTA confirmed a dissecting aneurysm in the atlantoaxial segment of the left VA and repeated occlusion of the BA (Figure 2G). In addition, cervical vertebral X-ray (Figure 2H) and CT three-dimensional reconstruction (3-D CT Figure 2I and J) both demonstrated os odontoideum. He was given subcutaneous heparin followed by rivaroxaban (20 mg daily) and made to wear a neck brace at discharge, without neurological deficit. He had had no stroke recurrence and underwent atlantoaxial fusion after 3 months’ recovery (Figure 2K). Repeat head CTA 3 months after surgery revealed a dissecting aneurysm on the V3 segment of the right VA had become smaller (Figure 2L).
Case 3
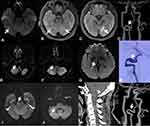
A 46-year-old woman who denied a history of hypertension, diabetes, hyperlipidemia, smoking, or alcohol use was admitted to our hospital because of spasmodically binocular amaurosis. At 20 days before admission, she had suffered an attack of bilateral blurred vision after sweating, and the symptoms were relieved 1 hour later. Her neurological and physical examination results were normal. Laboratory examination showed no significant abnormalities in routine blood. Cranial MRI showed new infarcts in the right temporal occipital junction, with multiple old infarcts in the bilateral medial temporal occipital lobe and left thalamus (Figure 3A–C). Head CTA showed aneurysmal dilation on the V3 segment of the right VA (Figure 3D). She was prescribed dual oral antiplatelet therapy and statins once hospitalized, and was discharged without neurological deficit. Approximately 2 months into recovery, she complained of vertigo accompanied by visual rotation. Cranial MRI showed new infarcts in the right cerebellar hemisphere and right medial temporal lobe (Figure 3E–G). DSA showed a dissecting aneurysm with a dual-lumen sign on the V3 segment of the right VA (Figure 3H). She was administered dabigatran and statins, and achieved gradual symptomatic relief. Approximately 5 months into recovery she suffered paroxysmal vertigo, weakness of the left limbs, and speechlessness, with new infarcts in the right cerebellar hemisphere and right pons on cranial MRI (Figure 3I and J). Cervical vertebra 3-D CT demonstrated atlanto-occipital fusion (Figure 3K). Her neurosurgeon suggested a conservative treatment first, and she was prescribed oral warfarin and a neck brace to prevent excessive neck activity. Repeat head CTA 7 months later revealed a smaller aneurysm on the V3 segment of the right VA (Figure 3L).
Case 4
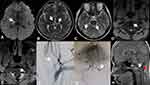
A 16-year-old boy was admitted to our hospital because of numbness, clumsiness in the left limbs, and unstable gait. He had no medical history of hypertension, diabetes, or heart disease. Seven months prior, he had been diagnosed with multiple acute infarcts in the left cerebellar hemisphere and thalamus, accompanied by a subacute infarction in the left thalamus and a complaint of speechlessness and numbness in the right limbs, and was treated with 100 mg aspirin daily. After admission, neurological physical examination indicated hypoesthesia in the left face and limbs and ataxia in the left limbs. Laboratory examination showed no significant abnormalities in routine blood or cerebrospinal fluid samples. Cranial MRI showed an acute infarct in the right thalamus and multiple old infarcts in the left cerebellar hemisphere and bilateral thalamus (Figure 4A–C). Cervical artery (CeA) ultrasound and HR MRI showed dissection in the atlantoaxial segment of the left VA (Figure 4D and E), while distal occlusion of the left VA was demonstrated on DSA (Figure 4F and G). In addition, sagittal cranial MRI showed atlantoaxial spinal canal stenosis and os odontoideum (Figure 4H). He was administered 100 mg aspirin plus 75 mg clopidogrel orally as antithrombotic therapy, and introduced to neurosurgical department for further examination after discharge.
Results
Demographics and Clinical Presentation
The average age of the 32 patients was 19.01±12.53 years (range 23 months to approximately 46 years), and there was a significant predominance of males (28/32, 87.5%). Only five of 32 cases (15.6%) had a history of CVJM before the onset of ischemic infarction or TIA in the posterior circulation (PC) or diagnosis of VAD, while 31/32 cases (96.8%) had no other cerebrovascular risk factors. Predisposing factors in the form of trivial neck trauma or sport was identified in 12/32 cases (37.5%).
There was one patient with only VAD without any ischemic attack. In total, 17/32 cases (53.1%) had multiple episodes of PCIS or TIA. Interestingly, 16/17 cases with multiple episodes had data on the temporal relationship between the recurrence of PCIS or TIA and diagnosis of CVJM, with 14/16 cases (87.5%) having recurrence before diagnosis and only three of 16 (18.8%) after diagnosis. Neck or head pain during presentation was noted in 16/32 cases (50%). The most common neurological symptoms were limb numbness/weakness (20/32), followed by ataxia (15/32) and dizziness/vertigo (12/32). Other symptoms included nausea and vomiting (eleven of 32), disturbance of consciousness or syncope (nine of 32), gait disturbance (eight of 32), language deficit (seven of 32), visual symptoms (six of 32), bulbar symptoms (six of 32), cognitive impairment (two of 32), irritating cough (one of 32), and tinnitus (one of 32).
Imaging Features
Except for one patient with no ischemic attack and one patient with no information, the remaining 30 patients had multiple infarcts scattered throughout the PC area (cerebellum, thalamus, medulla oblongata, pons, temporal occipital lobe) in various combinations, as shown on CT and MRI. In sum, 22 of 30 cases (73.3%) showed infarcts located in areas supplied by more than one vessel branch of the PC.
Vascular wall damage caused by VAD was diagnosed or considered in all patients using CeA ultrasound, MRA, HR MRI, CTA, or DSA in 31 patients, while one patient did not have detailed information on VAD. Dissection was located in the V3 segment (around the atlantoaxial joint) of the VA in 29/31 cases (93.5%), including 27 cases with complete dissection in the V3 segment and two cases in the V3+V4 segment. Only two of 31 cases (6.5%) had dissection damage in the V2 segment of the VA. The affected VA was on the right side in 16/31 cases (51.5%), on the left in nine of 31 cases (29%), and bilaterally in six of 31 cases (19.4%). Further, a dissecting pseudoaneurysm was diagnosed in eight of 31 cases (25.8%).
The most common congenital CVJMs were atlantoaxial dislocation (AAD) and atlantoaxial subluxation, which were found in 21/32 cases (65.6%), followed by os odontoideum in 12/32 cases (37.5%), Klippel–Feil anomaly in ten of 32 cases (31.3%), basilar invagination in six of 32 cases (18.8%), and atlanto-occipital fusion or assimilation in six of 32 cases (18.8%). The other CVJMs included posterior-arch anomaly of the atlas in three, disunited odontoid in one, odontoid aplasia in one, rudimentary lateral mass in one, malalignment of the lateral masses of the atlas with C2 in two, high standing tip of the odontoid in one, and C1 disunion in one case. Most patients (27/32, 84.3%) had multiple combined abnormalities, and AAD or atlantoaxial subluxation with os odontoideum was most common (12/27 cases, 44.4%).
Congenital CVJMs were confirmed by routine cervical spinal X-rays with or without flexion/extension in 23/32 cases (71.8%), 12/23 of whom (52.2%) received further examination, including cervical spinal CT, 3-D CT, or CTA. In addition, seven of 32 cases (21.9%) were confirmed directly by cervical spinal CT or 3-D CT, and two of 32 cases (6.2%) by spinal MRI. Further, dynamic changes in the VA were evaluated only in four cases, with a positive change in vessel caliber with anteroposterior extension and flexion neck movement in two cases and lateral and rotational neck movement in two cases.
Treatment and Prognosis
There were 22/32 cases with detailed data on antithrombotic therapy. In eleven patients with initial antiplatelet therapy and eleven with initial anticoagulation therapy, seven of eleven (63.6%: four with aspirin and three with dual antiplatelet) and one of eleven (9.1%), respectively, experienced recurrence of PCIS or TIA. Further, as a result of conversion to anticoagulant treatment, one patient with recurrence on initial aspirin treatment still experienced a recurrent PCIS.
After diagnosis of VAD and CVJM, there were 30/32 cases with treatment data. Targeted etiological treatment, including fusion or vertebral fixation, was performed in 16/30 cases, while aneurysm resection was performed in one case. There were two cases with recurrence of PCIS before surgery. However, there was no recurrence after surgery in 13 patients with follow-up data. Correspondingly, eleven of 13 cases with conservative treatment or who had rejected surgery had follow-up data, with one case of recurrence, one of new proximal occlusion of the PCA, and one with aneurysm enlargement on DSA.
Discussion
VAD can occur secondarily to blunt cervical trauma1 and connective-tissue disorders2 or be spontaneous. However, as patients with VAD derived from underlying CVJM have been occasionally reported,4–29 the occurrence of CVJM with PC stroke may be seriously underestimated, given that the necessary radiographic studies are rarely performed.8 Here, we report four cases with a definite diagnosis of VAD with congenital CVJM. Because all four cases had no family history of CeA dissection (CeAD) or hereditary connective-tissue disorders and did not meet clinical diagnostic criteria for established hereditary connective-tissue disorder, these cases had not had any genetic analysis performed for these disorders. In the present systematic review based on four new cases and similar reported cases, we analyzed and summarized the clinical profiles of VAD combined with CVJM to emphasize the significance of early recognition of CVJM in VAD patients.
We found several characteristics on etiology, clinical and imaging features, and treatment. Patients were mainly children, adolescents, and young adults, with a significant predominance of males. The majority of cases had no prior history of congenital CVJM, and only approximately a third of cases had predisposing factors in the form of trivial neck trauma or sporting activities. More than 50% of patients had multiple PCIS or TIAs over a period of several days to several months. The recurrence of PCIS or TIA also occurred before diagnosis of congenital CVJM more than after diagnosis. Further, the most common neurological symptoms were limb numbness/weakness, followed by ataxia and dizziness/vertigo, while neck or head pain during presentation was noted in approximately 50% of cases. Head MRI or CT showed infarcts scattered in the PC area in various combinations. In approximately 70% of patients, the infarcts were located in areas supplied by more than one vessel branch of the PC. Further, in 90% of these patients, vascular wall damage caused by dissection or pseudoaneurysm was located mainly in the V3 segment (around the atlantoaxial joint), wall damage on the bilateral side was found in approximately 20% of cases, and dissection pseudoaneurysm was diagnosed in approximately 25% of cases. The most common congenital CVJMs were AAD or atlantoaxial subluxation (found in 65.6% of cases), followed by os odontoideum, Klippel–Feil anomaly, and basilar invagination. More than 80% of patients had multiple combined abnormalities, and AAD or atlantoaxial subluxation combined with os odontoideum was most common (observed in 33% of cases). Patients who accepted initial anticoagulation therapy showed lower recurrence of PCIS or TIA than patients who accepted initial antiplatelet therapy. In addition, targeted surgery was effective in preventing further recurrence in patients after careful evaluation by a neurosurgeon.
As described, the patients in this study were predominantly children and young people and male. This demographic characteristic was consistent with coexisting CVJM. We found that AAD or atlantoaxial subluxation — os odontoideum, Klippel–Feil anomaly, and basilar invagination — were the most common types of CVJM, with the majority of cases showing coexistence of multiple types. This coexistence of multiple types of anomaly and lack of history of secondary etiology, such as severe trauma and rheumatic or degenerative osteoarthritis, suggests a congenital etiology for these disorders. A similar marked preponderance of males in such CVJMs as odontoid dysgenesis has previously been reported.30
The majority of patients in the present study had no history of congenital CVJM, although obvious skeleton marks were reported in some patients.22,27 Interestingly, >50% of patients had multiple episodes of PCIS or TIA, and ischemic episodes typically recurred before the diagnosis of CVJM. These findings suggest that VAD patients with underlying CVJM might have a greater risk of ischemic recurrence than patients without CVJM, because ischemic recurrence is quite rare in CeAD, and only 1.4%–2% of patients had experienced recurrent stroke at 3 months.31,32 In addition, there is poor awareness of CVJM in patients with VAD in general clinical practice. Nevertheless, the early recognition and targeted treatment of CVJM in VAD patients can prevent further worsening of neurological symptoms. As previously reported, the common neurological symptoms associated with CVJM are likely a result of mechanical compression of the spinal cord or nerve root,33 while most cases in the present study did not show mechanical compression of the spinal cord. As such, stabilization of the cervical spine with fusion at appropriate levels in these symptomatic patients with compression of the spinal cord may be successful in preventing further episodes of stroke. This may explain the relative rarity of stroke complications in patients with CVJM and the lack of history of congenital CVJM in the majority of patients.
As shown on CT or MRI, the majority of these patients exhibited repeated and multiple infarcts located in areas supplied by more than one vessel branch of the PC. These findings support a predominantly ischemic mechanism involving artery-to-artery embolisms in most cases. Another striking imaging feature was that vascular wall damage caused by dissection or pseudoaneurysm was mainly located in the V3 segment (ie, the C1–C2 level of the VA — >90% of cases), suggesting a causal relationship between CVJM and VAD. The mechanism of vascular wall damage in VAD caused by CVJM has been well described, and involves repeated motion, kinking, and stretching of a hypercurved VA because of an unstable occipitoatlantoaxial complex, which triggers intimal tears, dissection, and thrombus formation.34 Therefore, trivial neck trauma or sports may be predisposing factors for VAD with CVJM and only occur in some patients. In addition, as shown by the dynamic changes in the VA evaluated in four cases in this systematic review, as well as other reported cases,35,36 in which rotational VA-occlusion syndrome may be typically diagnosed, neck activity may also repeatedly crush and damage the VA wall, causing thrombus formation and hemodynamic changes, and might be contributing etiologies, other than the arterial dissections.37
Of note, this characteristic injury site may represent a radiological marker for recognizing underlying CVJM in VAD patients. Radiography of the cervical spine is a convenient and useful technique for detecting bony abnormalities. In the present study, CVJM was confirmed by routine cervical spinal X-ray with or without flexion/extension in 70% of cases. Considering that only two patients with a coexisting Klippel–Feil anomaly had VAD located in the V2 segment, patients with general VAD should undergo routing cervical spinal X-ray to screen for the Klippel–Feil anomaly. However, for patients with VAD at the V3 segment, further assessment of CVJM using spinal X-ray with flexion/extension and cervical spinal 3-D CT is required, as these techniques have greater resolution and provide valuable anatomical information on the mechanism of VAD formation induced by bone compression. In addition, dynamic changes in the VA assessed using CeA ultrasound, CTA, or DSA should be considered to assess potential mechanisms of VA damage.
With regard to therapeutic strategies for VAD, numerous meta-analyses based on case series and observation studies have been published in recent years showing no significant difference in stroke recurrence, symptomatic intracerebral hemorrhages, other major bleeds, or mortality with anticoagulation versus antiplatelet therapy in CeAD patients.38–40 The multicenter randomized controlled CADISS trial also found no differences in the efficacy of antiplatelet versus anticoagulant drugs for preventing stroke and death in patients with symptomatic carotid-artery and VAD.41,42 Furthermore, a recent study with data from CADISS aimed to determine whether dissecting aneurysm is associated with increased stroke-recurrence risk and whether the type of antithrombotic drug (antiplatelets vs anticoagulants) modifies the persistence or development of dissecting aneurysm. The results showed that the presence of a dissecting aneurysm did not indicate that an individual with dissection was at higher risk of recurrent stroke, and there was no difference in the persistence of dissecting aneurysms or development of new dissecting aneurysms for antiplatelet vs anticoagulant therapy.43 Current 2018 American Heart Association–American Heart Association guidelines, based on CADISS results and prior observational studies, suggest that 3- to 6-month therapy with either antiplatelet or anticoagulant therapy may be reasonable (class of recommendation IIB, level of evidence B-R).44 However, the question of choice of antithrombotic therapy in CeAD remains unanswered and controversial in some cases.45 No direct oral anticoagulants (DOACs) were used in these studies, and only very limited information on treatment of CeAD with DOACs is available. Treatment of VAD with DOACs in our reported cases was off-label, and their role in CeAD patients has yet to be investigated. The antiplatelet regimens used in the CADISS trial do not allow a clear conclusion on the efficacy of various antiplatelet regimens and is probably obscuring the potential beneficial effect of dual-antiplatelet therapy. In addition, it is still unclear whether CeAD patients with certain characteristics, such as microembolic signals, may have early recurrence of ischemic events and need more aggressive treatment.
In our systematic review of cases with combined VAD and CVJM, 63.6% of patients who accepted initial antiplatelets and 9.1% who accepted initial anticoagulation therapy experienced recurrence of PCIS or TIA, respectively. Therefore, the respective efficacy of antiplatelet and anticoagulation treatment was different for patients with combined VAD and CVJM. These benefits of anticoagulation therapy may be because the vascular wall of the VA is more prone to injury in CVJM patients than in those with VAD without CVJM, resulting in generation of more emboli. Further, as supported by our findings, the recurrence of ischemic episodes may be decreased after diagnosis of underlying CVJM in VAD patients. Based on the potential mechanism of vascular wall damage of VAD caused by CVJM, patients diagnosed with CVJM are usually required to limit their head and neck motion by wearing a neck brace to avoid vascular wall damage. In particular, surgery using an internal fixation system can reduce C1–C2 luxation. After this reduction in C1 and C2 foramina displacement, retraction strength on the VA is markedly reduced, and in combination with avoidance of dynamic injury because of the atlanto-odontoid joint fusion reduces the recurrent risk of VAD.21
Of note, some patients still showed recurrence while on antithrombotic treatment before surgery, and new proximal occlusion of the PC or aneurysm enlargement was observed in some patients who had had conservative treatment or refused surgery after diagnosis of CVJM. By contrast, no recurrence was reported in follow-up after surgery. Therefore, after careful neurosurgical evaluation, surgical treatments (eg, fusion and internal fixation) should be considered in patients with a combination of VAD and CVJM to strengthen the stability of the craniovertebral junction and protect the vascular wall.
Conclusion
Underlying CVJM is a rare but overlooked etiology in patients with VAD. VAD patients with underlying CVJM may be at greater risk of ischemic recurrence and require more active antithrombotic therapy. A striking imaging feature was that vascular wall damage caused by dissection or pseudoaneurysm was located mainly in the V3 segment (ie, the C1–C2 level of the VA), which may represent a radiological marker for underlying CVJM in VAD patients. In patients with VAD at the V3 segment, spinal X-ray with flexion/extension and cervical spinal 3-D CT should be considered for assessment of CVJM. Such timely assessment is important for determining the specific cause, and enables targeted intervention to prevent worsening of neurological symptoms. Further prospective radiographic studies are required to assess the clinical significance of CVJM in patients with VAD.
Ethics
Approval from the Ethics Committee of Xuanwu Hospital was obtained for this study. Consent was obtained from study participants prior to study commencement, and they provided written informed consent for publication of these case reports.
Acknowledgments
We thank Liwen Bianji, Edanz Editing China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
Disclosure
The authors report no conflicts of interest for this work.
References
1. Bartels E. Dissection of the extracranial vertebral artery: clinical findings and early noninvasive diagnosis in 24 patients. J Neuroimaging. 2006;16(1):24–33.
2. Toyota S, Wakayama A, Fujimoto Y, et al. Dissecting aneurysm of the radiculomedullary artery originating from extracranial vertebral artery dissection in a patient with rheumatoid cervical spine disease: an unusual cause of subarachnoid hemorrhage. Case report. J Neurosurg Spine. 2007;7(6):660–663. doi:10.3171/SPI-07/12/660
3. Cacciola F, Phalke U, Goel A. Vertebral artery in relationship to C1–C2 vertebrae: an anatomical study. Neurol India. 2004;52:178–184.
4. Singer WD, Haller JS, Wolpert SM. Occlusive disease associated with cervical spine anomaly. Am J Dis Child. 1975;129(4):492–495.
5. Fraser RAR, Zimbler MS. Hindbrain stroke in children caused by extracranial vertebral artery trauma. Stroke. 1975;6(2):153–159. doi:10.1161/01.STR.6.2.153
6. Ross CA, Curnes JT, Greenwood RS. Recurrent vertebrobasilar embolism in an infant with Klippel-Feil anomaly. Pediatr Neurol. 1987;3(3):181–183. doi:10.1016/0887-8994(87)90090-7
7. Born CT, Petrik M, Freed M, et al. Cerebrovascular accident complicating Klippel-Feil syndrome: a case report. J Bone Joint Surg Am. 1988;70(9):1412–1415. doi:10.2106/00004623-198870090-00025
8. Phillips PC, Lorentsen KJ, Shropshire LC, et al. Congenital odontoid aplasia and posterior circulation stroke in childhood. Ann Neurol. 1988;23(4):410–413. doi:10.1002/ana.410230421
9. Bhatnagar M, Sponseller PD, Carroll C, et al. Pediatric atlantoaxial instability presenting as cerebral and cerebellar infarcts. J Pediatr Orthop. 1991;11(1):103–107. doi:10.1097/01241398-199101000-00020
10. Kikuchi K, Nakagawa H, Watanabe K, et al. Bilateral vertebral artery occlusion secondary to atlantoaxial dislocation with os odontoideum: implication for prophylactic cervical stabilization by fusion–case report. Neurol Med Chir (Tokyo). 1993;33(11):769–773. doi:10.2176/nmc.33.769
11. Takakuwa T, Hiroi S, Hasegawa H, et al. Os odontoideum with vertebral artery occlusion. Spine (Phila Pa 1976). 1994;19(4):460–462. doi:10.1097/00007632-199402001-00015
12. Ando S, Matsui Y, Fujii J, et al. Cerebellar infarctions secondary to cranio-cervical anomalies: a case report. Nippon Geka Hokan. 1994;63(4):148–154.
13. Randall JM, Griffiths PD, Gardner-Medwin D, et al. Thalamic infarction in childhood due to extracranial vertebral artery abnormalities. Neuropediatrics. 1994;25(5):262–264. doi:10.1055/s-2008-1073033
14. Miyata I, Imaoka T, Masaoka T, et al. Pediatric cerebellar infarction caused by atlantoaxial subluxation–case report. Neurol Med Chir (Tokyo). 1994;34(4):241–245. doi:10.2176/nmc.34.241
15. Panda S, Ravishankar S, Nagaraja D, et al. Bilateral vertebral artery dissection caused by atlantoaxial dislocation. J Assoc Physicians India. 2010;58:187–189.
16. Sasaki H, Itoh T, Takei H, et al. Os odontoideum with cerebellar infarction: a case report. Spine (Phila Pa1976). 2000;25(9):1178–1181. doi:10.1097/00007632-200005010-00020
17. Zotter H, Zenz W, Gallistl S, et al. Stroke following appendectomy under general anesthesia in a patient with basilar impression. Acta Anaesthesiol Scand. 2000;44(10):1271–1272. doi:10.1034/j.1399-6576.2000.441015.x
18. Hasan I, Wapnick S, Kutscher ML, et al. Vertebral arterial dissection associated with Klippel-Feil syndrome in a child. Childs Nerv Syst. 2002;18(1–2):67–70. doi:10.1007/s003810100511
19. Fukuda M, Aiba T, Akiyama K, et al. Cerebellar infarction secondary to os odontoideum. J Clin Neurosci. 2003;10(5):625–626. doi:10.1016/S0967-5868(03)00131-0
20. Karimi M, Razavi M, Fattal D. Rubral lateropulsion due to vertebral artery dissection in a patient with Klippel- Feil syndrome. Arch Neurol. 2004;61(4):583–585. doi:10.1001/archneur.61.4.583
21. Chen Z, Jian FZ, Wang K. Diagnosis and treatment of vertebral artery dissection caused by atlantoaxial dislocation. CNS Neurosci Ther. 2012;18(10):876–877. doi:10.1111/j.1755-5949.2012.00376.x
22. Kulkarni GB, Mustare V, Pruthi N, et al. Profile of patients with craniovertebral junction anomalies with posterior circulation strokes. J Stroke Cerebrovasc Dis. 2014;23(10):2819–2826. doi:10.1016/j.jstrokecerebrovasdis.2014.07.003
23. Dornbos D
24. Ouyang ZY, Qiu MJ, Zhao Z, et al. Congenital anomaly of the posterior arch of the atlas: a rare risk factor for posterior circulation stroke. J Neurointerv Surg. 2017;9(7):e27. doi:10.1136/neurintsurg-2016-012731.rep
25. Hu Y, Du J, Liu Z, et al. Vertebral artery dissection caused by atlantoaxial dislocation: a case report and review of literature. Childs Nerv Syst. 2019;35(1):187–190. doi:10.1007/s00381-018-3948-x
26. Jadeja N, Nalleballe K. Pearls & Oy-sters: bow hunter syndrome: a rare cause of posterior circulation stroke: do not look the other way. Neurology. 2018;91(7):329–331. doi:10.1212/WNL.0000000000006009
27. Dirik E, Yis U, Dirik MA, et al. Vertebral artery dissection in a patient with Wilderwank syndrome. Pediatr Neurol. 2008;39(3):218–220. doi:10.1016/j.pediatrneurol.2008.06.005
28. Dickinson LD, Tuite GF, Colon GP, et al. Vertebral artery dissection related to basilar impression: case report. Neurosurgery. 1995;36(4):835–838. doi:10.1227/00006123-199504000-00026
29. Rajesh V, Ritesh S, Ojha BK, et al. Thalamic syndrome as the heralding manifestation of atlantoaxial dislocation. BMJ Case Rep. 2013;bcr2012007712.
30. Greenberg AD. Atlanto-axial dislocations. Brain. 1968;91(4):655–684. doi:10.1093/brain/91.4.655
31. von Babo M, De Marchis GM, Sarikaya H, et al. Differences and similarities between spontaneous dissections of the internal carotid artery and the vertebral artery. Stroke. 2013;44(6):1537–1542. doi:10.1161/STROKEAHA.113.001057
32. The CADISS trial investigators. Antiplatelet treatment compared with anticoagulation treatment for cervical artery dissection (CADISS): a randomised trial. Lancet Neurol. 2015;14(4):361–367. doi:10.1016/S1474-4422(15)70018-9
33. Bharucha EP, Dastur HM. Craniovertebral anomalies (a report on 40 cases). Brain. 1964;87(3):469–480. doi:10.1093/brain/87.3.469
34. Bell HS. Basilar artery insufficiency due to atlanto-axial instability. Am Surg. 1969;35(10):695–700.
35. Salunke P, Karthigeyan M, Ahuja CK, et al. An unusual cause of vertebrobasilar insufficiency in a case of atlantoaxial dislocation with anomalous vertebral artery. World Neurosurg. 2020;138:
36. Malik P, Salunke P, Kataria M, et al. Anomalous V3 segment aneurysm associated with congenital atlantoaxial dislocation: case report and discussing the challenges in management. World Neurosurg. 2019;121:
37. Cornelius JF, George B, N’dri Oka D, et al. Bow-hunter’s syndrome caused by dynamic vertebral artery stenosis at the cranio-cervical junction–a management algorithm based on a systematic review and a clinical series. Neurosurg Rev. 2012;35(1):127–135. doi:10.1007/s10143-011-0343-4
38. Menon R, Kerry S, Norris JW, et al. Treatment of cervical artery dissection: a systematic reviewand meta-analysis. J Neurol Neurosurg Psychiatry. 2008;79(10):1122–1127. doi:10.1136/jnnp.2007.138800
39. Arauz A, Ruiz A, Pacheco G, et al. Aspirin versus anticoagulation in intra- and extracranial vertebral artery dissection. Eur J Neurol. 2013;20(1):167–172. doi:10.1111/j.1468-1331.2012.03825.x
40. Daou B, Hammer C, Mouchtouris N, et al. Anticoagulation vs antiplatelet treatment in patients with carotid and vertebral artery dissection: a study of 370 patients and literature review. Neurosurgery. 2017;80(3):368–379. doi:10.1093/neuros/nyw086
41. CADISS trial investigators, Markus HS, Hayter E. Antiplatelet treatment compared with anticoagulation treatment for cervical artery dissection (CADISS): a randomised trial. Lancet Neurol. 2015;14(4):361–367.
42. Markus HS, Levi C, King A, et al. Cervical artery dissection in stroke study (CADISS) investigators. Antiplatelet therapy vs anticoagulation therapy in cervical artery dissection: the cervical artery dissection in stroke study (CADISS) randomized clinical trial final results. JAMA Neurol. 2019;76(6):657–664. doi:10.1001/jamaneurol.2019.0072
43. Larsson SC, King A, Madigan J, et al. Prognosis of carotid dissecting aneurysms: results from CADISS and a systematic review. Neurology. 2017;88(7):646–652. doi:10.1212/WNL.0000000000003617
44. Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American heart association/American stroke association. Stroke. 2018;2018(49):e49–e110.
45. Serkin Z, Le S, Sila C. Treatment of extracranial arterial dissection: the roles of antiplatelet agents, anticoagulants, and stenting. Curr Treat Options Neurol. 2019;21(10):48.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.