Back to Journals » International Journal of Women's Health » Volume 6
Profile of pazopanib and its potential in the treatment of epithelial ovarian cancer
Authors Davidson B, Secord A
Received 21 November 2013
Accepted for publication 9 January 2014
Published 13 March 2014 Volume 2014:6 Pages 289—300
DOI https://doi.org/10.2147/IJWH.S49781
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Brittany A Davidson, Angeles Alvarez Secord
Division of Gynecologic Oncology, Duke Cancer Institute, Department of Obstetrics and Gynecology, Duke University Medical Center, Durham, NC, USA
Abstract: Epithelial ovarian cancer (EOC) is the most lethal gynecological cancer. Recently, clinical trials have focused on novel antiangiogenic agents in combination with chemotherapy or alone in women with primary and recurrent ovarian cancer. Antiangiogenic agents include monoclonal antibodies, tyrosine-kinase inhibitors, and peptibodies. Many of these agents, including bevacizumab, pazopanib, nintedanib, cediranib, and trebananib, have been evaluated in randomized Phase III clinical trials, and all have demonstrated a progression-free survival (PFS) benefit. Specifically, maintenance pazopanib was shown to improve PFS in women with newly diagnosed EOC. Pazopanib, an oral TKI, inhibits several kinase receptors, including those for vascular endothelial growth factor (-1,-2,-3), platelet-derived growth factor (-α and -β), and fibroblast growth factor. It also targets stem cell-factor receptor (c-kit), interleukin 2-inducible T-cell kinase, lymphocyte-specific protein tyrosine kinase, and colony-stimulating factor 1 receptor. Pazopanib has been investigated in several Phase II and III clinical trials, with results indicating a potential role in the management of EOC. This article provides an overview of pazopanib in the treatment of EOC.
Keywords: pazopanib, antiangiogenic agents, ovarian carcinoma
Introduction
Worldwide, ovarian cancer is the third-most common gynecologic malignancy and ninth overall, with an estimated 225,000 new diagnoses each year. Owing in part to its late stage at diagnosis, it is the eighth-most common cancer cause of death for women.1 Carboplatin and paclitaxel have been used as the treatment backbone for epithelial ovarian cancer (EOC) since the pivotal Gynecologic Oncology Group (GOG)-158 and Arbeitsgemeinschaft Gynäkologische Onkologie (AGO)-OVAR-3 trials. Carboplatin/paclitaxel compared to cisplatin/paclitaxel demonstrated a noninferior median progression-free survival (PFS) for the carboplatin/paclitaxel group (GOG, 20.7 versus 19.4, response rate [RR] 0.88, 95% confidence interval [CI] 0.75–1.03; OVAR-3, 17.2 versus 19.1, hazard ratio [HR] 1.05, 95% CI 0.89–1.23) and median overall survival (OS) (GOG, 57.4 versus 48.7, RR 0.84, 95% CI 0.7–1.02; OVAR-3, 43.3 versus 44.1, HR 1.045, 95% CI 0.869–1.257). Carboplatin/paclitaxel is easier to administer and has a favorable toxicity profile compared to cisplatin/paclitaxel.2,3 Despite these therapies, over 70% of patients develop recurrent disease and ultimately die of progressive cancer,2 thus the impetus to evaluate novel treatment strategies and maintenance therapies for standard chemotherapy to improve disease control in women with advanced ovarian cancer. Given the antitumor activity seen with antiangiogenesis therapies in preclinical studies and Phase II trials, these agents have been added to frontline and/or maintenance phases of treatment.
The management of ovarian cancer has changed dramatically over the last few years as a result of several landmark clinical trials4–7 (GOG172, Japanese Gynecologic Oncology Group [JGOG]-3016, GOG218, International Collaborative Ovarian Neoplasm [ICON]-7). GOG172 demonstrated that intravenous (IV) and intraperitoneal (IP) cisplatin and paclitaxel in women with optimally debulked, advanced-stage disease resulted in a 16 month median survival benefit compared to IV therapy (RR 0.75, 95% CI 0.58–0.97; P=0.03).4 More recently, the JGOG reported a significant PFS and OS benefit in patients receiving a regimen with carboplatin and dose-dense paclitaxel (80 mg/m2 on days 1, 8, and 15 every 3 weeks) compared to the standard every-3-week carboplatin/paclitaxel regimen (PFS 28.2 versus 17.5 months, OS 100.5 versus 62.2 months).5 Several chemotherapeutic agents, including paclitaxel, have been found to have an inhibitory effect on angiogenesis, a phenomenon that is seen at lower drug doses but necessitates more frequent dosing intervals.8–10 Weekly paclitaxel has even demonstrated antitumor activity in patients with resistance to the standard 3-week paclitaxel dosing interval.11 Multiple Phase II/III trials are ongoing to evaluate the efficacy of dose-dense treatments in EOC.12
The addition of bevacizumab, a monoclonal antibody with pure anti-vascular endothelial growth factor (VEGF) activity, was studied in GOG218 and ICON7. GOG 218 compared a control arm of IV carboplatin (area under curve [AUC] 6) and paclitaxel (175 mg/m2) for six cycles. The bevacizumab-initiation arm used bevacizumab during cycles 2–6 followed by placebo through cycle 22, while the bevacizumab-throughout arm incorporated the drug during cycles 2–22. Bevacizumab-throughout therapy prolonged median PFS by 3.8 months compared to chemotherapy alone (HR 0.77, 95% CI 0.68–0.87). Notably, gastrointestinal perforations (GIPs) occurred more frequently in patients treated with this antiangiogenic agent (2.8% initiation, 2.6% throughout versus 1.2% control), with hypertension occurring in nearly 23% of patients in the bevacizumab-throughout arm (7.2% in control).6 ICON7 had similar aims, adding bevacizumab to a backbone regimen of IV carboplatin/paclitaxel. In a subgroup analysis, patients at highest risk of progression (International Federation of Gynecology and Obstetrics13 stage IV disease or FIGO stage III with >1.0 cm residual tumor at time of debulking) experienced the greatest benefit.7 Those randomized to bevacizumab had a 5.5-month increase in median PFS (HR 0.73, 95% CI 0.6–0.93; P=0.002) and a 7.8-month increase in median OS (HR 0.64, 95% CI 0.48–0.85; P=0.002). Grade 3 or 4 adverse events (AEs) in the bevacizumab group included hypertension (6%), GIPs (1%), thrombolytic events (7%), and neutropenia (17%).7
Based on these landmark trials, the current standard of care for the treatment of women with newly diagnosed EOC (stage II–IV) as per National Comprehensive Cancer Network guidelines involves different treatment strategies: IV/IP combination paclitaxel and cisplatin, IV paclitaxel and carboplatin, IV docetaxel and carboplatin, dose-dense IV paclitaxel on days 1, 8, and 15 with carboplatin on day 1, and a bevacizumab-containing regimen as per ICON7 or GOG218.14
GOG262, a randomized Phase III trial in patients with newly diagnosed EOC, fallopian tube cancer (FTC), or primary peritoneal carcinoma (PPC), compared IV carboplatin/paclitaxel every 3 weeks to dose-dense paclitaxel with carboplatin. Bevacizumab was optional. Initial results indicated that weekly dose-dense paclitaxel does not increase PFS (HR 0.97, 95% CI 0.79–1.18); however, a subgroup analysis demonstrated a 4 month PFS benefit for those treated with dose-dense paclitaxel who did not receive bevacizumab (HR 0.60, 95% CI 0.37–0.96; P=0.033). OS data are not yet available.15
While bevacizumab is the most studied antiangiogenic agent in EOC, there are other types of antiangiogenic agents, including tyrosine-kinase inhibitors (TKIs) and peptibodies, which have also been evaluated in ovarian cancer. Agents such as pazopanib, nintedanib, cediranib, and trebananib have been studied in randomized Phase III clinical trials and have demonstrated a PFS benefit.16–19 The improvement in PFS indicates a possible important role for the use of such drugs in the treatment of EOC.
The landscape of the treatment of EOC has significantly progressed throughout the last decade and continues to advance with the findings from pivotal phase III trials indicating a PFS benefit with the use of antiangiogenic therapies. This article reviews the role of angiogenesis in ovarian cancer and the rationale for targeting angiogenic pathways, specifically providing an overview of the preclinical and clinical development of pazopanib, an oral TKI, in the treatment of EOC.
The role of angiogenesis in epithelial ovarian cancer
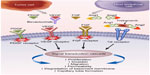
Angiogenesis plays a major role in the progression and metastasis of solid tumors. Tumor-related angiogenesis, based on microvessel density, has been demonstrated to have prognostic significance in patients with a variety of solid malignancies, including EOC.20–27 The regulation of angiogenesis is complex, and involves multiple pathways and targets (Figure 1). In normal ovarian tissue, this process occurs conditionally stimulated by locally released growth factors in response to various insults.28 VEGF is one of the most potent proangiogenic factors. The VEGF family consists of seven different ligands, and VEGF-A has emerged as the dominant player in angiogenesis.29,30 These different isoforms bind preferentially to three different VEGF receptors, known as VEGFR-1, -2 and -3, and appear to exert their roles on multiple intracellular pathways. Expressed in both normal and malignant ovaries, VEGF is notably upregulated in ascites and peritoneal metastases.31,32 Binding of VEGF to its receptor causes phosphorylation of the VEGFR, leading to activation of downstream signaling pathways involved in proliferation of endothelial cells.33 High preoperative VEGF levels correlate with tumor grade, disease stage, and OS.34 In vitro, carboplatin treatment induces VEGF expression by endothelial cells, supporting a mechanism for chemotherapy resistance and suggesting a need for VEGF inhibition in combination with cytotoxic therapies.35
Other growth factors and chemokines are involved in angiogenesis, and thus may be potential therapeutic targets. These include fibroblast growth factor (FGF), angiopoietins, endothelins, interleukin (IL)-8, macrophage chemotactic proteins, and platelet-derived growth factor (PDGF). Several studies have shown correlations between the expression of these factors and ascites36 and OS in EOC.37,38 The correlation of these proangiogenic factors and survival makes them attractive therapeutic targets.
Though tumors may have an initial response to antiangiogenesis agents, resistance to these drugs can and does occur. This phenomenon is unlike the classic mechanisms for resistance against conventional chemotherapeutic agents. As tumor endothelial cells are not prone to mutation, they adapt to VEGF inhibition using secondary signaling pathways (ie, PDGF, FGF) to recruit vasculature. Given that VEGF is the principal angiogenic pathway, continued blockade despite evidence of progression may confer an advantage, a concept supported in several Phase II trials of patients with metastatic colorectal cancer treated with bevacizumab.39,40 Patients treated with bevacizumab who continued treatment past their first progression experienced a survival benefit compared to those not receiving bevacizumab postprogression (HR 0.48, P<0.001).40 A study of patients with recurrent EOC who had previously experienced a complete response to a bevacizumab-containing regimen indicated that those who received bevacizumab at the time of recurrence had improved PFS compared to those who did not (20 versus 6 months, P=0.0019).41 Theoretically, agents like pazopanib that can simultaneously inhibit both the VEGF pathway and ancillary angiogenic pathways (ie, FGF, PDGF, c-kit) may overcome resistance to VEGF blockade.
The remainder of this review focuses specifically on the role of pazopanib in EOC.
Preclinical data
Pazopanib is an oral TKI of multiple kinase protein receptors, including those for VEGF, PDGF, and FGF. It also targets stem cell-factor receptor (c-kit), IL-2-inducible T-cell kinase, lymphocyte specific tyrosine kinase, and colony-stimulating factor 1 receptor.42 Pazopanib binds to intracellular adenosine triphosphate-binding pockets, thus inhibiting the phosphorylation of these target receptors and impeding downstream pathways.33 Pharmacokinetic testing has shown pazopanib tumor-growth inhibition to correlate with steady-state concentration rather than peak plasma concentration. The concentration required for inhibition of tumor growth in vivo is approximately 40 μM.43 TKIs target multiple angiogenic pathways, which may provide more effective tumor growth and metastatic inhibition.44
Multiple studies have evaluated the in vitro and in vivo effects of pazopanib alone and in combination with cytotoxics in a variety of different tumor types. Merritt et al assessed the effects of metronomic topotecan and pazopanib in human EOC lines. At high doses of single-agent pazopanib in vivo, there was a noticeable decrease in VEGFR-2 phosphorylation in as early as 4 hours. In contrast, there was no effect on phosphorylation with topotecan. Moreover, tumor growth was significantly reduced by 79%–84% in orthotopic murine models treated with combination topotecan/pazopanib. Similarly, mouse survival was significantly increased in the group treated with metronomic topotecan and pazopanib, compared to single-agent arms (P<0.001).45 Antitumor activity of pazopanib has been reported in several other types of tumor xenografts.43,46
Clinical experience with pazopanib
Since its discovery by Harris et al,47 pazopanib has received US Food and Drug Administration approval for two indications: renal cell carcinoma (RCC) and soft-tissue sarcomas.48,49 Patients with EOC were included in the initial Phase I/II studies of pazopanib (Table 1), and recently larger Phase II and III trials have focused on gynecologic malignancies (Table 2).
Phase I experience
Given the encouraging preclinical activity of pazopanib, a Phase I dose-escalation clinical trial in patients with advanced, refractory solid tumors was conducted. Pazopanib demonstrated a manageable toxicity profile and clinical activity in a range of solid tumors. No maximum tolerated dose (MTD) was found, as steady-state plasma drug concentrations plateaued above 800 mg daily. Drug-related AEs (all grades) included hypertension (33%), diarrhea (33%), reversible hair depigmentation (32%), and nausea (32%). Similar to other angiogenesis inhibitors, hypertension was the most frequent grade 3 toxicity. Of the 63 patients included, 17 had a partial response (PR) or stable disease (SD) for more than 6 months. Confirming in vivo studies, the clinical activity of pazopanib correlated with serum concentration ≥15 μg/mL (34 μM/L), indicating the importance of steady-state rather than peak concentration. Over 90% of patients were able to achieve a drug concentration ≥15 μg/mL using a daily dose of 800 mg.50
Several Phase I studies have evaluated MTD, AE profile, and pharmacokinetics of pazopanib in combination with other antitumor therapies.51–53 The bioavailability of pazopanib was notably increased by 50%–60% when used in concert with high-dose lapatinib in patients with advanced solid tumors. This was considered to be the result of a decrease in pazopanib metabolism secondary to inhibition of cytochrome P450 (CYP) 3A4 by lapatinib. Despite this increase in drug availability, only two of the 17 patients at the highest dose studied (pazopanib 800 mg/lapatinib 1,500 mg) experienced dose-limiting toxicities (DLTs) (grade 3 alanine transaminase [ALT] increase, grade 2 abdominal pain/nausea). Diarrhea was the most common AE, seen in 96% of study participants. Hypertension occurred in 21% of study participants, though it is difficult to assess drug effect, because poorly controlled hypertension was not an exclusion criteria for study participation.53
The MTD and toxicity profile of concomitant paclitaxel and pazopanib were studied in a Phase I trial of 26 patients with metastatic solid tumors. Twelve patients had received prior taxane treatment. Dose titration was performed, achieving maximum levels of 800 mg daily pazopanib and 80 mg/m2 weekly paclitaxel. A 26% increase in mean plasma paclitaxel AUC (36% increase in peak concentration) was noted with the combination, including the highest dose of both drugs. As expected from previous studies, fatigue, nausea, and diarrhea were the most common AEs (most grade 1 or 2). Previously not seen in other studies, one gastrointestinal hemorrhage occurred in a patient with hepatocellular carcinoma, portal hypertension, and esophageal varices. Of 17 patients receiving the MTDs (800 mg, 80 mg/m2), five achieved a PR. The most common reason for dose reduction or interruption was hepatic enzyme elevation. With drug discontinuation and subsequent resumption at reduced doses, these changes were found to be reversible.52 Dose-dense paclitaxel with pazopanib has not yet been studied specifically in patients with EOC; however, these early Phase I results indicate the combination has tolerable side effects.
Another Phase I/II trial evaluated daily pazopanib at 800 mg with paclitaxel 175 mg/m2 and carboplatin AUC 5. Two of six patients experienced DLTs: one GIP in a patient with extensive small-bowel tumor involvement, and grade 3 abdominal cramping. Subsequent participants were treated with 400 mg pazopanib daily, and two of six had DLTs: one GIP and one grade 2 skin necrosis. A planned arm using pazopanib with standard paclitaxel and carboplatin with AUC 6 was not performed given these toxicities; the trial was subsequently discontinued.19 In a separate trial of pazopanib with carboplatin and paclitaxel in advanced solid tumors, an MTD was reached (pazopanib 200 mg daily, carboplatin AUC 5, and paclitaxel 175 mg/m2). Despite this lower MTD, 30% of patients experienced a treatment response.51 Further studies are needed to define the role of pazopanib in combination with cytotoxic agents to improve the side-effect profile while maximizing drug efficacy. However, pazopanib therapy in combination with carboplatin and paclitaxel may not be a feasible regimen.
Preliminary data suggest that daily pazopanib in combination with weekly oral topotecan is tolerable, though with an increase in topotecan exposure compared to monotherapy. DLTs included hand–foot syndrome, neutropenia, and fatigue. Expansion studies of these agents are ongoing.54 Early studies suggest pazopanib may also be used in combination with gemcitabine at monotherapy doses with a similar AE profile55; a randomized Phase II study of gemcitabine with or without pazopanib in women with platinum-resistant EOC is ongoing.56
Combinations of pazopanib with antiangiogenic agents are also being investigated. A Phase I study of pazopanib with bevacizumab in patients with advanced solid tumors was conducted. The reported MTD was 400 mg/day pazopanib with 7.5 mg/kg bevacizumab every 2 weeks. In this study, bevacizumab administration did not affect pazopanib pharmacokinetics.57 Other Phase I studies of pazopanib in combination with a variety of chemotherapeutic and biologic agents are ongoing (Table 3).
Phase II experience of pazopanib in epithelial ovarian cancer
A Phase II trial evaluated single-agent pazopanib in patients with recurrent EOC, FTC, or PPC. All patients had small-volume (≤4 cm on imaging) disease, had received no more than two previous chemotherapy regimens, and had had a complete cancer antigen (CA)-125 response to prior platinum-based therapy. Participants received 800 mg daily oral pazopanib until clinical or radiologic evidence of disease progression. The primary outcome was biochemical response rate (defined as ≥50% decrease in CA-125 from baseline level). Overall response rate, SD rate, and PFS were included as secondary outcomes. All participants had elevated baseline CA-125, while 47% had measurable disease at enrollment. Eleven of 36 patients (31%, 95% CI 16%–48%) had a CA-125 response to pazopanib. Twenty patients (56%) had SD, with a median duration of response of 80 days. Patients with measurable disease at baseline achieved a less robust response to treatment. Three of 17 patients experienced a PR by CA-125 criteria, but failed to reach Response Evaluation Criteria in Solid Tumors criteria for response.58,59 Interestingly, 86% of all study patients achieved serum levels greater than 34 μM/L compared with 100% of CA-125 responders.58 Given differences in metabolism across individuals, certain patients may require increased daily doses to achieve this serum concentration. Identifying these patients may improve overall response and outcomes with pazopanib. The most common grade 3 AEs were diarrhea (8%), ALT elevation (8%), fatigue (11%) and γ-glutamyl transpeptidase elevation (11%). Of the ten patients on antihypertensive regimens prior to study enrollment, four required an increase in their medications during the study period. Ten patients experienced AEs that resulted in drug discontinuation.58
A Phase II trial of pazopanib in patients with recurrent platinum-resistant EOC, FTC, or PPC evaluated clinical benefit rate (CBR; complete response + PR + SD). Median PFS was 1.83 months (95% CI 1.67–2) with a 40% CBR (ten of 25). The most frequent AEs were asthenia (56%), hypertension (36%), and diarrhea, nausea, and anorexia (20% each). Six patients required a dose reduction to 600 mg due to toxicities. The study was discontinued because the observed CBR did not meet the planned statistical hypothesis.60
PACOVAR (A Phase I/II Study of Pazopanib [GW786034] and Cyclophosphamide in Patients with Platinum-Resistant Recurrent, Pre-treated Ovarian Cancer) is currently enrolling patients to evaluate the activity and feasibility of pazopanib in combination with metronomic cyclophosphamide in patients with recurrent, platinum-resistant EOC (NCT01238770).65 Other trials are evaluating pazopanib in combination with paclitaxel, gemcitabine, or liposomal doxorubicin (NCT01600573,61 NCT01644825,62 NCT01610206,56 NCT0103565863). Another trial is evaluating mechanisms of pazopanib-induced hypertension, specifically the relationship between nitric oxide bioavailability and blood pressure (NCT01392352).64
Phase III experience of pazopanib in epithelial ovarian cancer
Given the promising Phase II results, maintenance pazopanib in women with advanced newly diagnosed EOC was evaluated. AGO-OVAR-16 was a double-blinded, multicenter Phase III study that randomized 940 women with advanced-stage EOC, FTC, or PPC to receive maintenance pazopanib versus placebo for 24 months. All patients had previously achieved a clinical complete response with first-line platinum-based therapy. Fifty-eight percent of patients had been previously debulked to no macroscopic residual disease. Median PFS was significantly longer in the pazopanib group (17.9 versus 12.3, HR 0.77, 95% CI 0.64–0.91, P=0.0021). The first OS interim analysis (20% of OS events) showed no difference between arms. Not unlike Phase I/II studies of pazopanib, hypertension was the most commonly reported AE, with grade 3 or 4 events occurring in 31% of the study group (compared to 6% in the control arm).17 Other toxicities included diarrhea (29% grade 2–4,12 8% grade 3/4), liver-related toxicities (grade 3/4, 9%) and neutropenia (grade 3/4, 10%).17 Dose reductions were required in 58% of patients randomized to pazopanib; the median daily dose for these patients was 607.4 mg. Mean time on the study drug was 8.9 months. Three fatal AEs were reported: two in the pazopanib group and one patient on placebo. Overall, 11% of patients were withdrawn from the study protocol: 14% and 8% for those receiving pazopanib and placebo, respectively.17 Given these data, maintenance pazopanib appears to extend the time to progression in patients with advanced disease and delays the need for second-line chemotherapy.
An AGO-OVAR-16 extension study evaluated pazopanib maintenance after first-line chemotherapy specifically in Asian women with EOC, FTC, or PPC. Three-quarters of Asian women required a dose reduction, compared to only 53% of their non-Asian counterparts.17 There was a higher incidence of both hypertension (76%) and neutropenia (64%) in women on pazopanib maintenance.66 A study of pazopanib versus sunitinib in patients with metastatic RCC demonstrated an increased incidence of hematologic toxicity, hypertension, hand–foot syndrome, proteinuria, and abnormal liver function tests increase in Asian women compared to their North American and European counterparts.67 The mechanisms behind these racial differences have not yet been determined.
Quality of life (QOL), treatment efficacy and health care-related toxicity were also evaluated in AGO-OVAR-16. Though a decrement in QOL was noted in patients randomized to pazopanib, progression of disease resulted in an even larger decrease in QOL. Quality-adjusted PFS supports the net value of pazopanib maintenance therapy in this population.68
Potential toxicities
Inhibition of angiogenesis can lead to a constellation of AEs, including hypertension, GIP, and vascular thrombi. Liver-related toxicities (9%–20%), diarrhea (4%–8%), and hypertension (1.4%–31%) are the most commonly reported Grade 3 or 4 AEs associated with pazopanib.17,69,70 A meta-analysis of pazopanib-related hypertension found that nearly 35% of patients develop blood pressure issues during the course of treatment. Most blood pressure elevations can be controlled with antihypertensive dosage adjustment or an additional agent.70 The mechanism of pazopanib-induced hypertension is still etiologically unclear, and a Phase II trial is currently recruiting patients to better elucidate this process (NCT01392352).64
Elevated liver enzymes are frequently reported in patients receiving pazopanib,17,50,58 though it appears that patients with baseline mild liver dysfunction (bilirubin >1.5–3× upper limit of normal (ULN), ALT > ULN) may be dosed at the typical 800 mg daily dose without significant alterations in hepatic enzyme profile. Those with moderate-to-severe dysfunction may require lower daily doses, with the possibility of subtherapeutic serum levels and lower likelihood of clinical drug activity.71 Other laboratory abnormalities reported with pazopanib use include hypothyroid, hypophosphatemia, and hyperglycemia.68
Dermatologic toxicities are increasingly reported with the use of TKIs. Hair hypopigmentation is the most common pazopanib-related cutaneous manifestation,58,69 although diffuse skin hypopigmentation has been described in patients treated with pazopanib.50,72 Cytopenias appear to be slightly increased in patients treated with pazopanib,17 and become more common when used in combination with chemotherapy.55,73
Though rare, reversible posterior leukoencephalopathy, a serious complication of poorly controlled hypertension, has been reported in patients treated with pazopanib. A loss of cerebral vascular autoregulation, reversible posterior leukoencephalopathy may present with headaches, seizures, or vision changes, and has characteristic magnetic resonance imaging findings.74,75 Though much less common, GIP is a serious complication of antiangiogenic therapy. The mechanism is unclear, though several hypotheses have been suggested including the presence of bowel micrometastases that undergo necrosis with treatment, leading to bowel-wall weakening.76,77 Definitive risk factors have not been elucidated, however, and patients with prior abdominal radiotherapy, significant tumor involvement of the bowel, and recent surgery may be predisposed to this potentially fatal complication.78 GIP with pazopanib appears to be less common than that of other anti-VEGF agents.79
These AEs are rarely clinically significant; pazopanib appears to have an overall acceptable tolerability. In studies of pazopanib in RCC, the most common AE requiring drug discontinuation was ALT increase (2%). Diarrhea, proteinuria, and aspartate transaminase increase were also reasons for discontinuation (all 1%).79 In a Phase II trial of pazopanib in EOC, 14 of 36 patients experienced AEs that led to drug discontinuation, the most common of which were aspartate transaminase and ALT increase (8% each).58
Patients must also be counseled on pazopanib–drug interactions. As a substrate of CYP 3A4,80 pazopanib metabolism may be altered by certain antifungals, antivirals, or macrolide antibiotics. Pazopanib may lower the serum levels of dexamethasone, certain antiepileptics, and rifampin.42 Increased hepatotoxicity has been reported in patients taking simvastatin and pazopanib.81 Grapefruit juice should also be avoided, as it can inhibit drug metabolism.42
Future directions
Phase II and III trials indicate that pazopanib may have a role in the treatment of select women with EOC. Questions remain regarding the duration of pazopanib therapy, optimal timing, the efficacy of combination therapy with cytotoxic agents and other biologics, use in women with bevacizumab-resistant disease, sequencing with other antiangiogenic agents, and the ideal patient population. Identification of biomarkers or nomograms to predict treatment outcomes will be necessary to rationally direct therapy to women most likely to benefit from therapy. Angiopoietin-1 and -2, stromal cell-derived factor, osteopontin, and IL-6 have all been reported to be either predictive of response to antiangiogenic therapies, prognostic for survival, or involved in ovarian carcinogenesis.82–84 Soluble VEGFR-2, baseline circulating tumor cells, IL-8, and may predict pazopanib efficacy and prove to be useful biomarkers to direct therapy.87,88
Specimens were obtained from women who participated in the AGO-OVAR-16 trial, and may identify predictive biomarkers to better target those who will achieve the most benefit. In addition, Kattan et al reported on an internally validated nomogram used to predict 12-month OS in patients with RCC treated with pazopanib. The clinical predictors used included neutrophil and platelet counts, lactate dehydrogenase, alkaline phosphatase, corrected calcium, albumin, hemoglobin, Eastern Cooperative Oncology Group performance status, months from diagnosis to treatment, number of metastatic sites, and presence of lung, liver, and bone metastases. Albumin, followed by corrected calcium and months from diagnosis to treatment, were the strongest predictors of survival.89 Future research is ongoing to develop a nomogram and identify biomarkers in women with EOC to direct pazopanib therapy.
Of paramount importance is patient preference as they weigh the risks and benefits of their available treatment options. As an oral agent requiring only once-daily dosing, pazopanib is a convenient option, yet cost and safety profile remain significant considerations. Future studies will hopefully include comparative and/or cost-effectiveness components in an effort to improve the care of patients with EOC.
Disclosure
The authors report no conflicts of interest in this work.
References
International Agency for Research on Cancer. Globocan 2012: Estimated cancer incidence, mortality and prevalence worldwide in 2012 – fact sheets by population. Available from: http://globocan.iarc.fr/Pages/fact_sheets_population.aspx. Accessed December 22, 2013. | |
Ozols RF, Bundy BN, Greer BE, et al. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2003;21(17):3194–3200. | |
du Bois A, Luck HJ, Meier W, et al. A randomized clinical trial of cisplatin/paclitaxel versus carboplatin/paclitaxel as first-line treatment of ovarian cancer. J Natl Cancer Inst. 2003;95(17):1320–1329. | |
Armstrong DK, Bundy B, Wenzel L, et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Eng J Med. 2006;354(1):34–43. | |
Katsumata N, Yasuda M, Isonishi S, et al. Long-term results of dose-dense paclitaxel and carboplatin versus conventional paclitaxel and carboplatin for treatment of advanced epithelial ovarian, fallopian tube, or primary peritoneal cancer (JGOG 3016): a randomised, controlled, open-label trial. Lancet Oncol. 2013;14(10):1020–1026. | |
Burger RA, Brady MF, Bookman MA, et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Eng J Med. 2011;365(26):2473–2483. | |
Perren TJ, Swart AM, Pfisterer J, et al. A phase 3 trial of bevacizumab in ovarian cancer. N Eng J Med. 2011;365(26):2484–2496. | |
Browder T, Butterfield CE, Kraling BM, et al. Antiangiogenic scheduling of chemotherapy improves efficacy against experimental drug-resistant cancer. Cancer Res. 2000;60(7):1878–1886. | |
Colleoni M, Rocca A, Sandri MT, et al. Low-dose oral methotrexate and cyclophosphamide in metastatic breast cancer: antitumor activity and correlation with vascular endothelial growth factor levels. Ann Oncol. 2002;13(1):73–80. | |
Linderholm BK, Lidbrink E, Tallroth E, et al. Angiogenic factors in relation to clinical effect in a phase II trial of weekly paclitaxel. Breast. 2013;22(6):1142–1147. | |
Fennelly D, Aghajanian C, Shapiro F, et al. Phase I and pharmacologic study of paclitaxel administered weekly in patients with relapsed ovarian cancer. J Clin Oncol. 1997;15(1):187–192. | |
Clinicaltrials.gov Available at: clinicaltrials.gov. Accessibility verified January 31, 2014 | |
FIGO Committee on Gynecologic Oncology: Current FIGO staging for cancer of the vagina, fallopian tube, ovary, and gestational trophoblastic neoplasia. Int J Gynaecol Obstet. 2009; 105(1):3–4 | |
National Comprehensive Cancer Network. Clinical practice guidelines in oncology: ovarian cancer including fallopian tube cancer and primary peritoneal cancer. Available from: http://www.nccn.org/professionals/physician_gls/pdf/ovarian.pdf. Accessed December 22, 2013. | |
Chan J, Brady M, Penson R, et al. Phase III trial of every-3-weeks paclitaxel vs. dose dense weekly paclitaxel with carboplatin +/− bevacizumab in epithelial ovarian, peritoneal, fallopian tube cancer: GOG 262. Oral presentation at: 18th European Society of Gynaecological Oncology International Meeting; October 19–22, 2013; Liverpool, UK. | |
Monk BJ, Poveda A, Vergote I, et al. A phase III, randomized, double-blind trial of weekly paclitaxel plus the angiopoietin 1 and 2 inhibitor, trebananib, or placebo in women with recurrent ovarian cancer: TRINOVA-1. Poster presented at: European Society for Medical Oncology Cancer Congress; September 27–October 1, 2013; Amsterdam, Netherlands. | |
duBois A, Floquet A, Kim J, et al. Randomized, double-blind, phase III trial of pazopanib versus placebo in women who have not progressed after first-line chemotherapy for advanced epithelial ovarian, fallopian tube, or primary peritoneal cancer (AEOC): results of an international Intergroup trial (AGO-OVAR16). Am Soc Clin Oncol. 2013; 31 Suppl:LBA5503. | |
Ledermann JA, Perren TJ, Raja FA, et al. Randomised double-blind phase III trial of cediranib (AZD 21717) in relapsed platinum sensitive ovarian cancer: results of the ICON6 trial. Poster presented at: European Society for Medical Oncology Cancer Congress; September 27–October 1, 2013; Amsterdam, Netherlands. | |
du Bois A, Vergote I, Wimberger P, et al. Open-label feasibility study of pazopanib, carboplatin, and paclitaxel in women with newly diagnosed, untreated, gynaecologic tumours: a phase I/II trial of the AGO study group. Br J Cancer. 2012;106(4):629–632. | |
Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis – correlation in invasive breast carcinoma. N Eng J Med. 1991;324(1):1–8. | |
Weidner N, Folkman J, Pozza F, et al. Tumor angiogenesis: a new significant and independent prognostic indicator in early-stage breast carcinoma. J Natl Cancer Inst. 1992;84(24):1875–1887. | |
Weidner N, Carroll PR, Flax J, Blumenfeld W, Folkman J. Tumor angiogenesis correlates with metastasis in invasive prostate carcinoma. Am J Pathol. 1993;143(2):401–409. | |
Zätterström UK, Brun E, Willén R, Kjellén E, Wennerberg J. Tumor angiogenesis and prognosis in squamous cell carcinoma of the head and neck. Head Neck. 1995;17(4):312–318. | |
de Jong JS, van Diest PJ, Baak JP. Hot spot microvessel density and the mitotic activity index are strong additional prognostic indicators in invasive breast cancer. Histopathology. 2000;36(4):306–312. | |
Hlatky L, Hahnfeldt P, Folkman J. Clinical application of antiangiogenic therapy: microvessel density, what it does and doesn’t tell us. J Natl Cancer Inst. 2002;94(12):883–893. | |
Rubatt JM, Darcy KM, Hutson A, et al. Independent prognostic relevance of microvessel density in advanced epithelial ovarian cancer and associations between CD31, CD105, p53 status, and angiogenic marker expression: a Gynecologic Oncology Group study. Gynecol Oncol. 2009;112(3):469–474. | |
Alvarez AA, Krigman HR, Whitaker RS, Dodge RK, Rodriguez GC. The prognostic significance of angiogenesis in epithelial ovarian carcinoma. Clin Cancer Res. 1999;5(3):587–591. | |
Ramakrishnan S, Subramanian IV, Yokoyama Y, Geller M. Angiogenesis in normal and neoplastic ovaries. Angiogenesis. 2005;8(2):169–182. | |
Burger RA. Overview of anti-angiogenic agents in development for ovarian cancer. Gynecol Oncol. 2011;121(1):230–238. | |
Nagy JA, Dvorak AM, Dvorak HF. VEGF-A and the induction of pathological angiogenesis. Annu Rev Pathol. 2007;2:251–275. | |
Gadducci A, Viacava P, Cosio S, et al. Vascular endothelial growth factor (VEGF) expression in primary tumors and peritoneal metastases from patients with advanced ovarian carcinoma. Anticancer Res. 2003;23(3C):3001–3008. | |
Zebrowski BK, Liu W, Ramirez K, Akagi Y, Mills GB, Ellis LM. Markedly elevated levels of vascular endothelial growth factor in malignant ascites. Ann Surg Oncol. 1999;6(4):373–378. | |
Hamberg P, Verweij J, Sleijfer S. (Pre-)clinical pharmacology and activity of pazopanib, a novel multikinase angiogenesis inhibitor. Oncologist. 2010;15(6):539–547. | |
Li L, Wang L, Zhang W, et al. Correlation of serum VEGF levels with clinical stage, therapy efficacy, tumor metastasis and patient survival in ovarian cancer. Anticancer Res. 2004;24(3b):1973–1979. | |
Wild R, Dings RP, Subramanian I, Ramakrishnan S. Carboplatin selectively induces the VEGF stress response in endothelial cells: potentiation of antitumor activity by combination treatment with antibody to VEGF. Int J Cancer. 2004;110(3):343–351. | |
Steele IA, Edmondson RJ, Bulmer JN, Bolger BS, Leung HY, Davies BR. Induction of FGF receptor 2-IIIb expression and response to its ligands in epithelial ovarian cancer. Oncogene. 2001;20(41):5878–5887. | |
Sallinen H, Heikura T, Laidinen S, et al. Preoperative angiopoietin-2 serum levels: a marker of malignant potential in ovarian neoplasms and poor prognosis in epithelial ovarian cancer. Int J Gynecol Cancer. 2010;20(9):1498–1505. | |
Henriksen R, Funa K, Wilander E, Bäckström T, Ridderheim M, Oberg K. Expression and prognostic significance of platelet-derived growth factor and its receptors in epithelial ovarian neoplasms. Cancer Res. 1993;53(19):4550–4554. | |
Tsutsumi S, Ishibashi K, Uchida N, et al. Phase II trial of chemotherapy plus bevacizumab as second-line therapy for patients with metastatic colorectal cancer that progressed on bevacizumab with chemotherapy: the Gunma Clinical Oncology Group (GCOG) trial 001 SILK study. Oncology. 2012;83(3):151–157. | |
Grothey A, Sugrue MM, Purdie DM, et al. Bevacizumab beyond first progression is associated with prolonged overall survival in metastatic colorectal cancer: results from a large observational cohort study (BRiTE). J Clin Oncol. 2008;26(33):5326–5334. | |
McCann GA, Smith B, Backes FJ, et al. Recurrent ovarian cancer: is there a role for re-treatment with bevacizumab after an initial complete response to a bevacizumab-containing regimen? Gynecol Oncol. 2012;127(2):362–366. | |
American Cancer Society. Pazopanib. Available at: http://www.cancer.org/treatment/treatmentsandsideeffects/guidetocancerdrugs/pazopanib. Accessed January 31, 2014. | |
Kumar R, Knick VB, Rudolph SK, et al. Pharmacokinetic-pharmacodynamic correlation from mouse to human with pazopanib, a multikinase angiogenesis inhibitor with potent antitumor and antiangiogenic activity. Mol Cancer Ther. 2007;6(7):2012–2021. | |
Bergers G, Song S, Meyer-Morse N, Bergsland E, Hanahan D. Benefits of targeting both pericytes and endothelial cells in the tumor vasculature with kinase inhibitors. J Clin Invest. 2003;111(9):1287–1295. | |
Merritt WM, Nick AM, Carroll AR, et al. Bridging the gap between cytotoxic and biologic therapy with metronomic topotecan and pazopanib in ovarian cancer. Mol Cancer Ther. 2010;9(4):985–995. | |
Podar K, Tonon G, Sattler M, et al. The small-molecule VEGF receptor inhibitor pazopanib (GW786034B) targets both tumor and endothelial cells in multiple myeloma. Proc Natl Acad Sci U S A. 2006;103(51):19478–19483. | |
Harris PA, Boloor A, Cheung M, et al. Discovery of 5-[[4-[(2,3-dimethyl-2H-indazol-6-yl)methylamino]-2-pyrimidinyl]amino]-2-methyl-benzenesulfonamide (pazopanib), a novel and potent vascular endothelial growth factor receptor inhibitor. J Med Chem. 2008;51(15):4632–4640. | |
US Food and Drug Administration. FDA approves new treatment for advanced form of kidney cancer [press release]. Silver Spring, MD: US FDA; 2009 [October 19]. Available from: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm187174.htm. Accessed December 22, 2013. | |
US Food and Drug Administration. FDA approves Votrient for advanced soft tissue sarcoma [press release]. Silver Spring, MD: US FDA; April 26, 2012. Available from: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm302065.htm. Accessed December 22, 2013. | |
Hurwitz HI, Dowlati A, Saini S, et al. Phase I trial of pazopanib in patients with advanced cancer. Clin Cancer Res. 2009;15(12):4220–4227. | |
Burris HA 3rd, Dowlati A, Moss RA, et al. Phase I study of pazopanib in combination with paclitaxel and carboplatin given every 21 days in patients with advanced solid tumors. Mol Cancer Ther. 2012;11(8):1820–1828. | |
Tan AR, Dowlati A, Jones SF, et al. Phase I study of pazopanib in combination with weekly paclitaxel in patients with advanced solid tumors. Oncologist. 2010;15(12):1253–1261. | |
de Jonge MJ, Hamberg P, Verweij J, et al. Phase I and pharmacokinetic study of pazopanib and lapatinib combination therapy in patients with advanced solid tumors. Invest New Drugs. 2013;31(3):751–759. | |
Kerklaan BM, Lolkema M, Devriese LA, et al. Phase I study of safety, tolerability, and pharmacokinetics of pazopanib in combination with oral topotecan in patients with advanced solid tumors. J Clin Oncol. 2013;31 Suppl:2536. | |
Plummer R, Madi A, Jeffels M, et al. A Phase I study of pazopanib in combination with gemcitabine in patients with advanced solid tumors. Cancer Chemother Pharmacol. 2013;71(1):93–101. | |
Duska, LR. A Randomized Study of Safety and Efficacy of Pazopanib and Gemcitabine in Persistent or Relapsed Ovarian Cancer. Available from: http://clinicaltrials.gov/show/NCT01610206. NLM identifier: NCT01610206. Accessed: January 31, 2014. | |
Negrier S, Imbs DC, Perol D, et al. Pazopanib (P) and bevacizumab (B) in patients with metastatic renal cell carcinoma (mRCC) or other advanced refractory tumors: Phase I combination study final analysis. J Clin Oncol. 2013;31 Suppl:4574. | |
Friedlander M, Hancock KC, Rischin D, et al. A Phase II, open-label study evaluating pazopanib in patients with recurrent ovarian cancer. Gynecol Oncol. 2010;119(1):32–37. | |
Response Evaluation Criteria in Solid Tumors. Available at: http://www.recist.com Accessibility verified: January 31, 2014. | |
Oaknin A, Gonzalez-Martin A, Garcia Y, et al. Phase II study of pazopanib in recurrent or persistent ovarian (EOC), peritoneal (PPC), or Fallopian tube cancer (FTC): a Spanish Ovarian Cancer Group (GEICO) study. J Clin Oncol. 2012;30 Suppl:5068. | |
JSehouli. Pazopanib and weekly topotecan in patients recurrent ovarian cancer (TOPAZ). Available from: http://clinicaltrials.gov/ct2/show/NCT01600573?term=NCT01600573&rank=1. NLM identifier: NCT01600573. Accessed January 31, 2014. | |
National Cancer Institute, Naples. Weekly paclitaxel with or without pazopanib in platinum resistant or refractory ovarian cancer (MITO-11). Available from: http://clinicaltrials.gov/ct2/show/NCT01644825?term=NCT01644825&rank=1. NLM identifier: NCT01644825. January 31, 2014. | |
SCRI Development Innovations, LLC. Study of pazopanib and doxil in patients with advanced relapsed platinum-sensitive or platinum-resistant ovarian, fallopian tube or primary peritoneal adenocarcinoma. Available from: http://clinicaltrials.gov/ct2/show/NCT01035658?term=NCT01035658&rank=1. NLM identifier: NCT01035658. Accessed January 31, 2014. | |
Cambridge University Hospitals NHS Foundation Trust. HYPAZ: Hypertension induced by pazopanib. Available from: http://clinicaltrials.gov/ct2/show/NCT01392352?term=NCT01392352&rank=1. NLM identifier: NCT01392352. Accessed January 31, 2014. | |
Eichbaum M, Mayer C, Eickhoff R, et al. The PACOVAR-trial: a phase I/II study of pazopanib (GW786034) and cyclophosphamide in patients with platinum-resistant recurrent, pre-treated ovarian cancer. BMC Cancer. 2011;11:453. | |
Zang R, Wu L, Zhu J, et al. Pazopanib (Paz) monotherapy in Asian women who have not progressed after first-line chemotherapy for advanced ovarian, fallopian tube, or primary peritoneal carcinoma. J Clin Oncol. 2013;31 Suppl:5512. | |
Guo J, Jin J, Huang Y, et al. Comparison of PFS and safety for Asian compared to North American and European populations in the phase III trial of pazopanib versus sunitinib in patients with treatment-naive RCC (COMPARZ). J Clin Oncol. 2013;31 Suppl 6:366. | |
Friedlander MK, Meier W, Lesoin A, et al. Quality of life in patients with advanced epithelial ovarian, fallopian tube, or primary peritoneal cancer (AEOC) receiving either pazopanib monotherapy or placebo after first-line chemotherapy: AGO-OVAR16 results. Poster presented at: European Society for Medical Oncology Cancer Congress; September 27–October 1, 2013; Amsterdam, the Netherlands. | |
Sternberg CN, Hawkins RE, Wagstaff J, et al. A randomised, double-blind phase III study of pazopanib in patients with advanced and/or metastatic renal cell carcinoma: final overall survival results and safety update. EurJ Cancer. 2013;49(6):1287–1296. | |
Qi WX, Lin F, Sun YJ, et al. Incidence and risk of hypertension with pazopanib in patients with cancer: a meta-analysis. Cancer Chemother Pharmacol. 2013;71(2):431–439. | |
Shibata SI, Chung V, Synold TW, et al. Phase I study of pazopanib in patients with advanced solid tumors and hepatic dysfunction: a National Cancer Institute Organ Dysfunction Working Group study. Clin Cancer Res. 2013;19(13):3631–3639. | |
Sideras K, Menefee ME, Burton JK, Erlichman C, Bible KC, Ivy SP. Profound hair and skin hypopigmentation in an African American woman treated with the multi-targeted tyrosine kinase inhibitor pazopanib. J Clin Oncol. 2010;28(19):e312–e313. | |
Semrad TJ, Eddings C, Dutia MP, Christensen S, Lara PN Jr. Phase I study of the combination of temsirolimus and pazopanib in advanced solid tumors. Anticancer Drugs. 2013;24(6):636–640. | |
Hinchey J, Chaves C, Appignani B, et al. A reversible posterior leukoencephalopathy syndrome. N Eng J Med. 1996;334(8):494–500. | |
Schwartz RB, Jones KM, Kalina P, et al. Hypertensive encephalopathy: findings on CT, MR imaging, and SPECT imaging in 14 cases. AJR Am J Roentgenol. 1992;159(2):379–383. | |
Stone RL, Sood AK, Coleman RL. Collateral damage: toxic effects of targeted antiangiogenic therapies in ovarian cancer. Lancet Oncol. 2010;11(5):465–475. | |
Randall LM, Monk BJ. Bevacizumab toxicities and their management in ovarian cancer. Gynecol Oncol. 2010;117(3):497–504. | |
Han ES, Monk BJ. What is the risk of bowel perforation associated with bevacizumab therapy in ovarian cancer? Gynecol Oncol. 2007;105(1):3–6. | |
GlaxoSmithKline. Investigator’s Brochure for Pazopanib (GW786034) for Oncology and Opthalmic Indications. 10th ed. Brentford, UK: GlaxoSmithKline; 2013. | |
Goh BC, Reddy NJ, Dandamudi UB, et al. An evaluation of the drug interaction potential of pazopanib, an oral vascular endothelial growth factor receptor tyrosine kinase inhibitor, using a modified Cooperstown 5+1 cocktail in patients with advanced solid tumors. Clin Pharm Ther. 2010;88(5):652–659. | |
Xu CF, Xue Z, Bing N, et al. Concomitant use of pazopanib and simvastatin increases the risk of transaminase elevations in patients with cancer. Ann Oncol. 2012;23(9):2470–2471. | |
Tran HT, Liu Y, Zurita AJ, et al. Prognostic or predictive plasma cytokines and angiogenic factors for patients treated with pazopanib for metastatic renal-cell cancer: a retrospective analysis of phase 2 and phase 3 trials. Lancet Oncol. 2012;13(8):827–837. | |
Goede V, Coutelle O, Neuneier J, et al. Identification of serum angiopoietin-2 as a biomarker for clinical outcome of colorectal cancer patients treated with bevacizumab-containing therapy. Br J Cancer. 2010;103(9):1407–1414. | |
Nixon AB, Pang H, Starr MD, et al. Prognostic and predictive blood-based biomarkers in patients with advanced pancreatic cancer: results from CALGB 80303 (Alliance). Clin Cancer Res. 2013;19(24):6957–6966. | |
Imperial College London. [18F]Fluciclatide-PET, pazopanib and paclitaxel in ovarian cancer (PAZPET-1). Available from: http://clinicaltrials.gov/ct2/show/NCT01608009?term=NCT01608009&rank=1. NLM identifier: NCT01608009. Accessed January 31, 2014. | |
European Organisation for Research and Treatment of Cancer - EORTC. Pazopanib hydrochloride, paclitaxel, and carboplatin in treating patients with refractory or resistant ovarian epithelial cancer, fallopian tube cancer, or peritoneal cancer. Available from: http://clinicaltrials.gov/ct2/show/NCT01402271?term=NCT01402271&rank=1. NLM identifier: NCT01402271. Accessed January 31, 2014. | |
Grande E, Casanovas O, Earl J, et al. sVEGFR2 and circulating tumor cells to predict for the efficacy of pazopanib in neuroendocrine tumors (NETs): PAZONET subgroup analysis. J Clin Oncol. 2013; 31 Suppl:4140. | |
Necchi A, Mariani L, Zaffaroni N et al. Pazopanib in advanced and platinum resistant urothelial cancer: an open-label, single group phase 2 trial. Lancet Oncol. 2012; 13(8):810–816. | |
Kattan MW, Chakrabarti D, Bhatt K, Mehmud F, Sternberg CN, Motzer RJ. Development and internal validation of a prognostic nomogram for overall survival in patients with advanced renal cell carcinoma (aRCC) treated with pazopanib (PAZ). J Clin Oncol. 2013;31(6):380. | |
Dy GK, Infante JR, Eckhardt SG et al. Phase Ib trial of the oral angiogenesis inhibitor pazopanib administered concurrently with erlotinib. Invest New Drugs. 2013; 31(4):891–899. | |
Fasolo A, Sessa C, Bauer JA et al. Phase Ib clinical and pharmacological study of multiple schedules of pazopanib and epirubicin in patients with advanced solid tumors. Presented at: 2010 American Society of Clinical Oncology Annual Meeting. 4–8 June; Chicago, IL. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.