Back to Journals » Neuropsychiatric Disease and Treatment » Volume 15
Profile Of Patients With Advanced Parkinson’s disease Suitable For Device-Aided Therapies: Restrospective Data Of A Large Cohort Of Romanian Patients
Authors Szász JA , Constantin VA , Orbán-Kis K, Rácz A, Bancu LA, Georgescu D, Szederjesi J, Mihály I, Fárr AM, Kelemen K , Vajda T, Szatmári S
Received 6 September 2019
Accepted for publication 29 October 2019
Published 13 November 2019 Volume 2019:15 Pages 3187—3195
DOI https://doi.org/10.2147/NDT.S230052
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Roger Pinder
József Attila Szász,1,2,* Viorelia Adelina Constantin,2,3,* Károly Orbán-Kis,1,2 Attila Rácz,4 Ligia Ariana Bancu,1,5 Dan Georgescu,1,6 János Szederjesi,1,7 István Mihály,1,2 Ana-Mária Fárr,1 Krisztina Kelemen,1,2 Tamás Vajda,8 Szabolcs Szatmári1,2
1University of Medicine and Pharmacy of Târgu Mures, Târgu Mureş, Romania; 22nd Clinic of Neurology, Târgu Mures County Emergency Clinical Hospital, Târgu Mures, Romania; 3Doctoral School, Victor Babes University of Medicine and Pharmacy, Timisoara, Romania; 42nd Clinic of Psychiatry, Târgu Mures County Emergency Clinical Hospital, Târgu Mures, Romania; 51st Clinic of Internal Medicine, Târgu Mures County Emergency Clinical Hospital, Târgu Mures, Romania; 6Department of Gastroenterology, Târgu Mures County Emergency Clinical Hospital, Târgu Mures, Romania; 7Department of Anesthesiology and Intensive Care, Târgu Mures County Emergency Clinical Hospital, Târgu Mures, Romania; 8Department of Computer Science, Faculty of Technical and Human Sciences, Sapientia Hungarian University of Transylvania, Târgu Mureș, Romania
*These authors contributed equally to this work
Correspondence: Károly Orbán-Kis Gh. Marinescu Street No 38, Targu Mures 540142, Romania
Tel +40743754525
Email [email protected]
Background: There is insufficient data in the literature regarding the real-life, daily clinical practice evaluation of patients with advanced Parkinson’s disease (APD). We are not sure what is the upper limit of dopaminergic medication, especially the levodopa (LD) dosage, and how it is influenced by access and suitability to the various add-on and device-aided therapies (DAT).
Objective: This retrospective study explored the profile of APD patients that were considered and systematically evaluated regarding the suitability for DAT.
Methods: We analyzed the data from 311 consecutive patients with APD hospitalized between 2011 and 2017 that 1) described at least 2 hrs/day off periods divided into at least two instances/day (except early morning akinesia), 2) were in stage 3 or above on the Hoehn and Yahr scale, 3) were with or without dyskinesia, and 4) received at least four levodopa doses/day combined with adjuvant therapy.
Results: Of the 311 patients enrolled initially, 286 patients showed up for the second visit, of which in 125 cases we assessed that DAT would be necessary. Finally, 107 patients were tested in our clinic to confirm the efficacy of LCIG. Patients selected for DAT had significantly longer off periods, more frequent dyskinesia, early morning akinesia, and freezing despite having significantly higher LD doses than those with an improved conservative therapy.
Conclusion: Patients with APD can have a variety of symptoms, and because symptoms and therapeutical efficacy can be manifested in many different combinations, it is not possible to decide using a single, rigid set of criteria which APD patient is eligible for DAT. Nevertheless, treating physicians should refer APD patients to a specialized movement disorder center when patients with an average daily dose of LD of at least 750–1000 mg and maximal complementary therapies present daily motor complications that significantly reduce the quality of life.
Keywords: advanced Parkinson’s disease, motor complications, levodopa doses, levodopa-carbidopa intestinal gel
Introduction
Parkinson’s disease (PD) is a neurodegenerative, progressively worsening disorder. In the advanced stages of the disease (advanced Parkinson’s disease, APD), besides the inevitable motor complications, a number of nonmotor symptoms reduce the quality of life and limit the therapeutic possibilities. As the disease progresses, the efficacy of traditional per oral as well as transdermal medication is gradually decreased and becomes unpredictable. Motor complications resulting from uneven drug absorption, narrowed therapeutic window, and drug level fluctuations severely impair the lives of patients, ultimately leading to a drastic deterioration in the quality of life, ability to work, and self-reliance.1 Traditionally, the staging of the disease has mainly followed motor symptoms (topography and severity of motor signs). Similarly, the efficacy and inefficiency of the different therapeutic options (including device aided) were evaluated and interpreted based on motor symptoms. This relatively “simplistic” approach fails to describe the multidimensional, complex, and constantly changing spectrum of PD symptoms (both motor and nonmotor). The lack of proper definitions and criteria delays applying therapeutic strategies and/or device-aided therapies (DAT) for eligible APD patients.2
In the later years, there have been a large number of articles/expert recommendations trying to offer practical ways for PD staging.1–5 The biggest challenge seems to be the definition of the optimal moment in the evolution of APD, when initiating advanced phase therapies – device-related treatment strategies using pump-based continuous drug delivery (CDD) aiming continuous dopaminergic stimulation (CDS) or deep brain stimulation (DBS) – that may provide maximum benefits by continuous compensation for the disease-related striatal dopaminergic denervation.6
There is insufficient data in the literature regarding the real-life, daily clinical practice evaluation of APD patients. We do not know for sure the upper limits of dopaminergic medication, especially levodopa (LD) dosage, and how these are influenced by the various adjuvant (add-on) therapies available. We also have little data on the spectrum of different motor and nonmotor complications and of course the proportions of patients considered being eligible for DAT in tertiary centers with a high patient turnover. Partial data from the experience of 6 years of our center, regarding the limits of treatment with levodopa (LD) respectively the last dopaminergic medication before establishing the indication for DAT have been presented previously.7–9
Objectives And Method
The aim of this work is to analyze the clinical features of the patients treated with APD as well as the applied treatment strategies in our clinic during a 6-year period, with particular emphasis regarding the data of patients recommended for DAT. In our retrospective study, we analyzed the data of 311 patients with advanced Parkinson’s disease, diagnosed according to the UK Brain Bank Criteria, who were examined and followed-up at our clinic between 1 June 2011 and 31 May 2017. The levodopa-carbidopa intestinal gel (LCIG) therapy became available in Romania starting from 2009; the apomorphine pump infusion is not available and DBS has been performed only in a handful of cases due to national financial limitations. Our Neurology Clinic in Târgu Mureș organized in May 2011 an interdisciplinary working group (neurologist, psychiatrist, gastroenterologist, anesthesiologist, nurse with special expertise in PD device-aided therapies) in order to evaluate the suitability for DAT and successfully introduce LCIG therapy. During the 6-year period covered by this analysis, we followed all APD patients still responding to LD who reported at least 2 hrs/day off periods (with ≥2 off episodes/day, except early morning akinesia), with or without dyskinesias, who were on ≥3 stage on Hoehn and Yahr scale during on periods and received LD at least four times daily, in some combination with dopamine agonist (DA), monoamine oxidase B inhibitor (MAO-Bi), catechol-O-methyl transferase inhibitor (COMTi), and/or amantadine. The candidate patients were referred by the neurologists who have been following the case, then our multidisciplinary expert team selected the APD patients who meet the criteria mentioned above. We analyzed the profile of motor complications, the specifics, and limitations of dopaminergic therapy, and, of course, the patients’ suitability for DAT. Suitable patients considered for DAT were presented with data regarding the availability of different DATs in Romania.
In our clinic, APD patients underwent a two-step evaluation in order to assess the suitability for device-aided therapy. During the first examination (screening visit), after a detailed medical and clinical examination, review of the final reports and medical letters, and after proper preparation (if necessary/often with relatives, demonstration videos, etc), the patient completes a diary (every 30 mins) based on which at least one transition from on to off state (or vice versa) can be confirmed. Both the patient and the caregiver were informed in detail on further therapeutic options and the available DAT alternatives. Patients then received three 24 hr logs that had to be completed 3 days prior to the next examination.
Statistical analysis was conducted between patient groups using either one-way ANOVA, t-test or Fisher’s exact test. Values are presented as mean±SD, unless otherwise specified.
Results
Of the 311 patients enrolled initially, only 286 patients showed up for the second (baseline) visit. Only the data of these patients are presented in Table 1. Of these, we assessed that in 125 cases DAT alternatives would be necessary (motor fluctuations and/or dyskinesias with a major impact on the quality of life, severe side effects during earlier attempts to increase dopaminergic medication, important burden of caregiver). In the end, 107 patients were tested in our clinic to confirm the efficacy of LCIG. Nevertheless, the data of the 161 patients deemed not to have reached the limits of conservative therapy are also presented in the table. The flowchart of clinical decision-making is shown in Figure 1.
 |
Table 1 Characterization Of Patients With APD |
Of the 107 patients that were initially tested eventually, 89 underwent PEG-J. Patients that did not receive PEG-J included 7 cases with lack of efficacy or minor improvement during titration period, 5 cases with psychotic episodes, 1 patient with a gastric tumor discovered during gastroscopy and 1 patient with severe cardiac arrhythmia during gastroscopy. In the remaining 4 cases, patients opted for temporization of LCIG treatment initiation despite a clearly improved clinical status during testing.
We did not find a significant difference in the Mini-Mental State Examination (MMSE) score between patients found suitable for DAT therapy and those who were treated conservatively.
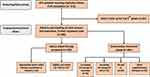 |
Figure 1 Flowchart of clinical decision-making. |
Discussion
In APD, a series of motor and nonmotor complications reduce the quality of life and limit the therapeutic possibilities. Traditionally, the staging of the disease has mainly followed motor symptoms (topography and severity of motor signs). Similarly, the efficacy as well as the inefficiency of the different therapeutic options (including device-aided) were evaluated and interpreted based on motor symptoms. This relatively “simplistic” approach failed to comprehensively describe the multidimensional, complex and constantly changing spectrum of PD symptomatology (both motor and nonmotor). Furthermore, everyday clinical practice makes it clear that the disease has many manifestations/features that are difficult to evaluate accurately, to assess and incorporate into recommendations/clinical guidelines. The different forms/subtypes of PD have different profiles, evolve and have different prognoses (different evolution curves), with the possibility of a rapidly aggravating clinical picture. Ideally, the severity of the disease should be evaluated based on the subtypes of PD, as suggested by the literature.10 However, the more detailed discussion of this topic is beyond the scope of this article.
In principle, we consider a patient to have APD when the motor performance fluctuates depending on the rate of administration of the dopaminergic medication or even independently of it. Thus, the main stages of APD are: the patient already has postural reflex disorders (Hoehn and Yahr stage 3), the patient needs assistance for most activities (Hoehn and Yahr stage 4), and the patient is immobilized in bed (Hoehn and Yahr stage 5). According to many authors, APD starts from the onset of motor complications.6 Nevertheless, during the further progression of the disease, motor complications’ importance may fade, especially with the onset of nonmotor symptoms (dementia, psychosis, falls, etc), which significantly impair the patient’s and caregiver’s quality of life. This is the so-called late-stage Parkinson’s disease (LSPD), which is usually reached by patients after an average disease evolution of 14 years.6,11 During LSPD, patients are often in need of constant care (at home or, where available, within an institutional setting).
The severity of motor complications is often assessed quite differently by the patient, family members, and, of course, by the attending physician. In general, the degree/severity of disability produced by PD should be considered, especially in the advanced phases. Disability refers to the limitation of the performance in carrying out activities reported to normal for the respective person, taking into account age, education, environment, and so on. From a practical point of view, however, we must accept that disability remains an “umbrella” concept, which can be very different from one patient to another. Even if the therapeutic strategy is continuously adapted to the patient’s profile and considering that the limits of the classical therapies have been reached, it is difficult to specify when DAT can be initiated to achieve maximum benefits, since there is no unanimously accepted definition of APD.
There is a very limited amount of data regarding the characterization of APD patients with or without DAT recommendations.
Only 44% (125/286) of our patients with APD were deemed suitable for DAT, which is significantly lower than the ratio reported in the recently published OBSERVE PD observational study (66%).12 It must be noted, however, that although we assessed APD patients for all DATs, realistically only LCIG is available in Romania: apomorphine pump infusion is not available and DBS has very limited availability (in a single center and with an average of 5–6 cases/year due to nationwide financial limitations). Of the 125 patients considered for DAT, actually only 107 were offered LCIG the rest needed unavailable DATs (or refused DAT). As the physicians assessing patients knew beforehand about this limitation, their evaluation of APD patients might be skewed, which can be a factor that explains the lower percentage of patients considered suitable for DAT.
The gender ratio was quite balanced in our study, when compared to OBSERVE PD where 61% of patients were male. The average age in our study group was higher with 1.5 years when compared to OBSERVE PD; the DAT group was significantly younger than the conservatively treated group.
The duration since APD diagnosis was 2 years shorter in our group compared to OBSERVE PD, and there is a significant difference between the DAT and CONS groups.
The modified Hoehn and Yahr score measured during the on phase was higher in our APD patients than in the OBSERVE PD; this difference is present also separately in the DAT and CONS groups.
The duration of motor fluctuations in our APD patients, even in those considered suitable for DAT, was shorter than in patients from the OBSERVE PD study.
Despite many proven symptomatic therapies for PD in the recent decades, substitution therapy with LD formulations (the gold standard of therapy) is key for the best clinical improvement at all stages of PD. Despite decades of clinical experience, there is a lack of clear recommendations, both in early and late stages of the disease (dosage should be strictly individualized). A major disadvantage of long-term LD treatment is the appearance of motor fluctuations and dyskinesias that significantly impair the quality of life; the rate of complications exceeds 90% after a 10-year disease history.13
The situation is further complicated by the fact that as the disease progresses, the therapeutic efficacy of levodopa is gradually narrowed and the side effects are more frequent. These disadvantages should be mitigated by drugs that prolong dopamine availability such as of third-generation monoamine oxidase type B inhibitors (MAO-Bi) and catechol-O-methyltransferase inhibitors (COMTi).14–16 Clinical studies evaluating the efficacy of inhaled LD may bring new data into this context.17 It cannot be confirmed at this time whether the magnitude of the clinical benefit of these preparations (used mainly in patients with moderate PD severity) is fully reproduced in APD. However, their value may be increasingly appreciated by the patients who will not accept DAT. Unfortunately, safinamide and opicapone are not available in Romania, nor is tolcapone, extended-release amantadine, or subcutaneous apomorphine injection for rapid relief of off episodes.18 Under these circumstances, as a logical consequence of the significantly limited therapeutical options available for clinicians to treat APD, the selected patients may start available device-aided therapies in an earlier disease stage than suggested by worldwide current clinical practice.
The exact delineation of APD and LSPD as well as the use of optimal therapy is often challenging even for movement disorder specialists. In many cases, we can only rely on expert opinions; nevertheless, changes to the treatment recommendations may be expected in the near future.2–5,12 At this stage, due to frequent side effects, the attending physician may be forced to reduce the dosage of dopaminergic agents. In these cases, the dilemma is whether the impairment of motor performance can be attributable to disease progression (and the consequent decline of levodopa responsiveness) or dose reduction.2 Therefore, we believe that it is practical to know the exact limits of LD therapy. Experience has shown that publications based on the market share of individual antiparkinsonian drugs and various budgetary parameters (which are often difficult to interpret for clinicians anyway) are in best case indicative in nature.19–21
There is probably a permanent dilemma between the natural tendency even retention (both from the patient’s and of the attending physician’s perspective) to delay the moment of the initiation of an invasive therapy (DAT) and the observation which is more and more outlined in the literature in the last years that the clinical benefits obtained with DAT are superior in patients with shorter disease duration.22 In other words, there is the possibility that in the case of “delayed” initiation, some of the benefits of an “on time” initiation may be lost. In this context, the characterization of the limits of conservative treatment strategies is of uppermost importance.
The body of scientific evidence regarding the daily/real-life clinical practice upper limits of LD dosage (full daily dose or dosing frequency) in APD is scant; furthermore, the influence on the dosage of LD brought on by the availability of DAT is not described properly. Even in reviews, there is no clear reference to the LD doses used; only the duration of treatment and the characteristics of each drug combination are presented.23 A further difficulty is that in many publications there are dose adjustments (levodopa equivalent dose – LED and levodopa equivalent daily dose – LEDD), from which it is sometimes difficult to infer LD doses.24,25
In previous publications, prior to the introduction of various DAT, mean LD doses in APD ranged from 884 to 1077 mg26–32 and 1152 and 1485 mg (LEDD), respectively.33,34 The LD doses that we report in this study are at the lower end of the range, but there are several explanations for this. First, we must emphasize that the LD/benserazide (200 mg/50 mg) combination disappeared from the Romanian drug market in the second half of the analyzed period, and the forced conversion to the remaining LD/carbidopa (250 mg/25 mg) preparations was in many cases difficult or impossible. Second, analysis of concomitant combination therapy reveals that in the whole study group but more evident in the patients deemed suitable for DAT the add-on therapy’ prevalence was the highest compared to literature (DA 79%, MAO-Bi 68% and COMTi 70%).
In a previous publication,8 we analyzed the average doses of LD used in different stages of intermediate/advanced PD (different selection parameters: duration of disease, presence of motor and non-motor fluctuations) described in the literature and found that doses varied between 511.4±200.1 mg/day and 910±384 mg/day (the latter considered as LEDD).35–38
In our study, the average LD doses of APD patients recommended for conservative therapy can be considered low. Nevertheless, in 42 of the 161 patients, an increase in the dose of LD was considered. This means that in about three-quarters of these patients, we assessed that a further increase is not possible due to various reasons: previous failure of LD dosage increase, side effects, possibility/risk of worsening dyskinesia, intolerance and/or depletion of amantadine efficiency, and so on.
Although the proportion of add-on therapies, especially DA usage, was higher than what was already reported in literature, further combination therapy was used to obtain improvement in conservatively treated patients (in 29 cases DA introduction, in 41 cases increase od DA dosage); in a further 49 cases, the treatment was completed with amantadine, rasagiline, and/or entacapone.9
Expert recommendations suggest that if the patient presents at least 2 hrs off periods and/or 1 hr of severe/troublesome dyskinesia, despite the administration of at least 5 doses of LD and with optimized oral/transdermal therapy, DAT should be taken into consideration.1,4,5 These characteristics were present in patients with APD (and potentially eligible for DAT) in the OBSERVE-PD study.12 However, in clinical practice, the duration and severity of the motor complications at the time of the decision for DAT may be even worse. In our study, the patients that we considered not to have exhausted the limits of conservative treatment had on average 2.8±0.8 hrs off periods, whereas those considered eligible for DAT had 4.7±1.1 hrs off (with an average of 3.62±1.3 hrs for the whole group of completely evaluated patients). Data published earlier showed even higher values (GLORIA register 6 hrs, Băjenaru et al 7.5 hrs).27,29 A similar trend is observed in the case of dyskinesias (see Table 1).
Regarding LD dosage, data from our previously published study suggest that approximately one-third of the patients considered for DAT had 4 doses/day, about one-third of them 5 doses/day, and the last 1/3 of patients had 6 or more doses/day.8 We wish to highlight that despite the experts’ recommendations, the daily practice has shown that an increase of dosage to more than 4/day can lead in many patients to a decrease in compliance (a phenomenon accentuated by the lack of a maximally involved caregiver or in case of institutionalization). On the other hand, there are cases where the increase of the dosage frequency does not significantly improve the symptoms and implicitly the quality of life of the patients or the increase of the doses is limited (it can be done only with the price of important side effects). We would like to emphasize that the majority of patients considered suitable for DAT presented early morning akinesia (118 out of 125) compared to “only” 70 of the 161 who continued with conservative treatment. Also, the incidence of the freezing phenomenon was significantly higher in the group considered suitable for DAT compared to the patients in whom we considered that the symptoms can be improved with oral/transdermal treatment.
In many cases, the management of PD can be done only through a multidisciplinary approach. This, however, is compulsory in APD or LSPD (a complex, multiphase, protracted process that is a constant challenge for physicians).3,39,40 Reduction in therapeutic adherence is a common observation at this stage. Insufficient cooperation leads to a further deterioration of the clinical condition of patients and consequently to the quality of life of both patients and their relatives.41–43 The fact that a significant proportion of patients are in need of institutional care further increases health-care costs.2 Timely device-aided therapeutical approaches can be a major breakthrough in the quality of life of many APD patients and may prolong the period during which the patient can be cared for within the confines of a family home. An indispensable element of this “situational recognition” is the assessment of the upper limits of the LD doses (still the basis for the medication of PD at all times).
We consider our study sample representative. We want to emphasize that in Romania the suitability of DAT for APD can only be assessed in a university teaching hospital setting. From our previous publications, it appears that the therapeutic strategies that we use are similar to those in the literature.44,45 Therefore, we believe that our analysis, based on the well-documented large number of patients from our clinic, over 6 years, faithfully reflects not only the Romanian, but maybe the Central-Eastern European therapeutic trends regarding APD care (LD therapeutic doses, limitations, opportunities, etc) and highlights the need for proper and timely assessment of DAT suitability.
Among the strengths of our study, we mention the accurate documentation, complex assessment, and analysis of motor complications. However, the retrospective method itself has its limitations. Also, this study only includes patients that were referred to the clinic by physicians. Furthermore, the fact that in Romania a number of well-established LD preparations and adjunctive therapies that are well suited to the treatment of APD are not available as well as the fact that in our region the only available DAT is LCIG can significantly influence the clinician’s decision-making.
Conclusion
Patients with APD can have a variety of symptoms, and because symptoms and therapeutical efficacy can be manifested in many different combinations, it is not possible to decide using a single, rigid set of criteria which APD patient is eligible for DAT. According to our data, patients with APD received lower and fewer LD doses. Nevertheless, the use of additional therapies was more prevalent when compared to literature. The authors consider that referral of APD patients by the treating physicians (neurologists, general practitioners) to a specialized movement disorder center with a multidisciplinary approach should be considered when the average daily dose of LD is at least 750–1000 mg, administered at least 5 times daily or, in justified cases even 4 times/day (poor compliance, severe burden of caregiver, limited availability of add-on therapeutical options, severe forms with rapid clinical progression), if motor complications (daily minimum 2 hrs off periods and/or more than 1 hr troublesome dyskinesia), that significantly reduce the quality of life despite maximal complementary therapies, persist.
Ethics Statement
This study enrolled patients admitted to the Neurological Clinics in Târgu Mureş. According to national legislation, all patients had to sign a written consent form of the teaching hospital. Furthermore, the study was approved by the Ethics Committee of the University of Medicine and Pharmacy from Târgu Mureș, approval no. 94/19.05.2017 (https://www.umfst.ro/universitate/comisii-de-etica/comisia-de-etica-a-cercetarii-stiintifice/avize/2017.html), which requires all human studies to be conducted entirely in accordance with the Declaration of Helsinki.
Acknowledgements
The authors are thankful for the help offered by Almási Emőke, Szatmári Szabolcs jr., Rad Paul, Ciorba Marius. This work has not been published elsewhere.
Author Contributions
B.L.A., G.D., S.J., R.A., M.I., F.AM., K.K: data gathering and analysis. S.J.A., C.V.A, K.K.O., S.S: final data analysis. All authors contributed to drafting or revising the article, gave final approval of the version to be published, and agreed to be accountable for all aspects of the work.
Funding
This work was supported by the joint project of Studium-Prospero and the Hungarian Academy of Sciences, project no. 138/2017.01.26.
Disclosure
Dr József Attila Szász reports personal fees from Abbvie, personal fees from Boehringer Ingelheim, personal fees from GSK, personal fees from Lundbeck, personal fees from Novartis, personal fees from Pfizer, personal fees from Teva, personal fees from UCB, grants from Studium-Prospero and the Hungarian Academy of Sciences, project no. 138/2017.01.26, during the conduct of the study. Dr Viorelia Adelina Constantin reports personal fees from Abbvie, personal fees from UCB, personal fees from WÖRWAG PHARMA, grants from Studium-Prospero and the Hungarian Academy of Sciences, project no. 138/2017.01.26, during the conduct of the study. Dr Tamás Vajda reports grants from Studium-Prospero and the Hungarian Academy of Sciences, project no. 138/2017.01.26, during the conduct of the study. Dr Szabolcs Szatmári reports grants from Studium-Prospero and the Hungarian Academy of Sciences, project no. 138/2017.01.26, during the conduct of the study. The authors report on other conflicts of interest in this work.
References
1. Odin P, Ray Chaudhuri K, Slevin JT, et al. Collective physician perspectives on non-oral medication approaches for the management of clinically relevant unresolved issues in Parkinson’s disease: consensus from an international survey and discussion program. Parkinsonism Relat Disord. 2015;21(10):1133–1144. doi:10.1016/j.parkreldis.2015.07.020
2. Titova N, Martinez-Martin P, Katunina E, Chaudhuri KR. Advanced Parkinson’s or “complex phase” Parkinson’s disease? Re-evaluation is needed. J Neural Transm. 2017;124(12):1529–1537. doi:10.1007/s00702-017-1799-3
3. Krüger R, Hilker R, Winkler C, et al. Advanced stages of PD: interventional therapies and related patient-centered care. J Neural Transm. 2016;123(1):31–43. doi:10.1007/s00702-015-1418-0
4. Luquin MR, Kulisevsky J, Martinez-Martin P, Mir P, Tolosa ES. Consensus on the definition of advanced Parkinson’s disease: a neurologists-based delphi study (CEPA Study). Parkinsons Dis. 2017;2017. doi:10.1155/2017/4047392
5. Antonini A, Stoessl AJ, Kleinman LS, et al. Developing consensus among movement disorder specialists on clinical indicators for identification and management of advanced Parkinson’s disease: a multi-country delphi-panel approach. Curr Med Res Opin. 2018;34(12):2063–2073. doi:10.1080/03007995.2018.1502165
6. Fabbri M, Coelho M, Abreu D, et al. Do patients with late-stage Parkinson’s disease still respond to levodopa? Parkinsonism Relat Disord. 2016;26:10–16. doi:10.1016/j.parkreldis.2016.02.021
7. Szász JA, Constantin VA, Orbán-Kis K, et al. Characteristics of dopaminergic treatments in advanced Parkinson’s before levodopa-carbidopa intestinal gel infusion: data from 107 tested patients. Mov Disord. 2018;33:171.
8. Szász JA, Szatmári S, Constantin V, et al. Characteristics of levodopa treatment in advanced Parkinson’s disease in the experiences of the neurology clinics of Târgu Mures, Romania. Orv Hetil. 2019;160(17):662–669. doi:10.1556/650.2019.31354
9. Szász JA, Constantin V, Orbán-Kis K, et al. Dopamine agonists in advanced Parkinson’s disease: data from a large cohort of Romanian patients. J Parkinsons Dis. 2019;9(1):128. doi:10.3233/JPD-199900
10. Van Rooden SM, Colas F, Martínez-Martín P, et al. Clinical subtypes of Parkinson’s disease. Mov Disord. 2011;26(1):51–58. doi:10.1002/mds.23346
11. Rosqvist K, Horne M, Hagell P, Iwarsson S, Nilsson MH, Odin P. Levodopa effect and motor function in late stage Parkinson’s disease. J Parkinsons Dis. 2018;8(1):59–70. doi:10.3233/JPD-171181
12. Fasano A, Fung VSC, Lopiano L, et al. Characterizing advanced Parkinson’s disease: OBSERVE-PD observational study results of 2615 patients. BMC Neurol. 2019;19(1):1–11. doi:10.1186/s12883-019-1276-8
13. Ahlskog JE, Muenter MD. Frequency of levodopa-related dyskinesias and motor fluctuations as estimated from the cumulative literature. Mov Disord. 2001;16:448–458. doi:10.1002/mds.1090
14. Borgohain R, Szasz J, Stanzione P, et al. Randomized trial of safinamide add-on to levodopa in Parkinson’s disease with motor fluctuations. Mov Disord. 2014;29(2):229–237. doi:10.1002/mds.25751
15. Borgohain R, Szasz J, Stanzione P, et al. Two-year, randomized, controlled study of safinamide as add-on to levodopa in mid to late Parkinson’s disease. Mov Disord. 2014;29(10):1273–1280. doi:10.1002/mds.25961
16. Scott LJ. Opicapone: a review in Parkinson’s disease. Drugs. 2016;76(13):1293–1300. doi:10.1007/s40265-016-0623-y
17. Patel AB, Jimenez-Shahed J. Profile of inhaled levodopa and its potential in the treatment of Parkinson’s disease: evidence to date. Neuropsychiatr Dis Treat. 2018;14:2955–2964. doi:10.2147/NDT.S147633
18. Szász J, Constantin V, Fazakas P, et al. The role of selective monoamine oxidase B inhibitors in the therapeutic strategy of Parkinson’s disease in the neurology clinics of Tirgu Mures County Emergency Clinical Hospital. Orv Hetil. 2017;158:2023–2028. doi:10.1556/650.2017.30914
19. Rosa MM, Ferreira JJ, Coelho M, Freire R, Sampaio C. Prescribing patterns of antiparkinsonian agents in Europe. Mov Disord. 2010;25(8):1053–1060. doi:10.1002/mds.23038
20. Morrish P. Prescribing in Parkinson’s disease: a story of hope and adverse events. Pract Neurol. 2012;12(5):335–340. doi:10.1136/practneurol-2012-000210
21. Osinaga EA, Inchaurregui LCA, Ikobaltzeta IE, Alonso NB, del Pozo JG. A pharmacoepidemiological study of the consumption of antiparkinson drugs in the Basque autonomous community (Spain) (1992–2004). Parkinsonism Relat Disord. 2007;13(8):500–504. doi:10.1016/j.parkreldis.2007.03.004
22. Regidor I, Santos-Garciá D, Catalán MJ, et al. Impact of disease duration in effectiveness of treatment with levodopa-carbidopa intestinal gel and factors leading to discontinuation. J Parkinsons Dis. 2019;9(1):173–182. doi:10.3233/JPD-181324
23. Clarke CE, Worth P, Grosset D, Stewart D. Systematic review of apomorphine infusion, levodopa infusion and deep brain stimulation in advanced Parkinson’s disease. Parkinsonism Relat Disord. 2009;15(10):728–741. doi:10.1016/j.parkreldis.2009.09.005
24. Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov Disord. 2010;25(15):2649–2653. doi:10.1002/mds.23429
25. Fülesdi B, Mitre C, Molnár C. Perioperative management of patients with Parkinson’s disease. Orv Hetil. 2015;156(34):1355–1359. doi:10.1556/650.2015.30233
26. Chaudhuri KR, Pirtosek Z, Pickut B, et al. Levodopa-carbidopa intestinal gel in routine care of advanced Parkinson’s disease patients: final long-term non-motor, quality of life and safety results from the GLORIA registry. Mov Disord. 2016;31(100):S661. doi:10.1002/mds.26688
27. Antonini A, Poewe W, Chaudhuri KR, et al. Levodopa-carbidopa intestinal gel in advanced Parkinson’s: final results of the GLORIA registry. Parkinsonism Relat Disord. 2017;45:13–20. doi:10.1016/j.parkreldis.2017.09.018
28. Olanow CW, Kieburtz K, Odin P, et al. Continuous intrajejunal infusion of levodopa-carbidopa intestinal gel for patients with advanced Parkinson’s disease: a randomised, controlled, double-blind, double-dummy study. Lancet Neurol. 2014;13(2):141–149. doi:10.1016/S1474-4422(13)70293-X
29. Băjenaru O, Ene A, Popescu BO, et al. The effect of levodopa-carbidopa intestinal gel infusion long-term therapy on motor complications in advanced Parkinson’s disease: a multicenter Romanian experience. J Neural Transm. 2016;123(4):407–414. doi:10.1007/s00702-015-1496-z
30. Juhász A, Aschermann Z, Ács P, et al. Levodopa/carbidopa intestinal gel can improve both motor and non-motor experiences of daily living in Parkinson’s disease: an open-label study. Parkinsonism Relat Disord. 2017;37:79–86. doi:10.1016/j.parkreldis.2017.02.001
31. Martinez-Martin P, Reddy P, Antonini A, et al. Chronic subcutaneous infusion therapy with apomorphine in advanced Parkinson’s disease compared to conventional therapy: a real life study of non motor effect. J Parkinsons Dis. 2011;1(2):197–203. doi:10.3233/JPD-2011-11037
32. Martinez-Martin P, Reddy P, Katzenschlager R, et al. EuroInf: a multicenter comparative observational study of apomorphine and levodopa infusion in Parkinson’s disease. Mov Disord. 2015;30(4):510–516. doi:10.1002/mds.26067
33. Katzenschlager R, Poewe W, Rascol O, et al. Apomorphine subcutaneous infusion in patients with Parkinson’s disease with persistent motor fluctuations (TOLEDO): a multicentre, double-blind, randomised, placebo-controlled trial. Lancet Neurol. 2018;17(9):749–759. doi:10.1016/S1474-4422(18)30239-4
34. Dafsari HS, Martinez-Martin P, Rizos A, et al. EuroInf 2: subthalamic stimulation, apomorphine, and levodopa infusion in Parkinson’s disease. Mov Disord. 2019;34(3):353–365. doi:10.1002/mds.27626
35. Möller JC, Körner Y, Dodel RC, et al. Pharmacotherapy of Parkinson’s disease in Germany. J Neurol. 2005;252(8):926–935. doi:10.1007/s00415-005-0784-1
36. Benbir G, Özekmekçi S, Apaydin H, Delil S, Erginöz E. A hospital-based study: risk factors in development of motor complications in 555 Parkinson’s patients on levodopa therapy. Clin Neurol Neurosurg. 2006;108(8):726–732. doi:10.1016/j.clineuro.2006.02.002
37. Brun L, Lefaucheur R, Fetter D, et al. Non-motor fluctuations in Parkinson’s disease: prevalence, characteristics and management in a large cohort of parkinsonian outpatients. Clin Neurol Neurosurg. 2014;127:93–96. doi:10.1016/j.clineuro.2014.10.006
38. Kadastik-Eerme L, Taba N, Asser T, Taba P. Factors associated with motor complications in Parkinson’s disease. Brain Behav. 2017;7(10):1–8. doi:10.1002/brb3.837
39. Szatmari S, Illigens BMW, Siepmann T, Pinter A, Takats A, Bereczki D. Neuropsychiatric symptoms in untreated parkinson’s disease. Neuropsychiatr Dis Treat. 2017;13:815–826. doi:10.2147/NDT.S130997
40. Csóka M, Molnár S, Kellos É, Domján G. Problem solving care models of Parkinson’s disease introduction: Parkinson’s disease. Orv Hetil. 2016;157(22):855–868. doi:10.1556/650.2016.30479
41. Hermanowicz N, Jones SA, Hauser RA. Impact of non-motor symptoms in Parkinson’s disease: a PMDAlliance survey. Neuropsychiatr Dis Treat. 2019;Volume 15:2205–2212. doi:10.2147/ndt.s213917
42. Zhang C, Zang Y, Song Q, et al. The efficacy of a “cocktail therapy” on Parkinson’s disease with dementia. Neuropsychiatr Dis Treat. 2019;15:1639–1647. doi:10.2147/NDT.S179453
43. Romosan A-M, Romosan R-S, Bredicean AC, Simu MA. Affective theory of mind in Parkinson’s disease: the effect of cognitive performance. Neuropsychiatr Dis Treat. 2019;15:2521–2535. doi:10.2147/NDT.S219288
44. Szász JA, Orbán-Kis K, Constantin VA, et al. Therapeutic strategies in the early stages of Parkinson’s disease: a cross-sectional evaluation of 15 years’ experience with a large cohort of Romanian patients. Neuropsychiatr Dis Treat. 2019;15:831–838. doi:10.2147/NDT.S197630
45. Szász JA, Constantin V, Mihály I, et al. Dopamine agonists in Parkinson’s disease therapy - 15 years of experience of the neurological clinics from Tirgu Mures. A cross-sectional study. Ideggyogy Sz. 2019;72(5–6):187–193. doi:10.18071/isz.72.0187
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
