Back to Journals » The Application of Clinical Genetics » Volume 16
Profile of BRAFV600E, BRAFK601E, NRAS, HRAS, and KRAS Mutational Status, and Clinicopathological Characteristics of Papillary Thyroid Carcinoma in Indonesian National Referral Hospital
Authors Harahap AS, Subekti I, Panigoro SS, Asmarinah , Lisnawati , Werdhani RA , Agustina H , Khoirunnisa D , Mutmainnah M , Salinah, Siswoyo AD, Ham MF
Received 17 March 2023
Accepted for publication 19 May 2023
Published 25 May 2023 Volume 2023:16 Pages 99—110
DOI https://doi.org/10.2147/TACG.S412364
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Martin Maurer
Agnes Stephanie Harahap,1– 3 Imam Subekti,4 Sonar Soni Panigoro,5 Asmarinah,6 Lisnawati,1 Retno Asti Werdhani,7 Hasrayati Agustina,8 Dina Khoirunnisa,1 Mutiah Mutmainnah,1 Salinah,1 Alvita Dewi Siswoyo,9 Maria Francisca Ham1,2
1Department of Anatomical Pathology, Faculty of Medicine Universitas Indonesia/Dr. Cipto Mangunkusumo Hospital, Jakarta, Indonesia; 2Human Cancer Research Center-Indonesian Medical Education and Research Institute, Faculty of Medicine Universitas Indonesia, Jakarta, Indonesia; 3Doctoral Program in Medical Sciences, Faculty of Medicine Universitas Indonesia, Jakarta, Indonesia; 4Department of Internal Medicine, Faculty of Medicine Universitas Indonesia/Dr. Cipto Mangunkusumo Hospital, Jakarta, Indonesia; 5Department of Surgery, Faculty of Medicine Universitas Indonesia/Dr. Cipto Mangunkusumo Hospital, Jakarta, Indonesia; 6Department of Medical Biology, Faculty of Medicine Universitas Indonesia, Jakarta, Indonesia; 7Department of Community Medicine, Faculty of Medicine Universitas Indonesia, Jakarta, Indonesia; 8Department of Anatomical Pathology, Faculty of Medicine Universitas Padjadjaran/Hasan Sadikin General Hospital, Bandung, Indonesia; 9Department of Radiology, Faculty of Medicine Universitas Indonesia/Dr. Cipto Mangunkusumo Hospital, Jakarta, Indonesia
Correspondence: Agnes Stephanie Harahap, Department of Anatomical Pathology, Faculty of Medicine Universitas Indonesia, Jl. Salemba Raya No. 6, Jakarta, 14320, Indonesia, Tel +62818765563, Email [email protected]
Introduction: BRAFV600E and RAS mutations are the most common gene mutations in papillary thyroid carcinoma (PTC) that may be correlated with its biological behavior. There are still limited data about BRAFV600E and RAS mutations in Indonesia. This study aims to determine the prevalence of BRAFV600E and RAS mutations, and their association with clinicopathologic characteristics.
Methods: Patients who had total thyroidectomy from 2019 to 2021 and those who met our study criteria underwent PCR and DNA sequencing analysis for BRAFV600E, BRAFK601E, exon 2 and 3 of NRAS, HRAS, and KRAS. Analyses were performed to determine the associations of BRAFV600E and RAS mutations with clinicopathologic characteristics.
Results: Of 172 PTC patients, BRAFV600E mutation was observed in 37.8% of the patients and RAS mutations were found in 21.5%. One patient harbored BRAFK601E mutation. There was a significant association of BRAFV600E with a high-stage (p = 0.033, OR: 3.279; 95% CI: 1.048– 10.259), tall-cell variants (p ≤ 0.001, OR: 41.143; 95% CI: 11.979– 141.308), non-encapsulated (p = 0.001, OR: 4.176; 95% CI: 2.008– 8.685), lymphovascular invasion (p = 0.043, OR: 1.912; 95% CI: 1.018– 3.592), extrathyroidal extension (p = < 0.001, OR: 3.983; 95% CI: 1.970– 8.054), and lymph node metastasis (p = 0.009, OR: 2.301; 95% CI: 1.224– 4.326). Follicular variant (p = 0.001, OR: 7.011; 95% CI: 2.690– 18.268), encapsulated (p = 0.017, OR: 2.433; 95% CI: 1.161– 5.100), and absent of extrathyroidal extension (p = 0.033, OR: 2.890; 95% CI: 1.052– 7.940) were associated with RAS mutations.
Conclusion: A significant association between BRAFV600E mutation and high clinical stage, tall-cell variants, non-encapsulated morphology, lymphovascular invasion, extrathyroidal extension, and lymph node metastasis in PTC was observed. RAS mutations were associated with the follicular variant, encapsulated tumor, and no extrathyroidal extension. HRAS-mutated PTC frequently exhibited tumor multifocality.
Keywords: papillary thyroid carcinoma, BRAFV600E, BRAFK601E, RAS, clinicopathological characteristics
Introduction
In recent decades, there has been a dramatic increase in the incidence of thyroid cancer.1,2 In terms of worldwide and Indonesian cancer incidence, it is currently ranked 7th and 12th, respectively.3 Papillary thyroid carcinoma (PTC) is a follicular cell-derived tumor attributed to 80–85% of thyroid cancer.1 Based on its distinctive histopathological characteristics, striking variants including tall and columnar cells, oncocytic, solid/trabecular, and those frequently display extrathyroidal extension (ETE), lymph node metastasis (LNM), and distant organ metastasis, are particularly aggressive.4
The presence of gene mutation is associated with the behavior and prognosis of the disease. B-Rapidly accelerating fibrosarcoma (BRAFV600E) and RAT Sarcoma (RAS) mutations are well-published driver mutations in the development of PTC. BRAFV600E mutation is typically present in the classic and tall-cell variants, commonly related to a higher level of aggression.5 Patients with BRAF mutation are twice as likely to experience a relapse of their illness and possess greater mortality rates than those without the mutation.6,7 The human RAS gene is divided into Kirsten RAT sarcoma (KRAS), neuroblastoma (NRAS), and Harvey (HRAS), as opposed to BRAFV600E mutation, are more prevalent in the follicular variant of PTC.5 Better prognosis and more indolent disease behavior have been associated with RAS mutation.8,9
The prevalence of BRAFV600E and RAS mutations varied between Western and Asian countries, which was believed to be caused by geographic heterogeneity, race, and other risk factors. Americans and Europeans carried BRAFV600E mutation in around 35–60% of the patients.10,11 Meanwhile, the prevalence of BRAFV600E mutations was quite high among Asian countries, though the numbers varied.12 On the other hand, a relatively similar prevalence of RAS-positive PTC was found in Europe, the USA, and Asian countries.13
Hitherto, studies about PTC and its mutational status are still limited in Indonesia. Yet, most of the studies used immunohistochemistry modality and/or cytology specimens with limited samples.14–18 Given the dearth of research describing the prevalence of BRAF and RAS mutations in PTC in Indonesia, this study aimed to assess the prevalence of BRAFV600E, BRAFK601E, and RAS (NRAS, HRAS, and KRAS) mutations and their association with the clinicopathological profiles of PTC that were related to the tumor behavior and prognosis in Indonesian population.
Subject and Methods
Subject Selection and Evaluation of Histologic Parameters
Every patient who underwent a complete thyroidectomy and was diagnosed with PTC at the Cipto Mangunkusumo Hospital-Faculty of Medicine Universitas Indonesia between 2019 and August 2021 was retrospectively collected. A total of 172 patients were included after eliminating patients with insufficient samples, inaccessible medical records, inappropriate hematoxylin and eosin (H&E) stained slides, and formalin-fixed paraffin-embedded (FFPE) tumor specimens. Medical records were used to acquire clinical information, such as age, gender, and clinical stage. Two certified pathologists from our institution blindly examined the pathological information microscopically, including the tumor size, histological variant, multifocality, nuclear score, LNM, ETE, and lymphovascular invasion (LVI). We excluded PTC with high-grade features (high mitosis index and necrosis) and all follicular variants were invasive. Interobserver agreement was analyzed by using Kappa analysis with nearly perfect agreement results.
BRAFV600E, BRAFK601E, HRAS, NRAS, and KRAS Mutational Analysis
DNA Isolation and Purification
Using the QIAamp DNA FFPE Tissue Kit (Qiagen, Valencia, CA) in the integrated Laboratory of FMUI-CHM, we extracted genomic material from 5-μm thick sections of FFPE tumor tissues. Following the manufacturer’s directions, we carried out the subsequent steps of melting the paraffin with xylene, lysing the tissue with Proteinase K, heating, DNA binding, and washing. After 8000 rpm centrifugation with a QIAamp MinElute Column, pure DNA products were obtained (Qiagen, Valencia, CA). Spectrophotometers called NanoDrop TM 2000/2000c were used to measure the final DNA product’s quantity (Thermo Fisher Scientific, Waltham, MA). Absorbance measurement was used to evaluate quality. An A260/A280 ratio of 1.8 to 2.0 indicates a high-quality DNA sample.
Polymerase Chain Reaction and DNA Sequencing
BRAF Exon 15 Mutation Analysis
KOD One Polymerase Chain Reaction (PCR) Master Mix was used to perform PCR (Toyobo KMM-201). As shown in Table 1, specific primers were employed to amplify exon 15 of the BRAF gene. In the Agilent Surecycler 8800 thermal cycler, PCRs were produced under the following conditions: (i) 94°C denaturation for 2 min, (ii) 40 cycles of 98°C denaturation for 15 sec, 53°C and 55°C annealing for 5 sec, and 68°C elongation for 1 sec, (iii) 68°C elongation for 10 sec, and then (iv) 4°C hold. To verify the quality of the PCR results, the presence of a band using 1% TBE agarose electrophoresis was assessed. DNA sequencing was done by using BigDye Terminator v3.1 Cycle Sequencing Kit (Thermo Fisher Scientific, Waltham, MA) and ABI PRISM 3730xl Genetic Analyzer (Applied Biosystems, Carlsbad, CA). By comparing sequences with the Basic Local Alignment Search Tool (BLAST) and manual reading confirmation, tumors harboring the BRAFV600E and BRAFK601E mutations were verified.
 |
Table 1 Primer Pairs for Mutational Analysis |
RAS Mutation Analysis
We used the MyTaq HS Red Mix Kit to perform PCR (Bioline). Exon 2 and exon 3 of the HRAS, NRAS, and KRAS genes were amplified using certain primers, as shown in Table 1. The following settings were used to achieve PCRs in the Veriti 96-Well Fast Thermal Cycler from Applied Biosystem in Carlsbad, California: (i) 95°C PCR initial activation step for 5 min (ii) 40 cycles of 95°C denaturation for 15 sec, 64°C annealing for 30 sec for exon 2 and 3 HRAS, 55°C annealing for 30 sec for exon 2 NRAS, 60°C annealing for 30 sec for exon 3 NRAS, 54°C annealing for 30 sec for exon 2 KRAS and 55°C annealing for 30 sec for exon 3 KRAS, and 72°C extension for 30 sec, (iii) 72°C final extension for 3 min, and (iv) 4°C hold before taken out of the machine. The presence of a band was assessed by using 1% TBE agarose electrophoresis to verify the quality of PCR results. DNA sequencing was done by using BigDye Terminator v3.1 Cycle Sequencing Kit (Thermo Fisher Scientific, Waltham, MA) and ABI PRISM 3730xl Genetic Analyzer (Applied Biosystems, Carlsbad, CA). By comparing sequences with the Basic Local Alignment Search Tool (BLAST) and manual confirmation, tumors harboring the NRAS, HRAS, and KRAS mutation were verified.
Statistical Analysis
All of the research data were processed using Statistical Program for Social Science (SPSS) ver.20. BRAF and RAS mutational status, patient gender, clinical stage, histological variation, and other categorical data were given as frequencies and percentages. Age and tumor size were presented as median values based on the distribution abnormality of the numerical data. The bivariate analysis employed the Chi-square test or Fisher’s exact test to look at the relationship between variables in categorical data. If the p-value for each test was less than 0.05, the analysis was regarded as significant.
Result
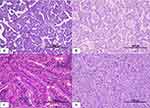
We summarized the patients’ baseline characteristics in Table 2. This study mostly consisted of patients <55 years old, female, low clinical stage, tumor size <4 cm, multifocal, unencapsulated, without LVI, and without ETE. Distant metastasis was found in the liver, lung, vertebrae, costae, and other bones. The most common histologic variant was the classic variant followed by follicular, tall-cell, solid, and oncocytic variants (Figure 1).
 |
Table 2 Baseline Characteristics of the Patients |
We examined exon 15 of the BRAF gene and exons 2 and 3 of all NRAS, HRAS, and KRAS genes (Table 3). This study revealed that 65 patients were BRAFV600E mutants and 37 patients were RAS mutants. Almost all BRAFV600E mutations were heterozygous and only one homozygous mutant was found. Only one patient harbored BRAFK601E mutation. BRAF and RAS mutations were mutually exclusive. Our result showed that substitution of glutamine-to-arginine at exon 3 NRAS mutation (CAA (Gln) > CGA (Arg); Q61R) was the most common type of mutation, followed by glutamine-to-arginine substitution at exon 3 HRAS (CAG (Gln) > CGG (Arg)) and glycine-to-arginine substitution at exon 2 NRAS (GGT (Gly) > CGT (Arg)) (Figure 2).
 |
Table 3 BRAF and RAS Mutations in PTC |
The association between clinicopathological characteristics of PTC and mutational status is displayed in Table 4. There was a significant association of BRAFV600E mutation with high clinical stage, tall-cell variant, non-encapsulated tumor, LVI, ETE, and LNM. Whereas only follicular variants, encapsulated tumors, and no ETE were associated with RAS mutation.
 |
Table 4 Differences Between Clinicopathological Characteristics of PTC and BRAFV600E and RAS Mutation |
Furthermore, we performed a subgroup analysis to investigate if there are any differences between clinicopathological characteristics and NRAS-HRAS mutations after excluding KRAS-mutated patients. Except for tumor multifocality, there were no differences in the majority of clinicopathological characteristics between NRAS and HRAS mutational status (Table 5).
 |
Table 5 Differences of Clinicopathological Characteristics of PTC Between NRAS and HRAS Mutation |
Discussion
RAS/RAF/MEK/ERK (MAPK, mitogen-activated protein kinase) signaling is a crucial route that controls cellular proliferation, differentiation, and survival. BRAF and RAS genes are well-known proto-oncogenes that contribute to the development of PTC. Missense mutations in codon 600 of exon 15 (V600E) account for most of the activating mutations in the BRAF gene. Their association with the behavior of the disease remains controversial despite prior publications concerning the issue.19,20
BRAFV600E had been known as the most prominent genetic mutation for thyroid carcinoma for decades. However, its worldwide prevalence in PTC ranges widely from 27.3% to 73.4%.21 A large meta-analysis study from the Asian population also showed a similar prevalence rate for BRAFV600E mutation, ranging from 23% to 83% in overall PTC cases.12 Different sample sizes, multiple tissue sources, various sequence reading techniques, and other geographic considerations including genetic and environmental status may contribute to the significant discrepancies among studies. In the present study, BRAFV600E mutation was detected in 37.8% of PTC patients. This result is similar to the previous finding from Southeast Asia by Navarro-Locsin et al,22 which reported the BRAFV600E mutation rate was 38.5%. Similar to our result, there were studies in Indonesia conducted by Heriyanto et al23 and Perdana AB et al18 that used molecular methods and reported BRAFV600E mutation rates were 40.3% and 31%, respectively. A single-center cohort study in Singapore reported BRAF mutation in 56% of the PTC patients.24 An even higher percentage of BRAF mutation was also found in Japanese (82.1%), Vietnamese (83%), and South Korean population (81.3%).25 Among other regions in Asian continents, BRAFV600E mutation was particularly more saturated in Southeast and East Asia.12 We hypothesize the difference between Indonesia and other Southeast Asian countries is because of geographic conditions including environmental factors. Although still controversial, a high iodine intake has been associated with a higher risk for the occurrence of BRAF mutation in PTC.26 A relatively poor iodine intake in certain provinces in Indonesia may be a plausible explanation for a lower percentage of BRAFV600E mutation in this country,27,28 which needs to be elucidated.
Beside BRAFV600E, a mutation that can occur in exon 15 of the BRAF gene is BRAFK601E. This rare variant showed mutation at nucleotide 1801 in which an A>G transition occurred, producing lysine to glutamic acid substitution.29 In contrast with BRAFV600E, BRAFK601E-mutated PTC is classified as a relatively low-grade tumor with no aggressive behavior such as ETE and metastases. In this study, we found only one case that harbored the BRAFK601E mutation. This particular case featured an encapsulated tumor, a follicular variant, no ETE, low stage, with neither distant nor LNM.
RAS was the second-most frequent genetic mutation discovered in thyroid carcinoma, primarily in follicular-cell-derived malignancies. The prevalence of RAS mutation in PTC was varied, ranging from 6.7% to 68.8%.30–32 Our study identified RAS mutation in 21.5% of the PTC patients. This result seems to be similar to a study conducted in India, which reported RAS mutation in 20% of the patients.33 Lower rate of RAS mutation was found in Saudi Arabia, in which the rate of RAS mutation was 6.1%.34 Among the three homologous isoforms, NRAS was the most frequent mutation displayed in thyroid nodules.30,34,35 This is similar to our finding where NRAS and HRAS mutation rates were 59.5% and 37.8%, respectively. Our study also supports evidence from Patel et al35 where the majority of both NRAS and HRAS mutations were detected in codon 61. Furthermore, one case of KRAS mutation was observed in codon 61.36 The KRAS mutation was higher in follicular adenoma than the NRAS mutation, which had a stronger oncogenic potential.30,35
In general, PTC has predominantly occurred in women, between the fourth to fifth decades of life.37–39 This is in line with our observation that 76.2% of PTC patients were female with the median age of diagnosis was 44 years. Despite the lack of association observed, BRAFV600E and RAS mutation were more prevalent in male and older patients. This pattern is similar to the previous studies.39–43 One phenomenon of this event could be the neoplastic transition that may begin in older individuals.44,45
In this cohort study, most of the patients were diagnosed in stage I and II of the disease. Several studies have explained the association between the clinical stage of PTC and BRAFV600E mutation.21,46 Our finding is consistent with the previous studies in which we found a significant correlation between BRAFV600E mutation and high clinical stage (p = 0.033). RAS mutation was slightly prevalent (28.6%) in the clinical stage III and IV (p = 0.502).
Consistent with the preceding literature,43,47 we observed the two most prevalent PTC variants were classic (34.9%) and follicular (30.2%). Interestingly, the number of tall-cell variants found in this study was quite high (21.5%). Our institution is a national referral hospital that is classified as a quaternary-level hospital. Mostly, we received advanced cases that cannot be solved at primary, secondary, and tertiary levels of care. Hence, the samples obtained were usually complicated cases with aggressive behavior.
BRAFV600E mutation has long been associated with aggressive variants of PTC.21,48,49 In the present study, BRAFV600E mutation was predominantly found in tall-cell (86.5%), and classic variants (36.7%). We also discovered a significant positive correlation between BRAFV600E mutation and histologic variants (p = <0.001), especially classic (OR: 3.722; 95% CI: 1.434–9.606) and tall-cell variants (OR: 41.143; 95% CI: 11.979–141.308). Compared to the BRAFV600E mutation, the RAS mutation is unlikely to be displayed in the aggressive types of thyroid tumors. It is commonly associated with follicular adenoma, follicular thyroid carcinoma, and follicular variant of PTC.34,50 This present study supports the previous evidence in which we observed that RAS mutation is more prevalent and showed a significant association with the follicular variant of PTC (p = <0.001).
The existence of tumor multifocality and capsules in PTC has been considered as the predictive marker for disease recurrence.41,51,52 Previous studies concluded that BRAFV600E mutation significantly affects tumor multifocality development.46,53,54 Those findings were incongruities to our present study in which we found no association between multifocality in both BRAFV600E and RAS mutation. However, HRAS-mutated patients were significantly inclined to show multifocality compared to NRAS-mutated patients (p = 0.016). We also found a significant association between the presence of tumor capsules in BRAFV600E and RAS mutation. BRAFV600E mutation was significantly more saturated in non-capsulated tumors (p = 0.001), whereas RAS mutation was significantly more prevalent in encapsulated tumors (p = 0.017). These findings also corroborate several existing studies.21,55
LVI, ETE, and LNM are important prognostic factors in cancer, reflecting the aggressiveness of disease behavior. Supporting previous evidences,21,46,48,54 a significant association between BRAFV600E mutation and the presence of LVI (p = 0.043), ETE (p = <0.001), and LNM (p = 0.009) were displayed in this study. Compared to the BRAFV600E mutation, we only found that RAS mutation is significantly associated with the absence of LNM (p = 0.033). The distribution of RAS mutation, which is more prevalent in encapsulated tumors, might be able to explain this finding.
Several limitations of this study may include a small sample size and limited data related to therapy and survival rate of the patients which may better represent disease aggressiveness. We also did not investigate TERT promoter mutation that frequently exists concurrently with BRAF or RAS mutation. Compared to a single mutation, some studies have reported that co-occurrence between TERT promoter mutation with either BRAFV600E or RAS mutation is associated with an increased PTC aggressiveness highlighted by a higher recurrence rate and decreased disease-free survival rate. It is known that the coexistence of TERT and BRAFV600E creates a special mechanism that increased the expression of the TERT mRNA in PTC.56,57
In conclusion, this cohort study consisted of 172 PTC patients with the majority of female, being diagnosed under 55 years, and low clinical stage. BRAFV600E mutation was found in 37.8% of the patients. RAS mutations were found in 21.5% of the patients. One patient harbored BRAFK601E mutation and displayed an indolent morphology. Among RAS genes, 59.5% of the patients harbored NRAS mutation, 37.8% of the patients harbored HRAS mutation, and 2.7% of the patients harbored KRAS mutation. There was a significant association between BRAFV600E mutation and high clinical stage, tall-cell variants, non-encapsulated morphology, LVI, ETE, and LNM. Only follicular variant, encapsulated morphology, and absence of ETE were associated with RAS mutations. HRAS-mutated patients tend to show multifocality.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by The Institutional Research Ethics Committee of the Faculty of Medicine, Universitas Indonesia − Cipto Mangunkusumo Hospital (FMUI-CMH) with the authorization number KET-253/UN2.F1/ETIK/PPM.00.02/2022 (date of approval: 14 March 2022). Our Institutional Review Board’s policy states that studies meeting a number of requirements—including those using existing data or documents, pathological specimens, or other diagnostic specimens, in which the documents are managed so that the identity of each subject is protected and cannot be identified—can have the direct informed consent form requirement waived (No.ND-532/UN2.FI/ETIK/PPM.00.02.2022).
Funding
This research was funded by Universitas Indonesia-Publikasi Terindeks Internasional (PUTI) grant with contract no. NKB-1334/UN2.RST/HKP.05.00/2020.
Disclosure
The authors declare no conflict of interest.
References
1. Lim H, Devesa SS, Sosa JA, Check D, Kitahara CM. Trends in thyroid cancer incidence and mortality in the United States, 1974-2013. JAMA. 2017;317(13):1338–1348. doi:10.1001/jama.2017.2719
2. Olson E, Wintheiser G, Wolfe KM, Droessler J, Silberstein PT. Epidemiology of thyroid cancer: a review of the National Cancer Database, 2000-2013. Cureus. 2019;11(2):e4127. doi:10.7759/cureus.4127
3. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249.
4. WHO Classification of Tumors Editorial Board. Endocrine and neuroendocrine tumors. Lyon (France): international Agency for Research on Cancer; 2022. Available from: https://tumourclassification.iarc.who.int/chapters/53.
5. Tang KT, Lee CH. BRAF mutation in papillary thyroid carcinoma: pathogenic role and clinical implications. J Chin Med Assoc. 2010;73(3):113–128.
6. Xing M. Prognostic utility of BRAF mutation in papillary thyroid cancer. Mol Cell Endocrinol. 2010;321(1):86–93.
7. Xing M, Alzahrani AS, Carson KA, et al. Association between BRAF V600E mutation and mortality in patients with papillary thyroid cancer. JAMA. 2013;309(14):1493–1501.
8. Xing M. Clinical utility of RAS mutations in thyroid cancer: a blurred picture now emerging clearer. BMC Med. 2016;14:1–4.
9. Póvoa AA, Teixeira E, Bella-Cueto MR, et al. Genetic determinants for prediction of outcome of patients with papillary thyroid carcinoma. Cancers. 2021;13(9):1–16.
10. Yip L, Nikiforova MN, Carty SE, et al. Optimizing surgical treatment of papillary thyroid carcinoma associated with BRAF mutation. Surgery. 2009;146(6):1215–1223. doi:10.1016/j.surg.2009.09.011
11. Cancer Genome Atlas Research Network. Integrated genomic characterization of papillary thyroid carcinoma. Endocrine Abstracts. 2014;159(3):676–690.
12. Rashid FA, Munkhdelger J, Fukuoka J, Bychkov A. Prevalence of BRAFV600E mutation in Asian series of papillary thyroid carcinoma—a contemporary systematic review. Gland Surg. 2020;9(5):1878–1900. doi:10.21037/gs-20-430
13. Odate T, Oishi N, Vuong HG, Mochizuki K, Kondo T. Genetic differences in follicular thyroid carcinoma between Asian and Western countries: a systematic review. Gland Surg. 2020;9(5):1813–1826. doi:10.21037/gs-20-356
14. Sekarputri CH, Hary Kusumastuti E, Mastutik G. Analysis of BRAF and TERT expressions in papillary thyroid carcinoma cases based on ATA risk stratification. Majalah Patologi Indonesia. 2020;29(3):131–138. doi:10.55816/mpi.v29i3.444
15. Kristiani E, Hardjolukito E, S Harahap A, Makes B. BRAF V600E Immunoexpression in papillary thyroid carcinoma and its association with prognostic factors and histopathologic variant. Medicinus. 2021;8(1):12–21. doi:10.19166/med.v8i1.3120
16. Rusmana MP, Sriwidyani NP, Mahendra Dewi IGAS. BRAF V600E expression found in aggressive papillary thyroid carcinoma (PTC), lymph node metastasis, and extra-thyroid extension. Bali Med J. 2018;7(3):658–662. doi:10.15562/bmj.v7i3.1201
17. Gestin D, Nizar RZ, Asri A, Yetti H. The correlation of BRAF V600E expressions with histopathological variant and lymphocyte infiltration in papillary thyroid carcinoma. Majalah Patologi Indonesia. 2021;30(2):272–279. doi:10.55816/mpi.v30i2.473
18. Perdana AB, Putri RI, Rachmawati R, Andinata B, Brahma B. Clinical utility of BRAF, NRAS, and TERT promoter mutation in preoperative thyroid fine-needle aspiration biopsy: a diagnostic study from Dharmais cancer hospital. Asian Pac J Cancer Prev. 2020;21(11):3267–3277. doi:10.31557/APJCP.2020.21.11.3267
19. Xing M. BRAF mutation in papillary thyroid cancer: pathogenic role, molecular bases, and clinical implications. Endocr Rev. 2007;28(7):742–762. doi:10.1210/er.2007-0007
20. Abdullah MI, Junit SM, Ng KL, Jayapalan JJ, Karikalan B, Hashim OH. Papillary thyroid cancer: genetic alterations and molecular biomarker investigations. Int J Med Sci. 2019;16(3):450–460. doi:10.7150/ijms.29935
21. Li C, Lee KC, Schneider EB, Zeiger MA. BRAF V600E mutation and Its association with clinicopathological features of papillary thyroid cancer: a meta-analysis. J Clin Endocrinol Metab. 2012;97(12):4559–4570. doi:10.1210/jc.2012-2104
22. Navarro-Locsin CG, Chang AMV, Daroy ML, Alfon AC, Andal JJ, Padua PF. Clinical and histopathological profile of BRAF V600E mutation in conventional papillary thyroid carcinoma in a Filipino population. The Malaysian Journal of Pathology. 2016;38(2):141–148.
23. Heriyanto DS, Laiman V, Limantara NV, et al. High frequency of KRAS and EGFR mutation profiles in BRAF-negative thyroid carcinomas in Indonesia. BMC Res Notes. 2022;15(1):1–6. doi:10.1186/s13104-022-06260-4
24. Goh X, Lum J, Yang S, et al. BRAF mutation in papillary thyroid cancer-Prevalence and clinical correlation in a South-East Asian cohort. Clin Otolaryngol. 2019;44(2):114–123. doi:10.1111/coa.13238
25. Vuong HG, Kondo T, Oishi N, et al. Genetic alterations of differentiated thyroid carcinoma in iodine-rich and iodine-deficient countries. Cancer Med. 2016;5(8):1883–1889. doi:10.1002/cam4.781
26. Guan H, Ji M, Bao R, et al. Association of high iodine intake with the T1799A BRAF mutation in papillary thyroid cancer. The Journal of Clinical Endocrinology & Metabolism. 2009;94(5):1612–1617. doi:10.1210/jc.2008-2390
27. Mahdian N, Sitoayu L. Factors associated with Iodine Deficiency Disorders (IDD) in elementary school 4 Krebet, Ponorogo, East Java. Scitepress. 2020;173–178.
28. Nur Fatimah S, Wan Nurdamia C. The Pattern of iodine food source intake in early adolescents in junior high school in Jatinangor. AMJ. 2019;6(4):159–163.
29. Afkhami M, Karunamurthy A, Chiosea S, et al. Histopathologic and clinical characterization of thyroid tumors carrying the BRAFK601E mutation. Thyroid. 2021;26(2):242–247.
30. Marotta V, Bifulco M, Vitale M. Significance of RAS mutations in thyroid benign nodules and non-medullary thyroid cancer. Cancers. 2021;13(15):1–16.
31. Garcia-Rostan G, Zhao H, Camp RL, et al. Ras mutations are associated with aggressive tumor phenotypes and poor prognosis in thyroid cancer. J Clin Oncol. 2003;21(17):3226–3235.
32. Giordano TJ, Beaudenon-Huibregtse S, Shinde R, et al. Molecular testing for oncogenic gene mutations in thyroid lesions: a case-control validation study in 413 postsurgical specimens. Hum Pathol. 2014;45(7):1339–1347.
33. George N, Agarwal A, Kumari N, Agarwal S, Krisnani N, Gupta SK. Mutational profile of papillary thyroid carcinoma in an endemic goiter region of North India. Indian J Endocrinol Metab. 2018;22(4):505–510.
34. Schulten HJ, Salama S, Al-Ahmadi A, et al. Comprehensive survey of HRAS, KRAS, and NRAS mutations in proliferative thyroid lesions from an ethnically diverse population. Anticancer Res. 2013;33(11):4779–4784.
35. Patel SG, Carty SE, McCoy KL, et al. Preoperative detection of RAS mutation may guide extent of thyroidectomy. In: Surgery (United States). Mosby Inc.; 2017:168–175.
36. Radkay LA, Chiosea SI, Seethala RR, et al. Thyroid nodules with KRAS mutations are different from nodules with NRAS and HRAS mutations with regard to cytopathologic and histopathologic outcome characteristics. Cancer Cytopathol. 2014;122(12):873–882.
37. Lee AW, Mendoza RA, Aman S, Hsu R, Liu L. Thyroid cancer incidence disparities among ethnic Asian American populations, 1990–2014. Ann Epidemiol. 2022;66:28–36.
38. Miranda-Filho A, Lortet-Tieulent J, Bray F, et al. Thyroid cancer incidence trends by histology in 25 countries: a population-based study. Lancet Diabetes Endocrinol. 2021;9(4):225–234.
39. Remer LF, Lee CI, Picado O, Lew JI. Sex differences in papillary thyroid cancer. J Surg Res. 2022;271:163–170.
40. Xie H, Wei B, Shen H, Gao Y, Wang L, Liu H. BRAF mutation in papillary thyroid carcinoma (PTC) and its association with clinicopathological features and systemic inflammation response index (SIRI). Am J Transl Res. 2018;10(8):2726–2736.
41. Al-Salam S, Sharma C, Afandi B, et al. BRAF and KRAS mutations in papillary thyroid carcinoma in the United Arab Emirates. PLoS One. 2020;15(4):1–16.
42. Basolo F, Torregrossa L, Giannini R, et al. Correlation between the BRAF V600E mutation and tumor invasiveness in papillary thyroid carcinomas smaller than 20 millimeters: analysis of 1060 cases. J Clin Endocrinol Metab. 2010;95(9):4197–4205.
43. Pessôa-Pereira D, da S MMF, Lima VMS, et al. Association between BRAF (V600E) mutation and clinicopathological features of papillary thyroid carcinoma: a Brazilian single-centre case series. Arch Endocrinol Metab. 2019;63(2):97–106.
44. Shen X, Zhu G, Liu R, et al. Patient age-associated mortality risk is differentiated by BRAF V600E status in papillary thyroid cancer. J Clin Oncol. 2018;36(5):438–445.
45. Subash A, Sinha P, Singh A. BRAF mutation and age in differentiated thyroid cancer risk stratification: two sides of the same coin. Oral Oncol. 2020;106(8):10–11.
46. Wei X, Wang X, Xiong J, et al. Risk and prognostic factors for BRAFV600E mutations in papillary thyroid carcinoma. Biomed Res Int. 2022;2022:1–13.
47. Yang J, Gong Y, Yan S, et al. Comparison of the clinicopathological behavior of the follicular variant of papillary thyroid carcinoma and classical papillary thyroid carcinoma: a systematic review and meta-analysis. Mol Clin Oncol. 2015;3(4):753–764.
48. Penna GC, Vaisman F, Vaisman M, Sobrinho-Simões M, Soares P. Molecular markers involved in tumorigenesis of thyroid carcinoma: focus on aggressive histotypes. Cytogenet Genome Res. 2016;150:194–207.
49. Dettmer MS, Schmitt A, Steinert H, et al. Tall cell papillary thyroid carcinoma: new diagnostic criteria and mutations in BRAF and TERT. Endocr Relat Cancer. 2015;22(3):419–429.
50. Jung CK, Little MP, Lubin JH, et al. The increase in thyroid cancer incidence during the last four decades is accompanied by a high frequency of BRAF mutations and a sharp increase in RAS mutations. J Clin Endocrinol Metab. 2014;99(2):276–285.
51. Haugen BR, Alexander EK, Bible KC, et al. 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26(1):1–133.
52. Woo J, Kim H, Kwon H. Impact of multifocality on the recurrence of papillary thyroid carcinoma. J Clin Med. 2021;10(21):1–8.
53. Qu HJ, Qu XY, Hu Z, et al. The synergic effect of BRAFV600E mutation and multifocality on central lymph node metastasis in unilateral papillary thyroid carcinoma. Endocr J. 2018;65(1):113–120.
54. Chen B, Zhang Z, Wang K, et al. Association of BRAFV600E mutation with ultrasonographic features and clinicopathologic characteristics of papillary thyroid microcarcinoma: a retrospective study of 116 cases. Clin Hemorheol Microcirc. 2019;73(4):545–552.
55. Rivera M, Ricarte-Filho J, Knauf J, et al. Molecular genotyping of papillary thyroid carcinoma follicular variant according to its histological subtypes (encapsulated vs infiltrative) reveals distinct BRAF and RAS mutation patterns. Mod Pathol. 2010;23(9):1191–1200.
56. Vuong HG, Altibi AMA, Duong UNP, Hassell L. Prognostic implication of BRAF and TERT promoter mutation combination in papillary thyroid carcinoma—A meta-analysis. Clin Endocrinol. 2017;87:411–417.
57. Xing M, Liu R, Liu X, et al. BRAF V600E and TERT promoter mutations cooperatively identify the most aggressive papillary thyroid cancer with highest recurrence. J Clin Oncol. 2014;32(25):2718–2726.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.