Back to Journals » Journal of Asthma and Allergy » Volume 13
Profile of Baricitinib and Its Potential in the Treatment of Moderate to Severe Atopic Dermatitis: A Short Review on the Emerging Clinical Evidence
Authors Napolitano M , Fabbrocini G, Cinelli E, Stingeni L , Patruno C
Received 12 November 2019
Accepted for publication 23 December 2019
Published 31 January 2020 Volume 2020:13 Pages 89—94
DOI https://doi.org/10.2147/JAA.S206387
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Luis Garcia-Marcos
Maddalena Napolitano,1 Gabriella Fabbrocini,2 Eleonora Cinelli,2 Luca Stingeni,3 Cataldo Patruno4
1Department of Medicine and Health Sciences Vincenzo Tiberio, University of Molise, Campobasso, Italy; 2Section of Dermatology, Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy; 3Dermatology Section, Department of Medicine, University of Perugia, Perugia, Italy; 4Department of Health Sciences, University Magna Graecia of Catanzaro, Catanzaro, Italy
Correspondence: Maddalena Napolitano
Department of Medicine and Health Sciences Vincenzo Tiberio, University of Molise, Campobasso, Italy
Tel +39 0817462439
Email [email protected]
Abstract: Atopic dermatitis (AD) is the most common chronic cutaneous inflammatory disease of childhood, affecting up to 25% of children; its prevalence in adulthood is currently unknown, since studies reported that AD may affect 0.3– 14.3% of adult population. In the last decade, the advanced understanding of AD molecular pathways along with patient’s and physician’s demand for more effective therapies, led to the introduction of new therapeutic agents. Baricitinib is an oral JAK inhibitor highly selective for JAK1 and JAK2. Treatment with baricitinib improved the signs and symptoms of moderate-to-severe AD compared to placebo, but it will be essential to better understand the safety profile of this drug.
Keywords: baricitinib, adult atopic dermatitis, small molecules, JAK/STAT
Introduction
Atopic dermatitis (AD) is the most common chronic cutaneous inflammatory disease of childhood, affecting up to 25% of children;1 its prevalence in adulthood is currently unknown, since studies reported that AD may affect 0.3–14.3% of adult population.2 AD has a typical chronic-relapsing course, featured by itch, erythema, and dry skin, which negatively affect patient’s quality of life.3,4 Up to one quarter of patients falls in the category of moderate-to-severe disease; for these cases, a systemic treatment is often mandatory.3
In the last decade, the advanced understanding of AD molecular pathways along with patient’s and physician’s demand for more effective therapies, led to the introduction of new therapeutic agents.5 The underlying etiopathogenesis is multifaceted with a central role played by the relationship between impaired skin barrier and dysregulated immune response.6 The first step in AD progress seems to be the altered skin barrier where environmental factors irrupt, leading to inflammatory response.6–8 The loss-of-function mutations in the structural protein filaggrin and other skin proteins induce perturbed barrier function, thus resulting in diminished epidermal defense mechanisms to allergens, microbes, and other environmental agents.9–11 The molecular chain reaction triggered by keratinocytes activate dendritic and Langerhans cells which in turn stimulate T-helper (Th) 2 cells to produce interleukin (IL)-4, IL-5, IL-13, IL-31, and IL-33.6,12–14 Th2 activation cause an additional impairment in keratinocyte and skin barrier integrity, with lowering of antimicrobial peptide (AMP) levels and subsequent reduced host defense mechanisms, increased inflammation, and pruritus mainly induced by IL-31.12–14 In addition, also Th17 and Th22 lymphocytes play an important role in the pathogenesis of AD. Indeed, they release IL-17, IL-19, and IL-22, which have a central importance in the chronic phase of the disease15 that is characterized by a mixed Th1 and Th2 response, and by the release of IL-22 from Th17 and Th22 cells.16 Therefore, AD might be considered a Th2/Th22-skewed disease, with an additional contribution from Th1 cytokines occurring in the chronic stages.15–18
In the AD molecular signaling, a critical role is played by the Janus kinase (JAK)/signal transducers and activators of transcription (STAT) pathway that is activated by the aforementioned Th2 cytokines, IL-4, IL-5 and TSLP.16 The JAKs comprise a group of 4 receptor-associated kinases [JAK1, JAK2, JAK3, and tyrosine kinase 2 (TYK2)] that mediate cytokine-stimulated transcriptional changes by phosphorylation of the STAT family of transcription factor.17 As proposed in a model by Bao et al, IL-4 influences Th2 differentiation and barrier proteins downregulation.19 Consequently, the Th2 milieu also promotes B cell differentiation with subsequent IgE secretion and, on the other hand, pro-inflammatory cytokines release, including IL-5, that recruits eosinophils, and IL-31, a histamine-independent itch mediator.20 Again, the itch pathway is enhanced by TSLP and type 2 cytokines through receptors of the JAK family.21 Furthermore, JAK/STAT activation downregulates structural epidermal proteins, eg FLG, involucrin, or loricrin, thus diminishing the skin barrier function.18,19,22 Additionally, downstream signalling in this pathway has been shown to prevent the induction of genes encoding innate immune response proteins, including β-defensins and cathelicidin,19,22 thus raising the vulnerability of patients to both viral and bacterial skin infections.
Dysregulation of JAK/STAT pathways is involved in inflammatory chronic diseases other than AD, such as psoriasis, lichen planus, cutaneous lupus erythematosus, alopecia areata, rheumatoid arthritis (RA), and autosomal dominant hyper-IgE syndrome.20 Currently, several pharmaceutical agents targeting TYK2, JAK1, JAK2, and JAK3 are being evaluated for the treatment of moderate-to-severe AD.4,5,20 This review will explore the current literature surrounding the use of baricitinib in AD.
Baricitinib is an oral JAK inhibitor highly selective for JAK1 and JAK2.23 In February 2017, baricitinib was approved in the EU, as monotherapy or in combination with methotrexate, for the treatment of moderate to severe active RA in adult patients who respond inadequately to, or who are intolerant to one or more disease-modifying anti-rheumatic drugs (DMARDs) and it is currently under investigation for other diseases, such as AD and systemic lupus erythematosus.19,20
Materials and Methods
A search was conducted using PubMed/MEDLINE, Embase, Cochrane Skin databases, and clinicaltrials.gov with the search terms ‘atopic dermatitis’ or “atopic eczema” and “baricitinib”. As the primary purpose of this review was the evaluation of safety and efficacy of the drug for the treatment of AD in human subjects, only randomized controlled trials (RCTs) or case reports and case series were selected from 1 January 2014 to 30 August 2019. Finally, this article is based on previously conducted studies and does not involve any new studies on human or animal subjects performed by any of the authors.
Pharmacokinetics and Pharmacodynamics of Baricitinib
Figure 1 shows the chemical structure of baricitinib. It strongly inhibits JAK1 and JAK2, with half-maximum inhibitory concentrations (IC50) of 5.9 and 5.7 nmol/L, respectively, while it has lower potency against Tyk2 and JAK3 (IC50 of 53 and 560 nmol/L).24,25 Steady-state plasma concentrations were attained within 48 h after the first dose in the multiple dose study.24,26
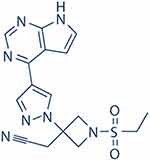 |
Figure 1 Structure of baricitinib. |
The pharmacokinetic profile of oral baricitinib was dose proportional over the dose range 1–20 mg in healthy volunteers in a single dose and a multiple-dose study.27 Its absolute bioavailability is of ~79% and administration of the drug with a high-fat meal had no clinically relevant effects on exposure. Baricitinib is distributed into tissues, with a mean volume of distribution of 76 L after intravenous infusion and it is ~50% bound to plasma proteins.27 Less than 10% of a dose of baricitinib undergoes biotransformation, mostly via CYP3A4 and OAT3. Co-administration with potent inhibitors of CYP3A4 or OAT3 like ketoconazole and ibuprofen or diclofenac could be associated to increase the hematic level of baricitinib and a reduction of renal clearance.24
Baricitinib is excreted via renal (~75% of a dose) and gastrointestinal (~20%) elimination, mainly as the unchanged drug.27 Exposure to it is increased in patients with mild or moderate renal impairment; dosage reduction is required in patients with creatinine clearance of 30–60 mL/min, while it is not recommended in patients with creatinine clearance <30 mL/min.27
Clinical Trials
Phase II
The efficacy of baricitinib as a treatment for AD has been established in a randomized, 16-week, double-blind, placebo-controlled study (NCT02576938), conducted at 13 centers in the United States and Japan between February 2016 and March 2017.28 Four weeks before randomization and throughout the study, patients discontinued any systemic treatment for AD and other medications prohibited for enrollment in the trial. Use of topical corticosteroid (TCS) triamcinolone 0.1% only was permitted. In this study 124 patients were randomized by using an interactive response technology (IRT) system in a 4:3:3 ratio to once-daily placebo plus TCS (49 patients), 2 mg of baricitinib once daily plus TCS (37 patients) or 4 mg of baricitinib once daily plus TCS (38 patients).28 The median Eczema Area and Severity Index (EASI) score for all randomized patients were 21.2 at baseline.28 The primary endpoint was the percentage of participants with a 50% or greater reduction in the Eczema Area and Severity Index (EASI 50) at week 16. It was achieved by a significant greater proportion of patients treated with baricitinib 4 mg plus a TCS, than those treated with 2 mg plus a TCS or placebo plus a TCS (p= 0.27).28 No significant difference was instead recorded when baricitinib 2 mg plus TCS group was compared to placebo plus TCS group (p= 0.065).27,28 Furthermore, after randomization patients receiving baricitinib used approximately 30% less TCS monthly than those of placebo group.28
The secondary outcome measures at week 16 were:
- Change from baseline in the Scoring Atopic Dermatitis (SCORAD): a significantly higher number of baricitinib 4-mg plus TCS patients achieved a reduction in SCORAD scores from baseline to week 16 (−47% in baricitinib 4-mg plus TCS; −41% of baricitinib 2 mg plus TCS and −21% of placebo group, p<0.001). Particularly, the patients reported a reduction of pruritus and sleep loss.28
- Change from baseline in the Dermatologic Life Quality Index (DLQI): total DLQI improved in both treatment groups relative to placebo, with significant improvement at week 4. The difference between baricitinib, 4 mg plus a TCS and both, placebo plus a TCS or baricitinib 2 mg plus TCS was also significant at weeks 8 and 12 (Figure 2).28
 |
Figure 2 Change from baseline in DLQI total score from baseline to week 16. P values: *P < 0.05, **P< 0.01, ***P< 0.001. Notes: Reprinted from J Am Acad Dermatol., 80(4), Guttman-Yassky E, Silverberg JI, Nemoto O, et al, Baricitinib in adult patients with moderate-to-severe atopic dermatitis: a Phase 2 parallel, double-blinded, randomized placebo-controlled multiple-dose study, 913–921, Copyright (2019), with permission from Elsevier.28 Abbreviations: TCS, topical corticosteroid; DLQI, dermatology life quality index. |
Treatment-dependent total adverse events (AEs) were noticed in 24/49 patients (49%) of placebo groups, 17/37 (46%) of baricitinib 2-mg group and 27/38 (71%) of baricitinib 4-mg group, respectively. The most common adverse events (AEs) in treatments’ groups were headache (5/38; 13%), increased blood creatinine phosphokinase (CPK) levels (5/38; 13%) and nasopharyngitis (8%). The treatment was interrupted by 5 patients (10%) of placebo group, and by 1 patient (8/38; 3%) receiving baricitinib 2 mg and by 5 patients (13%) receiving baricitinib 4 mg. Only one patient in the groups of baricitinib 4 mg experienced a serious adverse event (benign polyp of the large intestine), but no deaths occurred. Increase in CPK level in patients treated with baricitinib reflects what already reported with other JAK inhibitors; it is not related to muscular AEs and it is thought to be an unknown pharmacological effect, so far.28–31 Unlike what is reported in clinical trials and open-label extension studies on RA, no other AEs associated with JAK inhibitors such as lymphopenia or herpes zoster reactivation were remarked in AD Phase II trial.28–31
Phase III
Recently, two Phase III studies confirmed a significant improvement in patients under baricitinib therapy.32 BREEZE-AD1 (NCT03334396) and BREEZE-AD2 (NCT03334422) are the first of seven Phase 3 studies of baricitinib for moderate-to-severe AD. The aforementioned multicenter, randomized, double-blind, placebo-controlled studies evaluated the efficacy of baricitinib in monotherapy.32 Inclusion criteria of these studies were:
♦ ≥18-years-old and diagnosis of AD for ≥12 months
♦ Moderate-to-severe AD at screening and randomization, defined as:
- Investigator’s Global Assessment (IGA) score of 3 or 4
- EASI ≥16
- Body Surface Area (BSA) ≥10%
♦ Inadequate response or intolerance to ≥1 existing topical medications for AD
One thousand two hundred and thirty-nine patients (624 patients in BREEZE-AD1 and 615 in BREEZE AD-2) were enrolled and randomized in 2:1:1:1 ratio (PBO: 1 mg: 2 mg: 4 mg). The primary endpoint (an improvement at 16 weeks to clear or almost clear skin assessed by the 5-point IGA) was achieved by a significantly higher proportion of patients in the baricitinib 4-mg group (16.8%) compared to those of placebo group (4.8%) or baricitinib 2-mg group (11.4%) (p<0.01).32 Also the percentage of participants with EASI75 at week 16 was significantly higher in the baricitinib 4-mg group compared to those of others groups (p=<0.0001). Baricitinib showed rapid onset of action, improving patient-reported outcome measures (Itch Numerical Rating Scale (NRS) in 22% of patients of treatment group compared to 12% of patients of baricitinib 2-mg group and 7% of patients of placebo group (p<0.001). Moreover, an improvement of Patient Oriented Eczema Measure (POEM) >4 was reported in a significantly higher proportion of patients (42%) of baricitinib 4-mg group compared to those of baricitinib 2-mg group (29%) or placebo group (14%) (p<0.001), as early as week 1. Safety data showed no deaths, as well as no venous thromboembolic events (VTEs), major cardiovascular events (MACE) and gastro-intestinal perforations in any subject of all the groups, which are the main adverse events reported in the clinical trials for the use of baricitinib in RA.32 In the group that received 4 mg of baricitinib, headache, nasopharyngitis, and increase in CPK levels were the most common adverse events. No patients in the baricitinib-plus-TCS groups had herpes zoster infections.
Current Status and Ongoing Clinical Trials
Actually, baricitinib received in Europe its first global approval as monotherapy or in combination with methotrexate for the treatment of moderate-to-severe active RA in adult patients who responded inadequately, or were intolerant to one or more DMARD. Some studies are ongoing, in order to evaluate the efficacy of baricitinib as a treatment for moderate-to-severe AD in children and adolescents (NCT02576938; NCT03952559; NCT0343508; NCT03334435; NCT03428100), systemic lupus erythematosus (NCT02708095), and chronic graft versus host disease (NCT02759731). A compassionate use program for chronic atypical neutrophilic dermatosis with lipodystrophy and elevated temperatures (CANDLE) is also in place (NCT01724580).
Conclusion
Treatment with baricitinib improved the signs and symptoms of moderate-to-severe AD compared to placebo, and may represent a novel oral treatment option for patients with moderate-to-severe AD. In phase II and phase III trials, very few serious adverse events and no death occurred. Notwithstanding, it will be essential to better understand the safety profile since severe adverse reactions and death associated with the use of 2-mg baricitinib have been described in RA patients. Indeed, food and Drug Administration in the USA inserted a Black Box Warning in the label of 2 mg baricitinib. Furthermore, the correct placement of baricitinib for the therapeutic management of a so complex disease must be carefully evaluated.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Kim BE, Leung DYM. Significance of skin barrier dysfunction in atopic dermatitis. Allergy Asthma Immunol Res. 2018;10(3):207–215.
2. Megna M, Patruno C, Balato A, Rongioletti F, Stingeni L, Balato N. An Italian multicenter study on adult atopic dermatitis: persistent versus adult-onset disease. Arch Dermatol Res. 2017;309:443–445.
3. Calzavara Pinton P, Cristaudo A, Foti C, et al. Diagnosis and management of moderate to severe adult atopic dermatitis: a consensus by the Italian Society of Dermatology and Venereology (SIDeMaST), the Italian Association of Hospital Dermatologists (ADOI), the Italian Society of Allergy, Asthma and Clinical Immunology (SIAAIC), and the Italian Society of Allergological, Environmental and Occupational Dermatology (SIDAPA). G Ital Dermatol Venereol. 2018;153(2):133–145.
4. Napolitano M, Marasca C, Fabbrocini G, Patruno C. Adult atopic dermatitis: new and emerging therapies. Expert Rev Clin Pharmacol. 2018;11(9):867–878.
5. Mobasher P, Heydari Seradj M, Raffi J, Juhasz M, Atanaskova Mesinkovska N. Oral small molecules for the treatment of atopic dermatitis: a systematic review. J Dermatolog Treat. 2018;2:1–8.
6. Boguniewicz M, Leung DY. Atopic dermatitis: a disease of altered skin barrier and immune dysregulation. Immunol Rev. 2011;242(1):233–246.
7. Kim J, Kim BE, Leung DYM. Pathophysiology of atopic dermatitis: clinical implications. Allergy Asthma Proc. 2019;40(2):84–92.
8. Weidinger S, Beck LA, Bieber T, Kabashima K, Irvine AD. Atopic dermatitis. Nat Rev Dis Primers. 2018;4.
9. Elias PM, Hatano Y, Williams ML. Basis for the barrier abnormality in atopic dermatitis: outside-inside-outside pathogenic mechanisms. J Allergy Clin Immunol. 2008;121:1337–1343.
10. Kaufman BP, Guttman-Yassky E, Alexis AF. Atopic dermatitis in diverse racial and ethnic groups. Variations in epidemiology, genetics, clinical presentation and treatment. Exp Dermatol. 2018;27:340–357.
11. O’Regan GM, Sandilands A, McLean WHI, Irvine AD. Filaggrin in atopic dermatitis. J Allergy Clin Immunol. 2008;122(4):689–693.
12. Nygaard U, Vestergaard C, Deleuran M. Emerging treatment options in atopic dermatitis: systemic therapies. Dermatology. 2017;233(5):344–357.
13. Vestergaard C, Deleuran M, Gesser B, Gronhoj Larsen CG. Expression of the T-helper 2-specific chemokine receptor CCR4 on CCR10-positive lymphocytes in atopic dermatitis skin but not in psoriasis skin. Br J Dermatol. 2003;149:457–463.
14. Vestergaard C, Deleuran M, Gesser B, Larsen CG. Thymus and activation-regulated chemokine (TARC/CCL17) induces a Th2-dominated inflammatory reaction on intradermal injection in mice. Exp Dermatol. 2004;13:265–271.
15. Klonowska J, Gleń J, Nowicki RJ, Trzeciak M. New cytokines in the pathogenesis of atopic dermatitis-new therapeutic targets. Int J Mol Sci. 2018;19(10):3086.
16. Czarnowicki T, Krueger JG, Guttman-Yassky E. Novel concepts of prevention and treatment of atopic dermatitis through barrier and immune manipulations with implications for the atopic march. J Allergy Clin Immunol. 2017;139:1723–1734.
17. Darnell JE, Kerr IM, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264:1415–1421.
18. Villarino AV, Kanno Y, O’Shea JJ. Mechanisms and consequences of Jak-STAT signaling in the immune system. Nat Immunol. 2017;18:374–384.
19. Bao L, Zhang H, Chan LS. The involvement of the JAK-STAT signaling pathway in chronic inflammatory skin disease atopic dermatitis. JAKSTAT. 2013;2(3):e24137.
20. Cotter DG, Schairer D, Eichenfield L. Emerging therapies for atopic dermatitis: JAK inhibitors. J Am Acad Dermatol. 2018;78:S53–S62.
21. Potenzieri C, Undem BJ. Basic mechanisms of itch. Clin Exp Allergy. 2012;42(1):8–19.
22. Thyssen JP, Kezic S. Causes of epidermal filaggrin reduction and their role in the pathogenesis of atopic dermatitis. J Allergy Clin Immunol. 2014;134:792–799.
23. Kubo S, Nakayamada S, Sakata K, et al. Janus kinase inhibitor baricitinib modulates human innate and adaptive immune system. Front Immunol. 2018;9:1510.
24. Markham A. Baricitinib: first global approval. Drugs. 2017;77(6):697–704.
25. Fleischmann R, Schiff M, van der Heijde D, et al. Baricitinib, methotrexate, or combination in patients with rheumatoid arthritis and no or limited prior disease-modifying antirheumatic drug treatment. Arthritis Rheum. 2017;69(3):506–517.
26. Shi JG, Chen X, Lee F, et al. The pharmacokinetics, pharmacodynamics, and safety of baricitinib, an oral JAK 1/2 inhibitor, in healthy volunteers. J Clin Pharmacol. 2014;54(12):1354–1361.
27. European Medicines Agency. Baricitinib (Olumiant): summary of product characteristics; 2017. Available from: http://ec.europa.eu/health/documents/communityregister/2017/20170213136870/anx_136870_en.pdf.
28. Guttman-Yassky E, Silverberg JI, Nemoto O, et al. Baricitinib in adult patients with moderate-to-severe atopic dermatitis: a Phase 2 parallel, double-blinded, randomized placebo-controlled multiple-dose study. J Am Acad Dermatol. 2019;80(4):913–921.
29. Taylor PC, Keystone EC, van der Heijde D, et al. Baricitinib versus placebo or adalimumab in rheumatoid arthritis. N Engl J Med. 2017;376(7):652–662.
30. Dougados M, van der Heijde D, Chen YC, et al. Baricitinib in patients with inadequate response or intolerance to conventional synthetic DMARDs: results from the RA-BUILD study. Ann Rheum Dis. 2017;76(1):88–95.
31. Genovese MC, Kremer J, Zamani O, et al. Baricitinib in patients with refractory rheumatoid arthritis. N Engl J Med. 2016;374(13):1243–1252.
32. Simpson EL, Lacour JP, Spelman L, et al. Efficacy and safety of baricitinib in moderate to severe atopic dermatitis: results of two Phase 3 monotherapy randomized, double-blind, placebo controlled 16-week trials (BREEZE-AD1 and BREEZE-AD2. Poster EADV; 2018.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
