Back to Journals » International Journal of General Medicine » Volume 14
Procedural Results and Long-Term Outcomes of Percutaneous Coronary Intervention for in-Stent Restenosis Chronic Total Occlusion Compared with de novo Chronic Total Occlusion
Authors Tang G , Zheng N , Yang G , Li H, Ai H , Zhao Y, Sun F, Zhang H
Received 12 July 2021
Accepted for publication 31 August 2021
Published 15 September 2021 Volume 2021:14 Pages 5749—5758
DOI https://doi.org/10.2147/IJGM.S328332
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Guodong Tang, Naixin Zheng, Guojian Yang, Hui Li, Hu Ai, Ying Zhao, Fucheng Sun, Huiping Zhang
Department of Cardiology, Beijing Hospital, National Center of Gerontology, Institute of Geriatric Medicine, Chinese Academy of Medical Sciences, Beijing, 100730, People’s Republic of China
Correspondence: Huiping Zhang
Department of Cardiology, Beijing Hospital, National Center of Gerontology, Institute of Geriatric Medicine, Chinese Academy of Medical Sciences, No. 1 DaHua Road, Dong Dan, Beijing, 100730, People’s Republic of China
Tel +86 13911879076
Email [email protected]
Background: In-stent restenosis (ISR) chronic total occlusion (CTO) represents a challenging subgroup for revascularization of CTO by percutaneous coronary intervention (PCI). There are limited data on the treatment and outcomes of PCI for ISR CTO.
Objective: We aimed to evaluate the procedural results and 2-year outcomes of PCI for ISR CTO compared with de novo CTO.
Methods: Patients undergoing attempted CTO PCI between January 2017 and December 2019 were prospectively enrolled. We analyzed the procedural results and 2-year major adverse cardiac events (MACE) in patients undergoing ISR CTO and those undergoing de novo CTO PCI.
Results: A total of 426 patients undergoing 484 consecutive CTO PCI (ISR CTO PCI, n=84; de novo CTO, n=400) were enrolled during the study period. Patients undergoing de novo CTO PCI had a significantly greater syntax score than those undergoing ISR CTO PCI [23.0 (17.5, 30.5) vs 21.5 (14.5, 27.0), p=0.039]. Technical (73.8% vs 79.0%, p=0.296) and procedural (73.8% vs 78.0, p=0.405) success rates, as well as the incidence of major procedural complications (1.2% vs 2.3%, p=0.842), were comparable between the two groups. After a median follow-up of 20 months, patients who underwent ISR CTO PCI had a significantly higher incidence of MACE (33.3% vs 10.3%, p< 0.001), mainly attributed to the higher TVR rates (24.7% vs 7.6%, p< 0.001). ISR CTO was the only independent predictor of MACE (hazard ratio, 4.124; 95% confidence interval, 1.951– 8.717; p< 0.001) during follow-up in patients who underwent CTO PCI.
Conclusion: ISR CTO PCI shows comparable technical and procedural success, as well as major procedural complications compared with de novo CTO PCI. However, patients who underwent ISR CTO PCI had a significantly worse prognosis than those who underwent de novo CTO PCI, in terms of MACE, driven by TVR. ISR CTO was the only independent predictor of MACE during the follow-up.
Keywords: percutaneous coronary intervention, in-stent restenosis, de novo, chronic total occlusion
Introduction
The existing literature has suggested an association between recanalization of chronic total occlusion (CTO) by percutaneous intervention and its beneficial effects on clinical outcomes.1–5 Meanwhile, CTO percutaneous coronary intervention (PCI) has undergone a remarkable improvement in procedural success during the past two decades.6 CTO secondary to in-stent restenosis (ISR) is not rare, and it is considered as a consequence of late thrombotic stenosis, in-stent atherosclerosis, or endothelium hyperproliferation.7,8 The incidence of ISR CTO has been reported to be 11–12% among all CTOs undergoing PCI.9–11
Among CTO PCIs, ISR CTO PCI is thought to be the most challenging. There are limited published data reporting suboptimal procedural success rates in ISR CTO PCI resulting from specific procedural challenges, including the presence of stent underexpansion or fracture, presence of more than one layer of stent struts, heterogeneous neoatherosclerosis of the stented segment, rather than intimal proliferation alone. The present study aimed to investigate the procedural results and clinical outcomes of PCI for ISR CTO compared with de novo CTO.
Methods
Study Population
From January 2017 to December 2019, a total of 426 patients undergoing 484 consecutive CTO PCI procedures (84 ISR CTO PCI in 69 patients and 400 de novo CTO PCI in 357 patients, respectively) were prospectively enrolled in this study. Patients were divided into the ISR CTO and de novo CTO groups based on the type of CTO. CTO PCIs were performed electively and were clinically indicated according to the presence of angina or myocardial ischemia. The study was approved by the Institutional Ethics Committee, and all patients signed informed consent to undergo the interventional procedure. All patients were treated with 100–300 mg of aspirin and a loading dose of 300 mg of clopidogrel or 180 mg of ticagrelor before the PCI procedure. Aspirin (100 mg/day) was prescribed indefinitely, and clopidogrel (75 mg/day) or ticagrelor (90 mg two times daily) was continued for at least 12 months after the stents were implanted. Baseline characteristics, procedural, and hospitalization information were documented.
Angiographic and Intervention Procedural Variables
All variables regarding angiographic and interventional techniques were recorded. CTO was defined as complete occlusion with thrombolysis in myocardial infarction (TIMI) anterograde flow grade 0 in a major epicardial coronary artery or its main branch (diameter ≥ 2 mm), and an estimated occlusion duration of at least 3 months. ISR CTO was defined as CTO occurring within a previously implanted stent or occlusive segments within 5 mm proximal or distal to the stent edges. The occlusion duration was estimated according to the onset of symptoms, myocardial infarction (MI) history for the same target vessel territory, comparison with a previous coronary angiogram, or changes in electrocardiographic finding. The Japanese-Chronic Total Occlusion (J-CTO) score was calculated for each CTO lesion, and a J-CTO score ≥ 3 was considered a complicated CTO.12 Collaterals were graded by the filling degree of the involved vessel beyond the CTO segment according to the Rentrop classification; Rentrop grade 3 was considered a good collateral.13 Three-vessel coronary disease was defined as stenosis ≥ 75% of the diameter of 3 major epicardial coronary arteries or their main branch, and left main (LM) disease was defined as LM stenosis ≥ 50% of the diameter. The syntax score was calculated for each patient to assess the severity of coronary artery disease.14
An anterograde approach by antegrade wire escalation (AWE) or antegrade dissection/re-entry (ADR) was the first option for crossing CTO lesions. If the anterograde approach failed, a retrograde approach by retrograde wire escalation or retrograde dissection/re-entry was adopted. Technical success was defined as the achievement of residual stenosis < 50% in the target lesion with antegrade TIMI flow grade 3 in the CTO vessel.15 Procedural success was defined as technical success without the occurrence of in-hospital adverse events, including all-cause death, tamponade requiring pericardiocentesis, MI, stroke, and repeat target vessel revascularization (TVR) with PCI or coronary artery bypass graft, during the index hospitalization. TVR was defined as any repeat percutaneous intervention or surgical bypass aimed at any segment of the target vessel, which included the original target CTO lesion and its proximal or distal segments. Stent thrombosis was defined according to the Academic Research Consortium criteria. All procedure-associated adverse events, including procedure-related death, stroke, periprocedural type 4aMI, major bleeding (bleeding requiring transfusion or vasopressors), coronary perforation with cardiac tamponade requiring intervention (pericardiocentesis or covered stent implantation), and contrast-induced nephropathy (increase in serum creatinine > 25% or > 0.5 mg/dL at 48 h post-procedure), were regarded as major procedural complications. Non-CTO lesion PCI was defined as PCI aimed at non-CTO lesions during the index procedure or at a staged procedure within 30 days after the index hospitalization. Complete revascularization (CR) was defined as successfully attempting all diseased lesions with ≥ 50% stenosis in major epicardial coronary vessels and their major branches (diameter ≥ 2 mm) during the index hospitalization at a staged procedure within 30 days after discharge from the index hospitalization.16
Clinical Follow-Up
Follow-up was performed through phone interview, review of medical records, or outpatient visits. The 6-month, 1-year, and 2-year follow-up data were obtained by patient interviews. The primary endpoint in this study was the incidence of major adverse cardiac events (MACE) at the 2-year follow-up. MACE was defined as the composite of cardiac death, target-vessel MI, and ischemia-driven TVR. Routine angiographic follow-up assessment was not mandatory, but the angiographic evaluation was performed in cases showing repeat symptomatic occurrence or presenting abnormal findings in non-invasive tests for myocardial ischemia or left ventricular systolic function.
Statistical Analysis
Continuous variables are presented as mean ± standard deviation (SD) or median (interquartile range), as appropriate. Categorical variables are expressed as numbers and percentages. The Chi-square test or Fisher’s exact test was used for the comparison of categorical variables. The Student’s t-test or Mann–Whitney rank-sum test was used to evaluate the differences among continuous variables according to their distributions. Cumulative MACE curves were generated using the Kaplan-Meier method, and the differences between groups were evaluated by the Log rank test. A multivariable analysis using a Cox regression model expressed as hazard ratio (HR) with 95% confidence interval (CI) was conducted to determine predictors of MACE. Variables showing a p<0.10 in univariate analysis or suggested to be related to the outcome of interest according to clinical consideration were adopted as candidate predictors for multivariate analysis. The c-statistic and goodness-of-fit with Hosmer and Lemeshow test were used to determine model discrimination. A 2-tailed p<0.05 was considered statistically significant for all tests. Statistical analyses were performed using the SPSS version 20 (IBM Corp., Armonk, NY, USA).
Results
Baseline Demographic Variables
A total of 426 patients undergoing 484 consecutive CTO PCI were enrolled during the study period. The prevalence of ISR CTO PCI was therefore 17.4%. The clinical characteristics of the entire cohort are shown in Table 1. There were no significant differences between patients who underwent ISR CTO and those who underwent de novo CTO PCI with regard to age, sex, prevalence of cardiovascular risk factors, renal function, left ventricular ejection fraction, low-density lipoprotein cholesterol (LDL-C), and medication. Participants with ISR CTO had a higher prevalence of previous MI (58.0% vs 17.9%, p<0.001) than those with de novo CTO. The proportion of patients who were clinically diagnosed with silent ischemia without symptoms was significantly higher in the de novo CTO group than that in the ISR CTO group (9.5% vs 0%, p=0.002).
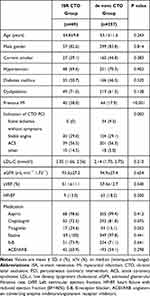 |
Table 1 Baseline Clinical Characteristics |
Angiographic Characteristics
The angiographic characteristics for both groups are listed in Table 2. The prevalence of LM disease, three-vessel disease, and LM plus three-vessel coronary disease was similar in both groups and presented with similar distribution in CTO location. Patients who underwent de novo CTO PCI had a greater syntax score than those who underwent ISR CTO [23.0 (17.5, 30.5) vs 21.5 (14.5, 27.0), p=0.039]. There were no significant differences between the two groups regarding the proportion of the target CTO vessel and branch vessel CTO. J-CTO score and the proportion of the J-CTO score ≥ 3 (17.9% vs 9.3%, p=0.021) were higher in the ISR CTO group than those in the de novo CTO group with less prevalence of Rentrop grade 3 (32.1% vs 48.5%, p=0.006). The prior attempt rates of target CTO were comparable between both groups.
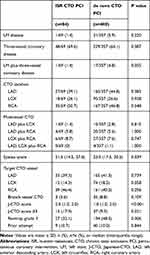 |
Table 2 Angiographic Characteristics |
Procedural Characteristics
Detailed procedural characteristics are also shown in Table 3. The rates of radial artery access were similar between the two groups. Dual injection was more frequently performed in patients who underwent de novo CTO interventions (32.5% vs 16.7%, p=0.004) with a trend for lower usage of 7Fr catheter. The retrograde approach was less often used in both groups (4.8% vs 6.5%, p=0.548). The incidences of cases involving intravascular ultrasound (IVUS) procedures were relatively low in the two groups (17.9% vs 15.0%, p=0.511). Technical (73.8% vs 79.0%, p=0.296) and procedural (72.6% vs 77.5%, p=0.405) success rates were comparable between both groups. The percentage of drug-eluting stent implantation was significantly higher in de novo CTO PCI than that in ISR CTO PCI (79.4% vs 48.4%, p<0.001), whereas drug-coated balloon (DCB) was frequently used following ISR CTO recanalization (51.6% vs 20.6%). There were no differences in the amount of contrast volume and fluoroscopy time during CTO PCI between the two groups. There were more patients in the de novo CTO group that underwent non-CTO lesion PCI compared with those in the ISR CTO group (40.6% vs 26.1%, p=0.023). There was no significant difference between the ISR CTO group and the de novo CTO group concerning the CR rates (58.0% vs 49.3%, p=0.187). The incidence of major procedural complications was also similar between both groups (1.2% vs 2.3%, p=0.842). Particularly, only one perforation with tamponade was observed in the ISR CTO group. No acute stent thrombosis or in-hospital death was observed in patients who underwent ISR CTO PCI, while three cases of acute stent thrombosis and two cases of in-hospital death occurred in patients who underwent de novo CTO PCI.
 |
Table 3 Procedural Characteristics |
Clinical Outcomes on Follow-Up
Table 4 shows clinical outcomes based on a 2-year follow-up. Clinical follow-up was available for 416 of 426 (97.7%) patients. The median follow-up length was 20 months (interquartile range: 10–40). There were more patients receiving successful CTO recanalization who underwent repeated coronary angiography during the follow-up period in the ISR CTO group (47.0% vs 32.0%, p=0.019), with a higher re-occlusion rate than that in the de novo CTO group (22.6% vs 8.9%, p=0.078). Target vessel MI rate was comparable in both groups (3.0% vs 2.0%, p=0.947). No significant difference was observed regarding cardiac death (1.5% vs 0.9%, p=0.500). Patients who underwent ISR CTO PCI had a significantly higher incidence of MACE (33.3% vs 10.3%, p<0.001), a finding that was mainly driven by TVR (24.7% vs 7.6%, p<0.001). Figures 1 and 2 show 2-year curves for the probability of MACE and TVR.
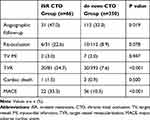 |
Table 4 Clinical Outcomes on 24-Month Follow-Up |
Predictors of 2-Year MACE in Patients Who Underwent CTO PCI
Candidate predictors in the univariate analysis included age, hypertension, diabetes mellitus, previous MI, LDL-C, estimated glomerular filtration rate (eGFR), number of diseased vessels, Syntax score, J-CTO score, prior attempt, crossing strategy, IVUS use, CR, major procedural complications, and ISR CTO. Final variables entered into the Cox regression model were previous MI, eGFR, Syntax score, J-CTO score, crossing strategy, CR, major procedural complications, and ISR CTO. Table 5 shows multivariate predictors of MACE based on the follow-up of patients who underwent CTO PCI. The multivariate analysis for MACE showed that ISR CTO (HR: 4.124, 95% CI: 1.951–8.717, p<0.001) is the only strong independent predictor for patients who underwent CTO PCI.
 |
Table 5 Independent Predictors of MACEs in Patients Who Underwent CTO PCI |
Discussion
The main findings of our study are the following: 1) ISR CTO PCI accounted for approximately 18% of attempted CTO PCI during the study period; 2) Technical and procedural success, CR, as well as major procedural complications rates, were comparable between ISR CTO and de novo CTO groups despite a higher J-CTO score in patients who underwent ISR CTO PCI; 3) Patients who underwent ISR CTO PCI had a significantly increased incidence of MACE, mainly attributed to the higher TVR rates; and 4) After adjusting for differences in clinical, angiographic, and procedural variables, ISR CTO was the only independent predictor of MACE in patients who underwent CTO PCI.
From the stent era restenosis, ISR CTO has been a challenging issue and is often not a rare situation. The prevalence of ISR CTO PCI ranged from 5–25% across all CTO PCIs in a series of studies,14,16,17 being similar to our current findings (17.4%). The proportion varies in different centers in accordance with PCI volume and the operator’s experience. In-stent restenosis more likely occurred in patients with diabetes, and the rate of complication due to diabetes mellitus might be higher among patients who underwent ISR CTO than those who underwent de novo CTO procedures.11,14 However, this result was not observed in our study. In the present study, all patients who underwent ISR CTO PCI received PCI for established diagnosis, while approximately 10% of patients in the de novo CTO group underwent percutaneous intervention for the indication of silent ischemia without symptoms. This implied a confounding association between CTO patient’s clinical manifestation and the indication of CTO PCI.
Compared with de novo CTO lesion, ISR CTO lesion features some peculiarities, including developed neoatherosclerosis and neointimal hyperplasia, which more likely occur when stent fracture or underexpansion is present.9,18 Furthermore, repeat in-stent micro-thrombosis associated with incomplete endothelialization might be involved in the formation of the ISR CTO.19 Regarding ISR CTO PCI, the previously implanted stent can provide a contour profile of the target vessel; thus, it facilitates finding the precise vessel route compared with de novo CTO PCI. In addition, a prior stent may prevent the target vessel dissection. Despite the technical advantages involved, ISR CTO represents a challenging subgroup for revascularization of CTO by PCI.
In our study, patients who underwent ISR CTO had a significantly higher proportion of the J-CTO score ≥ 3 than those who underwent de novo CTO PCI. In the early stage, PCI for ISR CTO was usually related to suboptimal procedural results. The success rates of ISR CTO PCI in the earlier period ranged between 63% and 71%.17,20 In Werner’s study, a lower success rate of approximately 70% in ISR-CTO PCI compared with 85% in de novo CTO PCI was reported.7 The most common reasons for failure in ISR CTO PCI were the inability of the wire to penetrate the stented occlusion segment and inability to advance the microcatheter or fully dilate the balloon. The prior stent struts might interfere with the wire and the microcatheter, which may be hindered by trapping in the struts of the prior stent, occasionally in more than one layer of stent struts.16 Even subintimal crossing of the stented occlusion segment, subintimal tracking, and true lumen re-entry could be performed with difficulty in cases of ISR CTO. In addition, even after balloon crossing, optimal balloon expansion may be difficult to achieve as a common result of the underlying underexpanded stent.17 All the above factors decreased the probability of succeeding recanalization of the ISR CTO.
In our study, technical and procedural success rates were 79.0% and 77.5% in the de novo CTO PCI group, 73.8% and 72.6% in the ISR CTO group, respectively. The percentages in the ISR CTO PCI group were aligned with that reported in previous literature but lower than in recent studies since 2017 (82.4–86.5%) using dedicated ADR devices, such as the CrossBoss or the combination of the Stingray catheter.9,16 The use of CrossBoss was reported to be associated with a shorter crossing time and a success rate even reaching 90%, as it could enable more effective true-to-true occlusion crossing by active dissection within the stented segment due to its higher crossing profile while avoiding the device entering to the subadventitial space.21,22 However, during the study period, CrossBoss and the Stingray catheter were unavailable in our center.
Technological progress in combination with the implementation of the hybrid algorithm in CTO PCI has achieved a sustained improvement in procedural success in recent years.2 For most CTO lesions, the antegrade approach, including AWE and ADR, was frequently adopted as the first choice to recanalize the CTO lesion, and in most situations, it could reach successful recanalization, especially for the cases with a tapered stump and without prior attempt. It was reported that the retrograde approach may contribute to improving the success rate in CTO interventional practice. In the PROGRESS-CTO registry, the retrograde approach was used in approximately one third of procedures in participants who underwent ISR-CTO PCI with a procedural success rate reaching 86%.14 While the fact that ISR CTOs were more likely to lack good collateral channels, or the visualized collaterals appeared extreme tortuosity, made it hard to be perceived as an interventional approach.11 In the ISR CTO group of the present study, the prevalence of Rentrop grade 3 was lower than in the de novo CTO group. As for selecting epicardial collaterals to perform retrograde CTO PCI, even for highly experienced operators using a complex technique, it may be a challenging process. In addition, retrograde CTO PCI was associated with a lower success rate and a higher risk for acute and long-term adverse events than antegrade CTO PCI.23 The retrograde approach was less used (4.8–6.5%) in the CTO PCI procedure in our study. This might reflect the discrepancy in success rate as compared to studies employing the retrograde approach more frequently.9,14
The incidence of major procedural complications was comparable between patients who underwent ISR CTO and those who underwent de novo CTO in our study, whereas the former participants had a markedly increased rate of MACE, mainly driven by a higher rate of TVR, which is consistent with prior reports.9,11 ISR was associated with a less benign clinical outcome with respect to recurrence of unstable angina, even MI often requiring re-hospitalization to receive repeat angiography.10,24,25 In our adjusted analysis for various related variables, ISR CTO was identified as the only independent predictor of MACE with a >4-fold risk increase (HR: 4.124, 95% CI: 1.951–8.717, <0.001) in patients undergoing CTO PCI. Indeed, ISR CTO was classified as a subgroup of in-stent restenosis, and it has been demonstrated that PCI for in-stent restenosis was a predictor of future adverse events.26 For ISR CTO, the situation appears more complicated given the ISR occlusion complexity.
Remarkably, de la Torre et al reported that 40% of recurrent restenosis were re-occluded after successful ISR CTO PCI.16 The re-occlusion rate tended to be higher in the ISR CTO group than in the de novo CTO group in our study, although the angiographic follow-up rates in participants who received successful CTO recanalization were less than 50% in both groups. The treatment for successful ISR CTO recanalization in the present study mainly included re-stenting and use of DCB. It was assumed that multi-layered stenting might generate abnormal vessel reactions and be associated with thrombus formation. Additionally, stent recoiling resulting from multi-stent layers might increase the risk of underexpansion.10 Therefore, DCB treatment for recanalization of ISR CTO might not adequately prevent restenosis progression. Optimal PCI treatment for ISR CTO deserves further investigation in future studies.
Study Limitations
There are several limitations to our study. First, this was an observational study performed in one single center. Not all the CTO lesions had undergone attempted percutaneous revascularization. The decision to perform CTO PCI was at the discretion of the interventionist, which would result in selection bias. Although we performed a risk-adjusted model to control for the confounding factors, we were not able to correct for the potential bias completely. Second, the success rates of CTO PCI in this registry were lower than those reported in top centers with high skilled operators (less than 80% vs 86%). CTO intervention techniques have undergone a sustained improvement across the study period; meanwhile, more dedicated CTO equipment is available for use nowadays. Therefore, the results of the study should be interpreted in certain PCI settings where the experience of percutaneous treatment for CTO lesion is more variable in diverse centers with different levels. However, our results are representative of the average practice. Third, the retrograde approach was less adopted in the present study. This reflects the disparity between the hybrid algorithm and daily practice in CTO PCI. However, the major procedural complication rates in our study, in which the antegrade approach was used in a larger proportion of the participants, were relatively lower than those in studies employing the retrograde approach more frequently. Considering the complexity and the peculiarities of the ISR CTO, the role of the retrograde approach for the treatment of ISR CTO deserves further investigation. Fourth, in our study, we used the J-CTO score as the predictive of CTO PCI success. While the J-CTO score was not specifically derived for ISR CTO, it was obtained from overall CTO lesions. A dedicated score system considering the complexity of ISR CTO validated to have predictive value in successful CTO PCI might be required. Fifth, the rates of IVUS use in both groups were relatively low in this registry, which was mainly due to cost-related issues. It was reported that IVUS can provide rich information on the mechanisms underlying ISR CTO formation, help understand the nature of occlusions, such as proliferation alone, neoatherosclerosis, or calcification distribution in the occluded segment. In addition, IVUS can be applied to guide wire crossing and to optimize stent implantation to decrease the risk of stent thrombosis or target-lesion revascularization (TLR).27 Sixth, the rate of non-CTO lesion PCI was higher in the de novo CTO group than in the ISR CTO group. As a result, the CR rate was less than 60% in the ISR CTO group and 50% in the de novo CTO group. It has been shown that less satisfactorily achieved CR was associated with an unfavorable survival benefit.28 However, it could be hard to reach an ideal CR rate in real world, and our study was representative of daily real-world practice. Finally, because angiographic evaluation during follow-up was not mandatory, only those patients presenting with recurrence of angina and/or new emerging ischemia evidence received repeat angiography, followed by subsequent TVR when indicated. It was difficult to perform routine angiography for asymptomatic patients, which might result in underestimating the rate of the target vessel failure. Although participants who underwent ISR CTO received treatment for CTO lesion in our study, the angiographic evaluation rate (47.0%) during follow-up was relatively higher than that in similar literature.
Conclusions
ISR CTO PCI was relatively frequent in contemporary practice with comparable technical and procedural success, as well as major procedural complications compared with de novo CTO PCI; however, ISR CTO PCI seemed more complex as shown by a high J-CTO score. Patients who underwent ISR CTO PCI had a significantly worse prognosis than those who underwent de novo CTO PCI, in terms of MACE, driven by TVR. ISR CTO was the only independent predictor of MACE based on the follow-up of patients who underwent CTO PCI. Special consideration should be taken regarding the strategy and algorithm of the recanalization of ISR CTO in future clinical practice.
Abbreviations
ISR, in-stent restenosis; CTO, chronic total occlusion; PCI, percutaneous coronary intervention; MACE, major adverse cardiac events; MI, myocardial infarction; TVR, target vessel revascularization; J-CTO, Japanese-Chronic Total Occlusion; LM, left main; CABG, coronary artery bypass graft; CR, Complete revascularization; HR, hazard ratio; AWE, antegrade wire escalation; ADR, antegrade dissection and re-entry; DCB, drug-coated balloon; TLR, target-lesion revascularization.
Data Sharing Statement
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
The study was conducted in accordance with the Declaration of Helsinki. The study was approved by the Institutional Review Board of Beijing Hospital, all patients signed informed consent to undergo coronary angiography and intervention procedure and to participate the registry (Approval No. 2019BJYYEC-022-02).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The present study was supported by Beijing Municipal Science & Technology Commission No. Z171100001017202.
Disclosure
The authors declare that they have no competing interests.
References
1. Ladwiniec A, Allgar V, Thackray S, et al. Medical therapy, percutaneous coronary intervention and prognosis in patients with chronic total occlusions. Heart. 2015;101:1907–1914. doi:10.1136/heartjnl-2015-308181
2. Wilson WM, Walsh SJ, Bagnall A, et al. One-year outcomes after successful chronic total occlusion percutaneous coronary intervention: the impact of dissection re-entry techniques. Catheter Cardiovasc Interv. 2017;90:703–712. doi:10.1002/ccd.26980
3. Valenti R, Migliorini A, De Gregorio MG, et al. Impact of complete percutaneous revascularization in elderly patients with chronic total occlusion. Catheter Cardiovasc Interv. 2020;95:145–153. doi:10.1002/ccd.28452
4. Pinto G, Fragasso G, Gemma M, et al. Long-term clinical effects of recanalization of chronic coronary total occlusions in patients with left ventricular systolic dysfunction. Catheter Cardiovasc Interv. 2020;96:831–838. doi:10.1002/ccd.28850
5. Teramoto T, Tsuchikane E, Yamamoto M, et al. Successful revascularization improves long-term clinical outcome in patients with chronic coronary total occlusion. Int J Cardiol Heart Vasc. 2017;14:28–32.
6. Azzalini L, Vo M, Dens J, Agostoni P. Myths to debunk to improve management, referral, and outcomes in patients with chronic total occlusion of an epicardial coronary artery. Am J Cardiol. 2015;116:1774–1780. doi:10.1016/j.amjcard.2015.08.050
7. Werner GS, Moehlis H, Tischer K. Management of total restenotic occlusions. EuroIntervention. 2009;5(SupplD):D79–D83.
8. Andreou I, Stone PH. In-stent atherosclerosis at a crossroads: neoatherosclerosis … or paleoatherosclerosis? Circulation. 2016;134:1413–1415. doi:10.1161/CIRCULATIONAHA.116.025129
9. Azzalini L, Dautov R, Ojeda S, et al. Procedural and long-term outcomes of percutaneous coronary intervention for in-stent chronic total occlusion. JACC Cardiovasc Interv. 2017;10:892–902. doi:10.1016/j.jcin.2017.01.047
10. Lee SH, Cho JY, Kim JS, et al. A comparison of procedural success rate and long-term clinical outcomes between in-stent restenosis chronic total occlusion and de novo chronic total occlusion using multicenter registry data. Clin Res Cardiol. 2020;109:628–637. doi:10.1007/s00392-019-01550-7
11. Lamelas P, Padilla L, Abud M, et al. In-stent chronic total occlusion angioplasty in the LATAM-CTO registry. Catheter Cardiovasc Interv. 2021;97:E34–E39. doi:10.1002/ccd.28937
12. Morino Y, Abe M, Morimoto T, et al. Predicting successful guidewire crossing through chronic total occlusion of native coronary lesions within 30 minutes: the J-CTO (Multicenter CTO Registry in Japan) score as a difficulty grading and time assessment tool. JACC Cardiovasc Interv. 2011;4:213–221. doi:10.1016/j.jcin.2010.09.024
13. Serruys PW, Morice MC, Kappetein AP, et al. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med. 2009;360:961–972. doi:10.1056/NEJMoa0804626
14. Christopoulos G, Karmpaliotis D, Alaswad K, et al. The efficacy of “hybrid” percutaneous coronary intervention in chronic total occlusions caused by in-stent restenosis: insights from a US multicenter registry. Catheter Cardiovasc Interv. 2014;84:646–651. doi:10.1002/ccd.25465
15. Hannan EL, Racz M, Holmes DR, et al. Impact of completeness of percutaneous coronary intervention revascularization on long-term outcomes in the stent era. Circulation. 2006;113:2406–2412. doi:10.1161/CIRCULATIONAHA.106.612267
16. de la Torre Hernandez JM, Rumoroso JR, Subinas A, et al. Percutaneous intervention in chronic total coronary occlusions caused by in-stent restenosis: procedural results and long-term clinical outcomes in the TORO (Spanish registry of chronic TOtal occlusion secondary to an occlusive in-stent RestenOsis) multicentre registry. EuroIntervention. 2017;13:e219–e226.
17. Abbas AE, Brewington SD, Dixon SR, et al. Success, safety, and mechanisms of failure of percutaneous coronary intervention for occlusive non-drug-eluting in-stent restenosis versus native artery Total occlusion. Am J Cardiol. 2005;95:1462–1466. doi:10.1016/j.amjcard.2005.01.098
18. Nakazawa G, Otsuka F, Nakano M, et al. The pathology of neoatherosclerosis in human coronary implants bare-metal and drug-eluting stents. J Am Coll Cardiol. 2011;57:1314–1322. doi:10.1016/j.jacc.2011.01.011
19. Cheneau E, Pichard AD, Stabile E, et al. Asymptomatic late stent thrombosis after sirolimus stent implantation. Cardiovasc Radiat Med. 2004;5:57–58.
20. Abdel-Karim AR, Lombardi WB, Banerjee S, et al. Contemporary outcomes of percutaneous intervention in chronic total coronary occlusions due to in-stent restenosis. Cardiovasc Revasc Med. 2011;12:170–176. doi:10.1016/j.carrev.2010.08.002
21. Wilson WM, Walsh S, Hanratty C, et al. A novel approach to the management of occlusive in-stent restenosis (ISR). EuroIntervention. 2014;9:1285–1293. doi:10.4244/EIJV9I11A218
22. Karacsonyi J, Tajti P, Rangan BV, et al. Randomized comparison of a CrossBoss first versus standard wire escalation strategy for crossing coronary chronic total occlusions: the CrossBoss First Trial. JACC Cardiovasc Interv. 2018;11:225–233. doi:10.1016/j.jcin.2017.10.023
23. Megaly M, Ali A, Saad M, et al. Outcomes with retrograde versus antegrade chronic total occlusion revascularization. Catheter Cardiovasc Interv. 2020;96:1037–1043. doi:10.1002/ccd.28616
24. Rathore S, Kinoshita Y, Terashima M, et al. A comparison of clinical presentations, angiographic patterns and outcomes of in-stent restenosis between bare metal stents and drug eluting stents. EuroIntervention. 2010;5:841–846. doi:10.4244/EIJV5I7A141
25. Magalhaes MA, Minha S, Chen F, et al. Clinical presentation and outcomes of coronary in-stent restenosis across 3-stent generations. Circ Cardiovasc Interv. 2014;7:768–776. doi:10.1161/CIRCINTERVENTIONS.114.001341
26. Singh M, Gersh BJ, McClelland RL, et al. Clinical and angiographic predictors of restenosis after percutaneous coronary intervention: insights from the Prevention of Restenosis with Tranilast and Its Outcomes (PRESTO) trial. Circulation. 2004;109:2727–2731. doi:10.1161/01.CIR.0000131898.18849.65
27. Hong SJ, Kim BK, Shin DH, et al. Usefulness of intravascular ultrasound guidance in percutaneous coronary intervention with second-generation drug-eluting stents for chronic total occlusions (from the Multicenter Korean-Chronic Total Occlusion Registry). Am J Cardiol. 2014;114:534–540. doi:10.1016/j.amjcard.2014.05.027
28. Goel PK, Khanna R, Pandey CM, et al. Long-term outcomes post chronic total occlusion intervention-implications of completeness of revascularization. J Interv Cardiol. 2018;31:293–301. doi:10.1111/joic.12480
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


