Back to Journals » Infection and Drug Resistance » Volume 12
Procalcitonin-guided antibiotic discontinuation in ventilator-associated pneumonia: a prospective observational study
Authors Wang Q , Hou D , Wang J, An K, Han C, Wang C
Received 15 October 2018
Accepted for publication 23 February 2019
Published 10 April 2019 Volume 2019:12 Pages 815—824
DOI https://doi.org/10.2147/IDR.S190859
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Joachim Wink
Qiushi Wang,1,2 Dapeng Hou,2 Jing Wang,3 Kai An,2 Chenghe Han,2 Chunting Wang1
1Department of Intensive Care Unit, Shandong Provincial Hospital Affiliated to Shandong University, Jinan, Shandong, People’s Republic of China; 2Department of Intensive Care Unit, Affiliated Hospital of Taishan Medical University, Taian, Shandong, People’s Republic of China; 3Department of Respiratory Medicine, Affiliated Hospital of Taishan Medical University, Taian, Shandong, People’s Republic of China
Background: Procalcitonin (PCT), an important biomarker, can be used for the guidance of antibiotic therapy in respiratory infection. However, it has been a problem that some patients might need antibiotic therapy restart because of infection recurrence after antibiotic discontinuation. To date, there are very few literature on the study of risk factors accounting for infection recurrence. Purpose of this clinical study: 1) To study on antibiotic discontinuation in ventilator-associated pneumonia (VAP) under the guidance of PCT; 2) To evaluate the possible risk factors leading to infection recurrence and antibiotic reuse.
Methods: Antibiotic discontinuation was performed when patients met the following criteria: (i) serum PCT<0.5 μg/L, (ii) temperature<38.5℃ and (iii) leukocyte count<15×109/L. Next, the patients were divided into infection recurrence group (infection recurring within 7 days after antibiotic discontinuation) or infection controlled group (no infection recurring after antibiotic discontinuation). Possible risk factors accounting for infection recurrence were evaluated using logistic regression analysis.
Results: Of the eligible 51 patients with VAP, 20 patients suffered infection recurrence. Clinical pulmonary infection score (CPIS) and characteristics of tracheal secretions were the independent risk factors (P=0.045 and P=0.041, respectively), accounting for infection recurrence. Simplified CPIS≥5 served a certain predictive value for infection recurrence in VAP when physicians considered antibiotic discontinuation (The area under the receiver operating characteristic curve 0.781, specificity 90.3%, sensitivity 55.0%, positive predictive value 78.6% and negative predictive value 75.7%). At the time of antibiotic discontinuation, differences between the two groups were not statistically significant in the proportion of patients with a tracheotomy and in the culture results of endotracheal aspirates (including semi-quantitative results and whether pathogens were multidrug-resistant [MDR] strains).
Conclusion: Simplified CPIS and characteristics of tracheal secretions can be used to predict infection recurrence following PCT-guided antibiotic discontinuation in VAP. These findings are important because physicians may not need to put too much care on semi-quantitative culture results of endotracheal aspirates and whether pathogens are MDRstrains.
Trial registration: The registration number of this clinical trial is: ChiCTR-OPC-17011228 (Trial registry name: Chinese Clinical Trial Registry; URL: http://www.chictr.org.cn).
Keywords: ventilator-associated pneumonia, procalcitonin, antibiotic therapy, antibiotic discontinuation, infection recurrence
Introduction
Ventilator-associated pneumonia (VAP) refers to pneumonia that occurs in patients receiving more than 48 hrs of mechanical ventilation via tracheal intubation or tracheotomy. Severe underlying diseases of VAP patients along with serious resistance of the pathogens resulted in prolonged antibiotic therapy. More importantly, both the recurrence rate and mortality of VAP are high, with the recurrence rate of 25–50% and the mortality of 14–50%.1,2 In addition to clinical features and microbial cultures, clinical pulmonary infection score (CPIS), C-reactive protein and procalcitonin (PCT) can be applied for assessing the duration and response of antibiotic therapy.3–5 According to the 2016 Clinical Practice Guidelines for Management of hospital-acquired pneumonia (HAP) and VAP by IDSA and ATS, PCT levels plus clinical criteria can be used to guide the discontinuation of antibiotic therapy (weak recommendation, low-quality of evidence).6 However, according to the recent ERS/ESICM/ESCMID/ALAT guidelines on HAP and VAP, routine measurement of serial serum PCT levels are not recommended to reduce the duration of the antibiotic course in patients with short anticipated duration (strong recommendation, moderate quality of evidence).7 Although the recommendations of the two guidelines are different, both of the two guidelines mentioned that there were few studies on serial serum PCT-guided discontinuation of antibiotic therapy for VAP. And in these studies,8,9 PCT-guided antibiotic therapy led to a high proportion of infection recurrence. To address this important issue, and to further optimize the antibiotic therapy for VAP, we have investigated risk factors that correlate with the recurrence of VAP after PCT-guided antibiotic discontinuation.
Methods
Study design and participants
This PCT-guided antibiotic discontinuation study was a prospective observational study in VAP patients requiring treatment at the intensive care unit (ICU) of a university hospital in China, performed between February 2016 and April 2017. Antibiotic therapy was given to patients according to clinical features, PCT concentration and microbial cultures, with antibiotic discontinuation guided by PCT concentration. According to both pulmonary infection recurrence and antibiotic reuse within 7 days after antibiotic discontinuation, patients were divided into the infection recurrence group and the infection controlled group, namely, the unsuccessful discontinuation group and the successful discontinuation group. This study was conducted in accordance with the Declaration of Helsinki. This study was approved by the ethics committee of the Affiliated Hospital of Taishan Medical University. The committee’s reference number is 2016002. All included patients or their legal representatives provided written informed consent.
Diagnostic criteria for VAP
With reference to guidelines for the prevention, diagnosis and treatment of VAP (2013) published by Society of Critical Care Medicine, Chinese Medical Association, the clinical diagnostic criteria for VAP was: new or persistent infiltrate on chest radiography associated with at least two of the following: purulent tracheal secretions, temperature >38℃ or <36℃, leukocyte count >10×109/L or <4×109/L. Physicians collected culture samples of endotracheal aspirates from patients who met the clinical diagnostic criteria, and then physicians combined the culture results and the clinical diagnostic criteria to give the diagnosis of VAP.10
Inclusion criteria
Eligible patients had to be at least 18 years of age, be admitted to the ICU, and met the diagnostic criteria for VAP. Patients were excluded if they had a coexisting extrapulmonary infection.
Exclusion criteria
1) Extrapulmonary infection occurred within 7 days after antibiotic discontinuation, including urinary infection, bloodstream infection, biliary tract infection and central nervous system infection, etc, 2) Drainage tubes of important viscera could not be removed within 14 days, such as ventricular drainage, lumbar cerebrospinal fluid drainage and pleural fluid drainage, etc, 3) Patients died, were discharged, or quit therapy, before antibiotic discontinuation or within 7 days after antibiotic discontinuation. 4) Patients or their legal representatives requested an early exit from the study. 5) After patients were transferred from the ICU to other departments, physicians in those departments did not approve of this study, changing the antibiotic regimen without any reason.
Antibiotic therapy
Most of the eligible patients had received some antibiotic therapy before inclusion to the study for the assumed VAP. Once meeting the diagnostic criteria for VAP, patients were given empiric antibiotic therapy by reference to local antimicrobial resistance surveillance, PCT concentration and simplified CPIS, etc. Then, sensitive antibiotics were selected according to the results of endotracheal aspirate cultures and antimicrobial susceptibility. During antibiotic therapy, PCT concentration was measured every other day, and chest imaging (CT or X-ray) was examined every 3–5 days. Antibiotics were discontinued when patients met the predefined criteria for antibiotic discontinuation. Chest imaging, endotracheal aspirate cultures and other laboratory tests were taken on the day of antibiotic discontinuation. Accordingly, antibiotic therapy would be started again when patients showed signs of infection recurrence and met the predefined criteria for infection recurrence. Initial therapy for the antibiotic restart was based on the results of endotracheal aspirate cultures taken on the day of antibiotic discontinuation, followed by sensitive antibiotic therapy based on the results of subsequent endotracheal aspirate cultures.
Criteria for antibiotic discontinuation
Patients reached each of the following three thresholds 48 hrs before the decision on antibiotic discontinuation: 1) The highest temperature<38.5 ℃; 2) leukocyte count>4.0×109/L and <15×109/L; 3) PCT<0.5 μg/L. And drainage tubes of important viscera had been removed.
Criteria for infection recurrence
New or persistent infiltrate on chest radiography associated with at least two of the following: purulent tracheal secretions, temperature >38°C or <36°C, leukocyte count >10×109/L or <4×109/L. It was considered infection relapse when pathogens of the subsequent endotracheal aspirate cultures were the same with the initially isolated pathogens before antibiotic discontinuation. It was considered superinfection when different pathogens were present in the subsequent endotracheal aspirate cultures.11 In our study, infection recurrence consisted of both infection relapse and superinfection. From the perspective of antibiotic therapy, antibiotic reuse is needed in both infection relapse and superinfection, both of which can be considered unsuccessful discontinuation, accordingly.
PCT measurement and antimicrobial susceptibility test
PCT (0.1–100 μg/L) was measured on an analyzer available at the ICU (Radiometer [Copenhagen, Denmark] AQT90 FLEX rapid immunoanalyser) that was maintained according to national quality standards. Pathogens were isolated from semi-quantitative cultures of endotracheal aspirates. The antimicrobial susceptibility of these pathogens was determined using AST-GP67 cards of VITEK-2 compact according to the manufacturer’s instructions [Bio-Merieux] by the agar diffusion method. MIC interpretations were based on 2012CLSI-M100-S22.12 Semi-quantitative reporting of microbial growth was based on Fujitani’s study.13
Simplified version of CPIS
Simplified version of the CPIS used in this study consisted of the following components:13 temperature, leukocyte count, tracheal secretions, oxygenation PaO2/FiO2 and chest radiography. On defining characteristics of tracheal secretions we referred to the same study:14 presence of moderate secretions=1 point; presence of large secretions=2 points; presence of large purulent secretions=3 points. Tracheal secretions were classified as few, moderate, large and purulent, and these data were obtained from the nurse report.
Statistical analysis
Measurement data of normal distribution were expressed as mean±SD; between-group differences were calculated using one-way ANOVA. Measurement data of abnormal distribution were expressed as median (interquartile range); between-group differences were calculated using Mann–Whitney U-test. Enumeration data were expressed as counts (%); between-group differences were calculated using a Chi-squared test or Fisher’s exact test. Logistic analysis was conducted on the statistically significant variables of univariate analyses to screen for independent risk factors. The independent risk factors were selected, and the receiver operating characteristic (ROC) curve was drawn with infection recurrence as the state variable to evaluate the predictive value. Diagnostic consistency was checked using Kappa analysis. All analyses were completed using SPSS, version17.0, with p-values of <0.05 deemed statistically significant.
Results
Baseline information of the patients
Eighty-five patients met the inclusion criteria including 34 patients who met the exclusion criteria, resulting in 51 patients enrolled in this study (Figure 1). According to both pulmonary infection recurrence and antibiotic reuse within 7 days after antibiotic discontinuation, patients were divided into the infection recurrence group (n=20; the unsuccessful discontinuation group) and the infection controlled group (n=31; the successful discontinuation group). Among the 20 patients in the infection recurrence group, 6 were patients of infection relapse and 14 were patients of superinfection. Baseline characteristics of the 51 patients were similar between the two groups (Table 1).
 | Table 1 Baseline information of the patients |
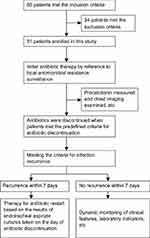 | Figure 1 Study profile. |
Information at the time of VAP diagnosis
At the time of VAP diagnosis, there were no significant differences between the two groups upon the presence of multidrug-resistant (MDR) bacteria and semi-quantitative culture results of endotracheal aspirates. Although the differences in PCT concentration at the time of VAP diagnosis were not significant, the simplified CPIS scores in infection recurrence group were significantly higher than those in the infection controlled group (P=0.005, Table 2).
 | Table 2 Information at the time of VAP diagnosis |
Information at the time of antibiotic discontinuation
At the time of reaching the criteria for antibiotic discontinuation, the differences between the two groups were not significant in PCT concentration, leucocyte count, plasma glucose, plasma albumin and prealbumin, blood urea nitrogen and serum creatinine (Table 3). Meanwhile, there were no statistically significant differences between the two groups upon the presence of MDR bacteria, semi-quantitative culture results of endotracheal aspirates, the highest temperature, APACHEⅡ score, Glasgow coma scale and days of antibiotic therapy (Tables 3 and 4). However, the proportion of purulent tracheal secretions was much higher in the infection recurrence group than that in the infection controlled group (40% vs 3.2%, Table 4). Furthermore, scores of simplified CPIS in the infection recurrence group were much higher than CPIS scores in the infection controlled group (P<0.001, Table 4).
 | Table 3 Laboratory indicators at the time of antibiotic discontinuation |
 | Table 4 Clinical indicators at the time of antibiotic discontinuation |
Prognosis of VAP patients
No 28-day mortality occurred in either group. The differences between the two groups were not significant in length of stay in the ICU, days of mechanical ventilation and the proportion of tracheotomy. Antibiotic-free days within the first 28 days was 10.48 (±4.50) in the infection controlled group vs 6.06 days (±3.69) in the infection recurrence group (P=0.001, Table 5).
 | Table 5 Prognosis of VAP patients |
Results of logistic analysis and ROC curve
Logistic analysis showed that CPIS scores and characteristics of tracheal secretions were the independent risk factors (P=0.045 and P=0.041, respectively), accounting for infection recurrence (Table 6).
 | Table 6 Risk factors for infection recurrence (Logistic analysis) |
The ROC curve showed that CPIS scores and characteristics of tracheal secretions were strong predictors for the antibiotic recurrence (areas under the ROC curve were 0.781 and 0.805, respectively, Table 7). On CPIS for predicting antibiotic recurrence, the best cutoff value was 4.5, and the best cutoff value about characteristics of tracheal secretions was 1.5.
 | Table 7 Results of ROC curve with infection recurrence as the state variable |
Kappa consistency
Both CPIS≥5 points and CPIS≥4 points were checked with the actual infection recurrence using Kappa consistency, for no 4.5 points existing in CPIS. Results showed that CPIS≥5 points had a certain consistency with the infection recurrence (kappa=0.479, P<0.001). Therefore, CPIS≥5 served a certain predictive value for infection recurrence when physicians considered antibiotic discontinuation (Specificity 90.3%, sensitivity 55.0%, positive predictive value 78.6% and negative predictive value 75.7%).
Discussion
Concerning antimicrobial management for critically ill patients with VAP, it is crucial that physicians should timely and sufficiently give patients the initial empiric therapy, followed by the microbiologically confirmed target therapy and appropriate duration of antibiotic therapy. A shorter course of antibiotic therapy and earlier antibiotic discontinuation could help lower unnecessary costs and reduce antibiotic exposure and antimicrobial resistance. Meanwhile, too short of a course of therapy may risk inadequate treatment and increased probability of infection recurrence. A meta-analysis15 on the duration of antibiotic therapy including eight studies (1,703 patients) compared short-course versus prolonged-course of antibiotic therapy for VAP (7–8 days vs 10–15 days), showing that shorter course of therapy was associated with the reduced antibiotic exposure in terms of 28-day antibiotic-free days, without significantly affecting mortality, recurrent pulmonary infection, therapeutic failure and duration of ICU stay, etc. However, VAP caused by non-fermenting, gram-negative bacilli might have a higher pulmonary infection recurrence rate when patients were given shorter courses of antibiotic therapy.11,16 Furthermore, the 2016 Clinical Practice Guidelines for Management of HAP and VAP by IDSA and ATS mentioned that, although a 7-day course of antimicrobial therapy rather than a longer duration was recommended for patients with VAP, short courses of antibiotics were associated with recurrent infection in the subgroup of patients with VAP due to a non-fermenting gram-negative bacillus. Consequently, the guidelines suggested that, in length of therapy there existed situations in which a shorter or longer duration of antibiotics might be indicated, depending upon the rate of improvement of clinical, radiologic, and laboratory parameters.6
Findings from several studies have shown that PCT has been advocated as a preferable biomarker for diagnosis and differential diagnosis of bacterial infections, displaying severity of bacterial infection and activity of inflammation.17–21 Moreover, PCT might assist physicians in making decisions on antibiotic therapy for infectious diseases.22–24 The ProVAP trial and the PRORAT trial suggested: 1) PCT<0.5 μg/L or a decrease by >80%, antibiotic discontinuation was encouraged; 2) PCT<0.25 μg/L or a suggested decrease by >90%, antibiotic discontinuation was strongly encouraged.9,25,26 Both of the two trials showed the number of antibiotic-free days within 28 days was significantly higher in the PCT group than that in the control group without increasing mortality.8,9,25 Between PCT-guided group and control group in the PRORATA trial, percentages of patients with relapse (12% vs 12.1%) and superinfection (48% vs 42%) were similar and high.8,25 Similar to the PRORATA trial, in our study, the infection recurrence rate was 39.2% within 7 days after the PCT-guided antibiotic discontinuation.
Statistical analysis showed that CPIS scores were the independent risk factors accounting for infection recurrence and unsuccessful antibiotic discontinuation. Results showed that patients with simplified CPIS≥5 points (at the time of antibiotic discontinuation) had a much higher VAP recurrence rate (78.6%) than the recurrence rate (24.3%) of patients with CPIS scores<5 points. Kappa consistency showed that CPIS≥5 points had a certain consistency with the infection recurrence. Therefore, if the simplified CPIS≥5 points, even though PCT might have reached the criteria for antibiotic discontinuation, antibiotic discontinuation could face a high risk of infection recurrence.
Generally, VAP patients clinical features including temperature, oxygenation, tracheal secretions and chest radiography are favorably improved by appropriate antibiotic therapy, while semi-quantitative culture results of endotracheal aspirates may still be positive (light/moderate/heavy growth threshold). In such a situation, it is often difficult for many physicians to decide on whether to give patients the de-escalation therapy or antibiotic discontinuation. Some studies have shown that semi-quantitative cultures of endotracheal aspirates were falsely positive at a certain percentage.13,27 In Fujitani’s study,13 an overall 17.5% of patients among the entire cohort had false-positive endotracheal aspirate cultures. Giantsou’s study27 showed that de-escalation therapy in VAP was negatively affected by the culture results of endotracheal aspirates. Our study showed no significant differences between the infection recurrence group and the infection controlled group upon semi-quantitative culture results of endotracheal aspirates. In other words, when PCT concentration was less than 0.5 μg/L, semi-quantitative culture results did not affect infection recurrence. In patients whose culture results of endotracheal aspirates were moderate/heavy, the infection recurrence rate was 37.5%, with no significant difference from the recurrence rate (40%) of patients whose culture results were rare/light. These results suggested that when PCT and clinical features meet the criteria for antibiotic discontinuation, semi-quantitative culture results of endotracheal aspirates are not reliable predictors of infection recurrence.
Similarly, VAP patients clinical features are improved by appropriate antibiotic therapy, but the culture results of endotracheal aspirates indicated MDR strains. How should physicians view such a situation, should they give patients the de-escalation therapy or antibiotic discontinuation? As is now well known, risk factors for MDR infections include the use of broad-spectrum antibiotics, prolonged mechanical ventilation, etc.28–30 MDR infections can lead to inadequate antibiotic therapy, prolonged course of therapy and duration of ICU stay, and increased mortality.31–35 Nevertheless, after VAP patients clinical features and infection-related indicators such as PCT and leucocyte counts are improved by appropriate antibiotic therapy, there is a doubt that the MDR strains obtained from endotracheal aspirate cultures are colonizations or infections. In Fujitani’s study,13 42.2% of patients were considered to have false-positive cultures for MDR pathogens, which would have been a negative impact on any strategy that emphasizes de-escalation. Our study showed no significant difference in proportions of MDR pathogens between the infection recurrence group and the infection controlled group (35.0% vs 32.3%, P=0.839). In other words, when PCT concentration was less than 0.5 μg/L, whether pathogens are MDR strains or not did not affect infection recurrence. Therefore, when PCT and clinical features meet the criteria for antibiotic discontinuation, antibiotic discontinuation can be considered even if culture results of endotracheal aspirates are MDR strains.
As the first stage of “PCT-guided antibiotic therapy in ventilator-associated pneumonia”, our study has several limitations. First, the criteria for antibiotic discontinuation set by this study are relatively conservative. Except for the level of PCT, 48 hrs before the decision on antibiotic discontinuation, the highest temperature should be less than 38.5℃, and leukocyte count should be more than 4.0×109/L and no more than 15×109/L. Meanwhile, drainage tubes of important viscera should have been removed. These combined criteria undoubtedly lead to longer days of antibiotic use. Secondly, most of the patients admitted to our ICU are surgical patients, especially neurosurgical patients (66.7%), which may have a certain effect on the results. Thirdly, patients who died before antibiotic discontinuation or within 7 days after antibiotic discontinuation were excluded. Consequently, no 28-day mortality occurred in our study, so 28-day mortality was not compared between the two groups. Lastly, because of the high quantity of patients who were excluded, the sample size of our study was small.
Conclusion
This prospective observational study showed when PCT was less than 0.5 μg/L and the simplified CPIS scores were 5 points or more, antibiotic discontinuation would face a high risk of infection recurrence. Thus, when applying PCT-guided antibiotic discontinuation in VAP, physicians should pay more attention to CPIS, without putting too much care on semi-quantitative culture results of endotracheal aspirates and whether pathogens are MDR strains.
Ethics approval and informed consent
This study was approved by the ethics committee of the Affiliated Hospital of Taishan Medical University. The committee’s reference number is 2016002. All included patients or their legal representatives provided written informed consent.
Abbreviation list
VAP, ventilator-associated pneumonia; PCT, procalcitonin; CPIS, clinical pulmonary infection score; CRP, C-reactive protein; ICU, intensive care unit; APACHE, Acute Physiology and Chronic Health Evaluation; GCS, Glasgow coma score; SD, standard deviation; IQR, interquartile range; ROC, receiver operating characteristic; OR, odds ratio; CI, confidence interval; BUN, blood urea nitrogen; MDR, multidrug-resistant.
Data availability
The datasets analyzed during the current study are available in the “ResMan” repository,
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgment
In the collection of data, this study was supported by grants from Science and Technology Development Program of Tai’an City (2016NS1129).
Author contributions
DPH and CTW designed the study; QSW and KA performed the study and collected data; DPH and JW analyzed the data; CHH contributed to the methods and analysis; QSW and DPH wrote the manuscript. All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Davis KA. Ventilator-associated pneumonia: a review. J Intensive Care Med. 2006;21(4):211–226. doi:10.1177/0885066606288837
2. Pugh R, Grant C, Cooke RP, Dempsey G. Short-course versus prolonged-course antibiotic therapy for hospital-acquired pneumonia in critically ill adults. Cochrane Database Syst Rev. 2015;(8):CD007577.
3. Singh N, Rogers P, Atwood CW, Wagener MM, Yu VL. Short-course empiric antibiotic therapy for patients with pulmonary infiltrates in the intensive care unit. A proposed solution for indiscriminate antibiotic prescription. Am J Respir Crit Care Med. 2000;162(2 Pt 1):505–511. doi:10.1164/ajrccm.162.2.9909095
4. Povoa P, Martin-Loeches I, Ramirez P, et al. Biomarkers kinetics in the assessment of ventilator-associated pneumonia response Biomarkers kineticsto antibiotics – results from the BioVAP study. J Crit Care. 2017;41:91–97. doi:10.1016/j.jcrc.2017.05.007
5. Povoa P, Coelho L, Almeida E, et al. C-reactive protein as a marker of ventilator-associated pneumonia resolution: a pilot study. Eur Respir J. 2005;25(5):804–812. doi:10.1183/09031936.05.00071704
6. Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63(5):e61–e111. doi:10.1093/cid/ciw353
7. Torres A, Niederman MS, Chastre J, et al. International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator-associated pneumonia: guidelines for the management of hospital-acquired pneumonia (HAP)/ventilator-associated pneumonia (VAP) of the European Respiratory Society (ERS), European Society of Intensive Care Medicine (ESICM), European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and Asociacion Latinoamericana del Torax (ALAT). Eur Respir J. 2017;50(3). doi:10.1183/13993003.00711-2017.
8. Luyt CE, Combes A, Trouillet JL, Chastre J. Value of the serum procalcitonin level to guide antimicrobial therapy for patients with ventilator-associated pneumonia. Semin Respir Crit Care Med. 2011;32(2):181–187. doi:10.1055/s-0031-1275530
9. Stolz D, Smyrnios N, Eggimann P, et al. Procalcitonin for reduced antibiotic exposure in ventilator-associated pneumonia: a randomised study. Eur Respir J. 2009;34(6):1364–1375. doi:10.1183/09031936.00053209
10.
11. Chastre J, Wolff M, Fagon JY, et al. Comparison of 8 vs 15 days of antibiotic therapy for ventilator-associated pneumonia in adults: a randomized trial. JAMA. 2003;290(19):2588–2598. doi:10.1001/jama.290.19.2588
12.
13. Fujitani S, Cohen-Melamed MH, Tuttle RP, Delgado E, Taira Y, Darby JM. Comparison of semi-quantitative endotracheal aspirates to quantitative non-bronchoscopic bronchoalveolar lavage in diagnosing ventilator-associated pneumonia. Respir Care. 2009;54(11):1453–1461.
14. Luna CM, Blanzaco D, Niederman MS, et al. Resolution of ventilator-associated pneumonia: prospective evaluation of the clinical pulmonary infection score as an early clinical predictor of outcome. Crit Care Med. 2003;31(3):676–682. doi:10.1097/01.CCM.0000055380.86458.1E
15. Pugh R, Grant C, Cooke RP, Dempsey G. Short-course versus prolonged-course antibiotic therapy for hospital-acquired pneumonia in critically ill adults. Cochrane Database Syst Rev. 2011;(10):CD007577.
16. Pinzone MR, Cacopardo B, Abbo L, Nunnari G. Optimal duration of antimicrobial therapy in ventilator-associated pneumonia: what is the role for procalcitonin? J Glob Antimicrob Resist. 2014;2(4):239–244. doi:10.1016/j.jgar.2014.06.004
17. Charles PE, Ladoire S, Aho S, et al. Serum procalcitonin elevation in critically ill patients at the onset of bacteremia caused by either Gram negative or Gram positive bacteria. BMC Infect Dis. 2008;8:38. doi:10.1186/1471-2334-8-38
18. Petrikkos GL, Christofilopoulou SA, Tentolouris NK, Charvalos EA, Kosmidis CJ, Daikos GL. Value of measuring serum procalcitonin, C-reactive protein, and mannan antigens to distinguish fungal from bacterial infections. Eur J Clin Microbiol Infect Dis. 2005;24(4):272–275. doi:10.1007/s10096-005-1312-z
19. Sugimoto K, Shimizu N, Matsumura N, et al. Procalcitonin as a useful marker to decide upon intervention for urinary tract infection. Infect Drug Resist. 2013;6:83–86. doi:10.2147/IDR.S47161
20. Schuetz P, Albrich W, Mueller B. Procalcitonin for diagnosis of infection and guide to antibiotic decisions: past, present and future. BMC Med. 2011;9:107. doi:10.1186/1741-7015-9-107
21. Pfister R, Kochanek M, Leygeber T, et al. Procalcitonin for diagnosis of bacterial pneumonia in critically ill patients during 2009 H1N1 influenza pandemic: a prospective cohort study, systematic review and individual patient data meta-analysis. Crit Care. 2014;18(2):R44. doi:10.1186/cc13712
22. Wongsurakiat P, Tulatamakit S. Clinical pulmonary infection score and a spot serum procalcitonin level to guide discontinuation of antibiotics in ventilator-associated pneumonia: a study in a single institution with high prevalence of nonfermentative gram-negative bacilli infection. Ther Adv Respir Dis. 2018;12:1753466618760134. doi:10.1177/1753466618760134
23. Schuetz P, Christ-Crain M, Thomann R, et al. Effect of procalcitonin-based guidelines vs standard guidelines on antibiotic use in lower respiratory tract infections: the ProHOSP randomized controlled trial. JAMA. 2009;302(10):1059–1066. doi:10.1001/jama.2009.1297
24. Zhang T, Wang Y, Yang Q, Dong Y. Procalcitonin-guided antibiotic therapy in critically ill adults: a meta-analysis. BMC Infect Dis. 2017;17(1):514. doi:10.1186/s12879-017-2757-2
25. Bouadma L, Luyt CE, Tubach F, et al. Use of procalcitonin to reduce patients’ exposure to antibiotics in intensive care units (PRORATA trial): a multicentre randomised controlled trial. Lancet. 2010;375(9713):463–474. doi:10.1016/S0140-6736(09)61879-1
26. Schuetz P, Chiappa V, Briel M, Greenwald JL. Procalcitonin algorithms for antibiotic therapy decisions: a systematic review of randomized controlled trials and recommendations for clinical algorithms. Arch Intern Med. 2011;171(15):1322–1331. doi:10.1001/archinternmed.2011.318
27. Giantsou E, Liratzopoulos N, Efraimidou E, et al. De-escalation therapy rates are significantly higher by bronchoalveolar lavage than by tracheal aspirate. Intensive Care Med. 2007;33(9):1533–1540. doi:10.1007/s00134-007-0619-x
28. Huang H, Chen B, Liu G, et al. A multi-center study on the risk factors of infection caused by multi-drug resistant Acinetobacter baumannii. BMC Infect Dis. 2018;18(1):11. doi:10.1186/s12879-018-3109-6
29. Masse J, Elkalioubie A, Blazejewski C, et al. Colonization pressure as a risk factor of ICU-acquired multidrug resistant bacteria: a prospective observational study. Eur J Clin Microbiol Infect Dis. 2017;36(5):797–805. doi:10.1007/s10096-016-2863-x
30. Seguin P, Fedun Y, Laviolle B, Nesseler N, Donnio PY, Malledant Y. Risk factors for multidrug-resistant bacteria in patients with post-operative peritonitis requiring intensive care. J Antimicrob Chemother. 2010;65(2):342–346. doi:10.1093/jac/dkp439
31. Dent LL, Marshall DR, Pratap S, Hulette RB. Multidrug resistant Acinetobacter baumannii: a descriptive study in a city hospital. BMC Infect Dis. 2010;10:196. doi:10.1186/1471-2334-10-196
32. Falagas ME, Rafailidis PI. Attributable mortality of Acinetobacter baumannii: no longer a controversial issue. Crit Care. 2007;11(3):134. doi:10.1186/cc5911
33. Hirsch EB, Tam VH. Impact of multidrug-resistant Pseudomonas aeruginosa infection on patient outcomes. Expert Rev Pharmacoecon Outcomes Res. 2010;10(4):441–451. doi:10.1586/erp.10.49
34. Tam VH, Rogers CA, Chang KT, Weston JS, Caeiro JP, Garey KW. Impact of multidrug-resistant Pseudomonas aeruginosa bacteremia on patient outcomes. Antimicrob Agents Chemother. 2010;54(9):3717–3722. doi:10.1128/AAC.00207-10
35. Zilberberg MD, Nathanson BH, Sulham K, Fan W, Shorr AF. Multidrug resistance, inappropriate empiric therapy, and hospital mortality in Acinetobacter baumannii pneumonia and sepsis. Crit Care. 2016;20(1):221. doi:10.1186/s13054-016-1362-x
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
