Back to Journals » Infection and Drug Resistance » Volume 9
Probiotics improve the efficacy of standard triple therapy in the eradication of Helicobacter pylori: a meta-analysis
Authors Lau CSM, Ward A, Chamberlain RS
Received 22 July 2016
Accepted for publication 9 September 2016
Published 7 December 2016 Volume 2016:9 Pages 275—289
DOI https://doi.org/10.2147/IDR.S117886
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Suresh Antony
Christine S M Lau,1,2 Amanda Ward,2 Ronald S Chamberlain1–4
1Department of Surgery, Saint Barnabas Medical Center, Livingston, NJ, USA; 2Saint George’s University School of Medicine, Grenada, West Indies; 3Department of Surgery, Banner MD Anderson Cancer Center, Gilbert, AZ, USA; 4Department of Surgery, New Jersey Medical School, Rutgers University, Newark, NJ, USA
Introduction: Helicobacter pylori colonization is present in half of the world’s population and can lead to numerous gastrointestinal diseases if left untreated, including peptic ulcer disease and gastric cancer. Although concurrent triple therapy remains the recommended treatment regimen for H. pylori eradication, its success rate and efficacy have been declining. Recent studies have shown that the addition of probiotics can significantly increase eradication rates by up to 50%. This meta-analysis examines the impact of probiotic supplementation on the efficacy of standard triple therapy in eradicating H. pylori.
Methods: A comprehensive literature search was conducted using PubMed, Cochrane Central Registry of Controlled Trials, and Google Scholar (time of inception to 2016) to identify all published randomized control trials (RCTs) assessing the use of probiotics in addition to triple therapy for the treatment of H. pylori. Searches were conducted using the keywords “probiotics”, “triple therapy”, and “Helicobacter pylori”. RCTs comparing the use of probiotics and standard triple therapy with standard triple therapy alone for any duration in patients of any age diagnosed with H. pylori infection were included. H. pylori eradication rates (detected using urea breath test or stool antigen) were analyzed as-per-protocol (APP) and intention-to-treat (ITT).
Results: A total of 30 RCTs involving 4,302 patients APP and 4,515 patients ITT were analyzed. The addition of probiotics significantly increased eradication rates by 12.2% (relative risk [RR] =1.122; 95% confidence interval [CI], 1.091–1.153; P<0.001) APP and 14.1% (RR =1.141; 95% CI, 1.106–1.175; P<0.001) ITT. Probiotics were beneficial among children and adults, as well as Asians and non-Asians. No significant difference was observed in efficacy between the various types of probiotics. The risk of diarrhea, nausea, vomiting, and epigastric pain was also reduced.
Conclusion: The addition of probiotics is associated with improved H. pylori eradication rates in both children and adults, as well as Asians and non-Asians. Lactobacillus, Bifidobacterium, Saccharomyces, and mixtures of probiotics appear beneficial in H. pylori eradication. Furthermore, the reduction in antibiotic-associated side effects such as nausea, vomiting, diarrhea, and epigastric pain improves medication tolerance and patient compliance. Given the consequences associated with chronic H. pylori infection, the addition of probiotics to the concurrent triple therapy regimen should be considered in all patients with H. pylori infection. However, further studies are required to identify the optimal probiotic species and dose.
Keywords: probiotics, Helicobacter pylori, triple therapy, meta-analysis
Introduction
Helicobacter pylori, previously referred to as Campylobacter pylori, is a Gram-negative, spiral bacterium that is present on the gastric epithelium mucus layer.1 H. pylori colonization almost always leads to acute gastritis, with neutrophilic and mononuclear infiltrates in the gastric mucosa.2 If left untreated, it can cause chronic gastritis, which is associated with various gastrointestinal diseases.2 Various extragastric manifestations of H. pylori have also been reported, including idiopathic thrombocytopenia purpura, vitamin B12 deficiency, and metabolic syndrome.3 Studies have reported H. pylori colonization to be as high as 90% among patients with gastric ulcers or cancer.4,5 Furthermore, virtually, all patients with mucosa-associated lymphoid tissue lymphomas (MALTomas) are colonized with H. pylori.6,7 It is estimated that over half of the current world population has H. pylori in their gastric flora.1 Early H. pylori eradication has been associated with a sixfold reduction in the recurrence of ulcers as well as a two- to threefold reduction in the risk of gastric carcinoma.8
Current treatment guidelines recommend concomitant triple therapy for the eradication of H. pylori, utilizing clarithromycin, either amoxicillin or metronidazole, as well as a proton pump inhibitor for 7–14 days.9–11 Despite initial successes, there has been a constant decline in H. pylori eradication rates with standard triple therapy in both adult and pediatric populations, from 75 to 55% between 2009 and 2014.12 Although several mechanisms have been proposed, most of the studies agree that the main reasons for the declining efficacy are the increasing resistance to clarithromycin and poor medication compliance as a result of antibiotic-induced nausea, vomiting, and diarrhea.13–15 While eradication rates of 88% are seen with clarithromycin-sensitive H. pylori strains, eradication rates are only 14% among strains resistant to clarithromycin.16 As with all antibiotics, H. pylori medications often cause diarrhea, nausea, and vomiting, which lead to poor tolerance and ultimately decreased patient compliance, the single most important factor in H. pylori eradication.14,17 Graham et al18 reported H. pylori eradication rates of 96% in high medication-compliant patients (taking ≥60% of the prescribed antibiotics), while only 69% eradication rates were observed among low medication-compliant patients (taking <60% of prescribed antibiotics). With the decline in H. pylori eradication rates, novel therapeutic alternatives are being studied and evaluated. Eradication rates are highest during the early phase of treatment when antibiotic sensitivity and patient compliance are greatest. Early treatment failure results in elevated risk of secondary antibiotic resistance due to the need for additional, less effective antibiotics used over longer periods of time with the possibility of additional medication side effects, thereby perpetuating the increase in antibiotic resistance and decreased medication compliance cycle.19 Since medication compliance has been considered the most important factor in H. pylori eradication, a major goal of therapy is aimed at improving the compliance. Recently, the use of probiotic supplementation has been proposed for both preventing and treating various gastrointestinal conditions, including antibiotic-induced side effects such as diarrhea, which may in turn increase medication tolerability and patient compliance.
Probiotics are defined by the World Health Organization (WHO) and the Food and Agriculture Organization (FAO) as living microorganisms that could potentially benefit health.20 Although a variety of different probiotic species have been studied, the Lactobacillus genus, Bifidobacterium genus, and Saccharomyces genus remain the most commonly studied.20 Probiotics act in numerous pathways, and both immune-mediated and nonimmune-mediated mechanisms have been documented.14
Gong et al21 reported lower odds of H. pylori eradication with triple therapy alone, compared to triple therapy with probiotic supplementation (odds ratio [OR] 0.58; 95% confidence interval [CI], 0.50–0.68; P<0.05). Significant reductions in side effects, including nausea, vomiting, bloating, epigastric pain, diarrhea, constipation, taste distortion, and skin rash, were also observed.21 Numerous randomized control trials (RCTs) not included in the Gong et al study have recently been published. Furthermore, no subgroup analysis has been conducted to determine whether probiotics are beneficial in all populations, such as adults and children, as well as Asians and non-Asians.
Given the grave long-term consequences associated with chronic H. pylori infection, this meta-analysis provides an updated analysis on the efficacy of probiotic supplementation to triple therapy on H. pylori eradication rates in both children and adults, as well as the Asian and non-Asian populations.
Methods
Study selection
A comprehensive literature search of PubMed, Cochrane Central Registry of Controlled Trials, and Google Scholar from the time of inception (1966) to the present day (2016) was conducted to identify all published RCTs evaluating the effect of probiotic supplementation on the efficacy of standard triple therapy in the treatment of H. pylori. Using the yielded search results, additional references and studies were searched. The last search was performed on February 22, 2016. Combinations of the keywords “probiotics”, “triple therapy”, “Campylobacter pylori”, “Helicobacter pylori”, and “H. pylori” were used. Studies comparing the use of probiotics and standard triple therapy with standard triple therapy alone for any duration in patients of any age diagnosed with H. pylori infection were included. If there were duplicate publications of the same study, only the most updated and comprehensive data set for the study was included.
Data extraction
Each article retrieved from the database searches as described earlier was reviewed and assessed for eligibility and study inclusion. Data related to the patients, comparison groups (probiotic and standard triple therapy group vs standard triple therapy alone group), clinical outcomes, and study methodology were extracted (Figure 1). The incidence rates of H. pylori eradication (detected via urea breath test or stool antigen) and adverse events (including nausea, vomiting, diarrhea, and epigastric pain) were assessed.
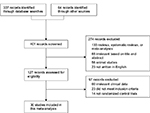  | Figure 1 CONSORT diagram detailing the study selection process. Abbreviation: CONSORT, consolidated standards of reporting trials. |
Statistical analysis
Relative risk (RR) along with a 95% CI for the incidence of H. pylori eradication and medication side effects was calculated for each included study. If any study reported a zero incidence in either the intervention (standard triple therapy and probiotic) or the control (standard triple therapy alone) group, a “0.5” continuity correction factor was applied to allow for calculation of RR and variance. Depending on the heterogeneity of the included study, either a fixed-effects model or a random-effects model was used. Both Cochrane’s Q statistic and I2 statistic were used to assess heterogeneity, and a P<0.05 or I2>50 was utilized for determining the presence of significant heterogeneity. Data were analyzed using a random-effects model when heterogeneity was deemed significant, while a fixed-effects model was used in the absence of heterogeneity. Sensitivity analysis to determine the influence of each individual included study on the overall effect size (RR estimates) was assessed by removing each study one-by-one and calculating the overall effect sizes. Publication bias for the pooled H. pylori eradication rates was evaluated, both visually using a funnel plot and quantitatively using Egger’s and Begg’s tests. Subgroup analysis was performed to determine any differences based on probiotic genus (Lactobacillus, Bifidobacterium, Saccharomyces, and mixed), patient age (children vs adults), ethnicity (Asians vs non-Asians), as well as the control group utilized (placebo vs no treatment). All meta-analyses of pooled study data were conducted using Comprehensive Meta-Analysis Software Version 3 (Biostat, Englewood, NJ, USA), and statistical significance was accepted at a level of P<0.05 (two tail).
Results
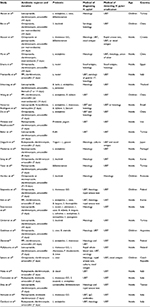
A total of 30 RCTs meeting the inclusion criteria were identified (Table 1). There were 4,302 patients when analyzed as-per-protocol (APP) and 4,515 patients when analyzed intention-to-treat (ITT).
Effects of probiotics on triple therapy efficacy in H. pylori eradication rates (APP treated)
All studies reported on H. pylori eradication rates in both the probiotic-supplemented and triple therapy alone groups. The addition of probiotics to the triple therapy regimen significantly increased eradication rates compared to triple therapy alone (1,786/2,140 [83.5%] vs 1,602/2,162 [74.1%]). No significant heterogeneity between trials (P=0.321, I2<8.993) was found, and a fixed-effects model was therefore utilized. There was a 12.2% increase in eradication rates with probiotic supplementation (RR =1.122; 95% CI, 1.091–1.153; P<0.001) (Figure 2).
Subgroup analysis by patient age revealed that probiotic supplementation improved the efficacy of triple therapy in both children (RR =1.176; 95% CI, 1.050–1.317; P=0.005) and adults (RR =1.118; 95% CI, 1.087–1.150; P<0.001) with no significant between group heterogeneity (P=0.491).
Subgroup analysis by the type of probiotic identified benefit for Lactobacillus (RR =1.142; 95% CI, 1.084–1.203; P<0.001), Saccharomyces (RR =1.088; 95% CI, 1.022–1.158; P=0.008), and mixture of probiotics (RR =1.135; 95% CI, 1.088–1.185; P<0.001). A trend toward an increase in eradication rates with Bifidobacterium (RR =1.094; 95% CI, 0.992–1.207; P=0.073) was also observed. There was no significant between group heterogeneity (P=0.589).
Subgroup analysis by ethnicity identified that probiotic supplementation improved the efficacy of triple therapy in both Asians (RR =1.108; 95% CI, 1.066–1.152; P<0.001) and non-Asians (RR =1.136; 95% CI, 1.092–1.181; P<0.001), with no statistically significant between group differences (P=0.826).
Subgroup analysis based on the type of control group utilized revealed a significant improvement in triple therapy efficacy in both the no treatment group (RR =1.152; 95% CI, 1.106–1.200; P<0.001) and placebo group (RR =1.122; 95% CI, 1.075–1.170; P<0.001), with no significant difference between the two groups (P=0.365).
Effects of probiotics on triple therapy efficacy in H. pylori eradication rates (ITT)
The addition of probiotics to the triple therapy regimen significantly increased eradication rates compared to triple therapy alone (1,744/2,222 [78.5%] vs 1,564/2,293 [68.2%]). Heterogeneity between trials was deemed not significant (P=0.459, I2<0.445), and therefore a fixed-effects model was employed. There was a 14.1% increase in eradication rates with the addition of probiotics (RR =1.141; 95% CI, 1.106–1.176; P<0.001) (Figure 3).
Subgroup analysis based on patient age showed that probiotic supplementation improved the efficacy of triple therapy in both children (RR =1.193; 95% CI, 1.106–1.176; P<0.001) and adults (RR =1.138; 95% CI, 1.102–1.174; P<0.001), with no significant between group heterogeneity (P=0.557).
Subgroup analysis based on the type of probiotic identified benefit for Lactobacillus (RR =1.153; 95% CI, 1.092–1.217; P<0.001), Bifidobacterium (RR =1.168; 95% CI, 1.031–1.324; P=0.015), Saccharomyces (RR =1.127; 95% CI, 1.050–1.211; P=0.001), and mixture of probiotics (RR =1.140; 95% CI, 1.086–1.197; P<0.001). There was no significant difference in heterogeneity between the groups (P=0.938).
Subgroup analysis by ethnicity identified that probiotic supplementation improved the efficacy of triple therapy in both Asians (RR =1.132; 95% CI, 1.084–1.182; P<0.001) and non-Asians (RR =1.150; 95% CI, 1.101–1.200; P<0.001), with no significant difference based on ethnicity (P=0.959).
Subgroup analysis of the control group utilized identified a significant improvement in triple therapy efficacy in both the no treatment group (RR =1.149; 95% CI, 1.103–1.197; P<0.001) and placebo group (RR =1.130; 95% CI, 1.079–1.184; P<0.001), with no significant difference between the two groups (P=0.636).
Adverse events
A total of 18 of the studies (N=2,916 patients) reported on the incidence of nausea, 14 of the studies (N=1,703 patients) reported on the incidence of vomiting, 19 of the studies (N=2,554 patients) reported on the incidence of diarrhea, and 14 of the studies (N=2,537 patients) reported on the incidence of epigastric pain. There was a significant reduction in the risk of nausea (RR =0.606; 95% CI, 0.520–0.705; P<0.001), vomiting (RR =0.724; 95% CI, 0.533–0.985; P=0.040), diarrhea (RR =0.549; 95% CI, 0.391–0.771; P=0.001), and epigastric pain (RR =0.812; 95% CI, 0.727–0.907; P<0.001) with the addition of probiotics to standard triple therapy compared to triple therapy alone (Figures S1–S4).
Sensitivity analysis
Sensitivity analysis revealed similar overall effect sizes and RR estimates for H. pylori eradication rates after the removal of each individual study. H. pylori eradication rates ranged from 11.1% increase (RR =1.111; 95% CI, 1.078–1.144; P<0.001) to 13.1% increase (RR =1.131; 95% CI, 1.098–1.165; P<0.001) (Figure S5).
Publication bias
Publication bias for H. pylori eradication rates was assessed utilizing both a funnel plot for qualitative analysis and Egger’s and Begg’s tests to quantitatively calculate the bias. There was no asymmetry on the funnel plot (Figure S6) and no significant publication bias calculated by either Egger’s test (P=0.784) or Begg’s test (P=0.566).
Discussion
H. pylori, a Gram-negative, spiral bacterium, was first discovered in 1982 by Warren and Marshall.1 Over half of the world’s population is colonized with H. pylori, which if left untreated potentially leads to chronic gastritis, gastric and duodenal ulcers, gastric cancer, and MALTomas.2,4,5,7,22 H. pylori induces an initial inflammatory response (histological gastritis), followed by chronic inflammation and gastritis, causing damage to the epithelium and atrophy of the gastric lining.2 Chronic inflammation leads to the production of reactive oxygen species (ROS), leading to DNA damage, which in turn leads to mutations, intestinal metaplasia and dysplasia, and further gastric pathology.2
Standard triple therapy remains the gold standard for eradicating H. pylori; however, more recent studies have shown a constant decline in H. pylori eradication rates, to as low as 50%.12,23,24 This decline has been attributed to increased clarithromycin resistance and low medication compliance secondary to medication side effects.13,14 Numerous alternative therapeutic regimens to enhance eradication rates have been proposed, including sequential therapy, which utilizes a dual 5-day therapy, with an initial 5-day regimen of amoxicillin and a proton pump inhibitor followed by 5 days of clarithromycin, metronidazole, and a proton pump inhibitor.25 Lau et al25 conducted a meta-analysis with 12 RCTs and 1,221 patients and revealed that sequential therapy improved eradication rates by 14.2%. Low medication compliance due to side effects has been deemed the most important factor in eradicating H. pylori, highlighting the need for novel treatments that increase medication tolerability and patient compliance.17
Probiotics, living commensal microorganisms naturally found in the host intestinal flora, exert a protective effect on the gastrointestinal tract.26,27 Although the precise mechanism of probiotics has not been fully elucidated, numerous mechanisms have been proposed. Each probiotic strain has a unique mechanism of action that may be more or less effective in increasing H. pylori eradication and reducing the side effects. Previous studies have shown that probiotics significantly reduce the risk of antibiotic-associated side effects, including nausea, vomiting, diarrhea, and epigastric pain.24,26 These commensal bacteria inhibit enteric pathogens and suppress pathogenic bacterial growth and invasion, ultimately improving intestinal barrier function.28 Probiotics also modulate proinflammatory cytokines, which help maintain homeostasis and regulate immune responses.28,29 Lactobacillus species have been shown to modify immune response by decreasing the levels of proinflammatory cytokines, stimulate mucin secretion, suppress bacterial growth, and inhibit H. pylori adhesion to the gastric epithelium.14,20 Studies have shown that Lactobacillus salavaris reduces interleukin (IL)-8 secretion from the gastric epithelial cells, Lactobacillus acidophilus inactivates the Smad7 and NFκB inflammatory pathways, and Lactobacillus bulgaricus inhibits the activation of the TLR4 signaling pathway and IL-8 production.20 Lactobacilli are also able to enhance the local IgA secretion and reduce specific anti-H. pylori IgG antibodies.20 Additionally, strains of Lactobacillus are responsible for increasing mucin production.20 Mucins protect the gastric epithelium, and H. pylori suppresses MUC5AC and MUC1 gene expressions. Lactobacillus plantarum 299v increases MUC2 expression while Lactobacillus rhamnosus GG stimulates MUC3 gene expression.20 Lactobacilli also secrete antibacterial substances, including lactic acid, hydrogen peroxidase, bactericines, and short-chain fatty acids.14,20 L. acidophilus contains an autolysin, a proteinaceous compound, and antibacterial that is released after the cell lyses.20 Lactobacillus reuteri produces reuterina that suppresses the growth of bacteria and also inhibits bacterial adhesion and colonization by binding of spiral bacterium to glycolipid protein receptors asialo-GMI and sulfatide.20 Bifidobacterium acts by inhibiting DNA gyrase enzymes involved in bacterial cell division.20 Saccharomyces contains neuroaminidase activity that removes the ligand for sialic acid-binding H. pylori adhesion to the gastric epithelium, α(2-3)-linked sialic acid.20
This current meta-analysis found that the addition of probiotics to the triple therapy is associated with a 13.8% increase in the chance of eradicating H. pylori infection compared to triple therapy alone, which is consistent with the results of a prior meta-analysis.21 Additionally, probiotics have shown a significant decrease in side effects, including nausea, vomiting, epigastric pain, and diarrhea.
Despite the improvements in H. pylori eradication with adjunct probiotics, the use of probiotics as monotherapy has been shown to reduce bacterial load but not effective in eradicating H. pylori.30,31 Bhatia et al32 discovered that H. pylori growth was inhibited in vitro if L. acidophilius was present. Michetti et al33 was the first to study the effect of L. acidophilus in vivo, concluding that the probiotic decreased the density of the bacterial load, but complete eradication was unsuccessful. Wang et al studied adult patients taking multispecies probiotic therapies that included L. acidophilus and concluded a decrease in urea breath test values but not in complete H. pylori eradication.31
Although probiotic efficacy has been studied extensively with numerous RCTs, the side effect profile for probiotics is not well documented. The Agency for Healthcare Research and Quality conducted a comprehensive review of 622 studies on the safety of probiotic use, and reported that a majority of the published studies only state the presence or absence of one or more specific side effect, but lack specific details, and only a third provided vague statements indicating that the probiotics were well tolerated.34 Case reports of sepsis, bacteremia, and fungemia with probiotic use have been reported; however, these adverse events are inconsistent and, when pooled together, are not statistically significant.34
Despite the significant and positive results from this meta-analysis, there are several limitations, mainly a result of the variation and heterogeneity of the included RCTs. Age, gender, ethnicity, and country of origin varied. The specific medications and dosage regimen, as well as diagnostic methods and any follow-up conducted, varied between studies. Similarly, the specific probiotic strain, dose, and treatment duration utilized were also slightly different. Additional RCTs are required to determine the best probiotic supplement for H. pylori eradication. Given the promise of probiotics in H. pylori eradication, further studies evaluating the bactericidal effects of different probiotic strains and potentially comparing the efficacy of probiotics alone vs probiotics in combination with triple therapy are warranted.
Despite the limitations discussed, this study identified that probiotic supplementation is associated with increased H. pylori eradication rates in adults and children, as well as Asians and non-Asians, compared to standard triple therapy alone. Lactobacillus, Bifidobacterium, Saccharomyces, and mixtures of probiotics appear beneficial in H. pylori eradication. Furthermore, the reduction in antibiotic-associated side effects, such as nausea, vomiting, diarrhea, and epigastric pain, improves medication tolerance and patient compliance. Given the significant increase in H. pylori eradication rate and reduction in side effects, probiotics should be administered concurrently with standard triple therapy.
Acknowledgments
The abstract of this study was presented at the Digestive Disease Week conference (May 21–24, 2016, San Diego, CA, USA) as a poster presentation with interim findings. The poster abstract was published in “Poster Abstracts” in Gastroenterology (Volume 150, Issue 4, Supplement 1, Page S878).
Disclosure
The authors report no conflicts of interest in this work and have not accepted financial sponsorship in producing and presenting this article.
References
Brown LM. Helicobacter pylori: epidemiology and routes of transmission. Epidemiol Rev. 2000;22(2):283–297. | ||
Kusters JG, van Vliet AH, Kuipers EJ. Pathogenesis of Helicobacter pylori infection. Clin Microbiol Rev. 2006;19(3):449–490. | ||
Goni E, Franceschi F. Helicobacter pylori and extragastric diseases. Helicobacter. 2016;21(suppl 1):45–48. | ||
Nomura A, Stemmermann GN, Chyou PH, Kato I, Perez-Perez GI, Blaser MJ. Helicobacter pylori infection and gastric carcinoma among Japanese Americans in Hawaii. N Engl J Med. 1991;325(16):1132–1136. | ||
Nomura A, Stemmermann GN, Chyou PH, Perez-Perez GI, Blaser MJ. Helicobacter pylori infection and the risk for duodenal and gastric ulceration. Ann Intern Med. 1994;120(12):977–981. | ||
Rauws EA, Langenberg W, Houthoff HJ, Zanen HC, Tytgat GN. Campylobacter pyloridis-associated chronic active antral gastritis. A prospective study of its prevalence and the effects of antibacterial and antiulcer treatment. Gastroenterology. 1988;94(1):33–40. | ||
Eidt S, Stolte M, Fischer R. Helicobacter pylori gastritis and primary gastric non-Hodgkin’s lymphomas. J Clin Pathol. 1994;47(5):436–439. | ||
Hung IF, Wong BC. Assessing the risks and benefits of treating Helicobacter pylori infection. Therap Adv Gastroenterol. 2009;2(3):141–147. | ||
Bazzoli F, Pozzato P. Therapy of H. pylori infection. J Physiol Pharmacol. 1997;48(suppl 4):39–46. | ||
Papastergiou V, Georgopoulos SD, Karatapanis S. Treatment of Helicobacter pylori infection: past, present and future. World J Gastrointest Pathophysiol. 2014;5(4):392–399. | ||
Lind T, Veldhuyzen van ZS, Unge P, et al. Eradication of Helicobacter pylori using one-week triple therapies combining omeprazole with two antimicrobials: the MACH I Study. Helicobacter. 1996;1(3):138–144. | ||
Kutluk G, Tutar E, Bayrak A, et al. Sequential therapy versus standard triple therapy for Helicobacter pylori eradication in children: any advantage in clarithromycin-resistant strains? Eur J Gastroenterol Hepatol. 2014;26(11):1202–1208. | ||
Megraud F, Doermann HP. Clinical relevance of resistant strains of Helicobacter pylori: a review of current data. Gut. 1998;43(suppl 1):S61–S65. | ||
Ruggiero P. Use of probiotics in the fight against Helicobacter pylori. World J Gastrointest Pathophysiol. 2014;5(4):384–391. | ||
Cremonini F, Di CS, Covino M, et al. Effect of different probiotic preparations on anti-Helicobacter pylori therapy-related side effects: a parallel group, triple blind, placebo-controlled study. Am J Gastroenterol. 2002;97(11):2744–2749. | ||
Venerito M, Krieger T, Ecker T, Leandro G, Malfertheiner P. Meta-analysis of bismuth quadruple therapy versus clarithromycin triple therapy for empiric primary treatment of Helicobacter pylori infection. Digestion. 2013;88(1):33–45. | ||
O’Connor JP, Taneike I, O’Morain C. Improving compliance with Helicobacter pylori eradication therapy: when and how? Therap Adv Gastroenterol. 2009;2(5):273–279. | ||
Graham DY, Lew GM, Malaty HM, et al. Factors influencing the eradication of Helicobacter pylori with triple therapy. Gastroenterology. 1992;102(2):493–496. | ||
Navarro-Rodriguez T, Silva FM, Barbuti RC, et al. Association of a probiotic to a Helicobacter pylori eradication regimen does not increase efficacy or decreases the adverse effects of the treatment: a prospective, randomized, double-blind, placebo-controlled study. BMC Gastroenterol. 2013;13:56. | ||
Homan M, Orel R. Are probiotics useful in Helicobacter pylori eradication? World J Gastroenterol. 2015;21(37):10644–10653. | ||
Gong Y, Li Y, Sun Q. Probiotics improve efficacy and tolerability of triple therapy to eradicate Helicobacter pylori: a meta-analysis of randomized controlled trials. Int J Clin Exp Med. 2015;8(4):6530–6543. | ||
Dooley CP, Cohen H, Fitzgibbons PL, et al. Prevalence of Helicobacter pylori infection and histologic gastritis in asymptomatic persons. N Engl J Med. 1989;321(23):1562–1566. | ||
Francavilla R, Lionetti E, Castellaneta SP, et al. Improved efficacy of 10-Day sequential treatment for Helicobacter pylori eradication in children: a randomized trial. Gastroenterology. 2005;129(5):1414–1419. | ||
Huang J, Zhou L, Geng L, et al. Randomised controlled trial: sequential vs. standard triple therapy for Helicobacter pylori infection in Chinese children-a multicentre, open-labelled study. Aliment Pharmacol Ther. 2013;38(10):1230–1235. | ||
Lau CS, Ward A, Chamberlain RS. Sequential (as Opposed to Simultaneous) antibiotic therapy improves Helicobacter Pylori eradication in the pediatric population: a meta-analysis. Clin Pediatr (Phila). 2016;55(7):614–625. | ||
Hempel S, Newberry SJ, Maher AR, et al. Probiotics for the prevention and treatment of antibiotic-associated diarrhea: a systematic review and meta-analysis. JAMA. 2012;307(18):1959–1969. | ||
Lau C, Chamberlain RS. Probiotics are effective at preventing Clostridium difficile-associated diarrhea: a systematic review and meta-analysis. Int J Gen Med. 2016;9:27–37. | ||
Rohde CL, Bartolini V, Jones N. The use of probiotics in the prevention and treatment of antibiotic-associated diarrhea with special interest in Clostridium difficile-associated diarrhea. Nutr Clin Pract. 2009;24(1):33–40. | ||
Jones SE, Versalovic J. Probiotic Lactobacillus reuteri biofilms produce antimicrobial and anti-inflammatory factors. BMC Microbiol. 2009;9:35. | ||
Namkin K, Zardast M, Basirinejad F. Saccharomyces boulardii in Helicobacter Pylori eradication in children: a randomized trial from Iran. Iran J Pediatr. 2016;26(1):e3768. | ||
Wang KY, Li SN, Liu CS, et al. Effects of ingesting Lactobacillus- and Bifidobacterium-containing yogurt in subjects with colonized Helicobacter pylori. Am J Clin Nutr. 2004;80(3):737–741. | ||
Bhatia SJ, Kochar N, Abraham P, Nair NG, Mehta AP. Lactobacillus acidophilus inhibits growth of Campylobacter pylori in vitro. J Clin Microbiol. 1989;27(10):2328–2330. | ||
Michetti P, Dorta G, Wiesel PH, et al. Effect of whey-based culture supernatant of Lactobacillus acidophilus (johnsonii) La1 on Helicobacter pylori infection in humans. Digestion. 1999;60(3):203–209. | ||
Hempel S, Newberry S, Ruelaz A, et al. Safety of probiotics used to reduce risk and prevent or treat disease. Evid Rep Technol Assess (Full Rep). 2011:1–645. | ||
Akcam M, Koca T, Salman H, Karahan N. The effects of probiotics on treatment of Helicobacter pylori eradication in children. Saudi Med J. 2015;36(3):286–290. | ||
Bin Z, Ya-Zheng X, Zhao-Hui D, Bo C, Li-Rong J, Vandenplas Y. The efficacy of Saccharomyces boulardii CNCM I-745 in addition to standard Helicobacter pylori eradication treatment in children. Pediatr Gastroenterol Hepatol Nutr. 2015;18(1):17–22. | ||
Hauser G, Salkic N, Vukelic K, JajacKnez A, Stimac D. Probiotics for standard triple Helicobacter pylori eradication: a randomized, double-blind, placebo-controlled trial. Medicine (Baltimore). 2015;94(17):e685. | ||
Ma F, Zhou C, Wang J, Liu T, Liu J. Probiotics in the treatment of peptic ulcer infected by helicobacter pylory and its safety. Pak J Pharm Sci. 2015;28(3 suppl):1087–1090. | ||
Emara MH, Mohamed SY, Abdel-Aziz HR. Lactobacillus reuteri in management of Helicobacter pylori infection in dyspeptic patients: a double-blind placebo-controlled randomized clinical trial. Therap Adv Gastroenterol. 2014;7(1):4–13. | ||
Francavilla R, Polimeno L, Demichina A, et al. Lactobacillus reuteri strain combination in Helicobacter pylori infection: a randomized, double-blind, placebo-controlled study. J Clin Gastroenterol. 2014;48(5):407–413. | ||
Srinarong C, Siramolpiwat S, Wongcha-um A, Mahachai V, Vilaichone RK. Improved eradication rate of standard triple therapy by adding bismuth and probiotic supplement for Helicobacter pylori treatment in Thailand. Asian Pac J Cancer Prev. 2014;15(22):9909–9913. | ||
Du YQ, Su T, Fan JG, et al. Adjuvant probiotics improve the eradication effect of triple therapy for Helicobacter pylori infection. World J Gastroenterol. 2012;18(43):6302–6307. | ||
Mirzaee V, Rezahosseini O. Randomized control trial: comparison of triple therapy plus probiotic yogurt vs. standard triple therapy on Helicobacter pylori eradication. Iran Red Crescent Med J. 2012;14(10):657–666. | ||
Bekar O, Yilmaz Y, Gulten M. Kefir improves the efficacy and tolerability of triple therapy in eradicating Helicobacter pylori. J Med Food. 2011;14(4):344–347. | ||
Deguchi R, Nakaminami H, Rimbara E, et al. Effect of pretreatment with Lactobacillus gasseri OLL2716 on first-line Helicobacter pylori eradication therapy. J Gastroenterol Hepatol. 2012;27(5):888–892. | ||
Medeiros JA, Goncalves TM, Boyanova L, et al. Evaluation of Helicobacter pylori eradication by triple therapy plus Lactobacillus acidophilus compared to triple therapy alone. Eur J Clin Microbiol Infect Dis. 2011;30(4):555–559. | ||
Song MJ, Park DI, Park JH, et al. The effect of probiotics and mucoprotective agents on PPI-based triple therapy for eradication of Helicobacter pylori. Helicobacter. 2010;15(3):206–213. | ||
Yasar B, Abut E, Kayadibi H, et al. Efficacy of probiotics in Helicobacter pylori eradication therapy. Turk J Gastroenterol. 2010;21(3):212–217. | ||
Hurduc V, Plesca D, Dragomir D, Sajin M, Vandenplas Y. A randomized, open trial evaluating the effect of Saccharomyces boulardii on the eradication rate of Helicobacter pylori infection in children. Acta Paediatr. 2009;98(1):127–131. | ||
Szajewska H, Albrecht P, Topczewska-Cabanek A. Randomized, double-blind, placebo-controlled trial: effect of Lactobacillus GG supplementation on Helicobacter pylori eradication rates and side effects during treatment in children. J Pediatr Gastroenterol Nutr. 2009;48(4):431–436. | ||
Kim MN, Kim N, Lee SH, et al. The effects of probiotics on PPI-triple therapy for Helicobacter pylori eradication. Helicobacter. 2008;13(4):261–268. | ||
Scaccianoce G, Zullo A, Hassan C, et al. Triple therapies plus different probiotics for Helicobacter pylori eradication. Eur Rev Med Pharmacol Sci. 2008;12(4):251–256. | ||
Cindoruk M, Erkan G, Karakan T, Dursun A, Unal S. Efficacy and safety of Saccharomyces boulardii in the 14-day triple anti-Helicobacter pylori therapy: a prospective randomized placebo-controlled double-blind study. Helicobacter. 2007;12(4):309–316. | ||
Goldman CG, Barrado DA, Balcarce N, et al. Effect of a probiotic food as an adjuvant to triple therapy for eradication of Helicobacter pylori infection in children. Nutrition. 2006;22(10):984–988. | ||
Ziemniak W. Efficacy of Helicobacter pylori eradication taking into account its resistance to antibiotics. J Physiol Pharmacol. 2006;57(suppl 3):123–141. | ||
Myllyluoma E, Veijola L, Ahlroos T, et al. Probiotic supplementation improves tolerance to Helicobacter pylori eradication therapy – a placebo-controlled, double-blind randomized pilot study. Aliment Pharmacol Ther. 2005;21(10):1263–1272. | ||
Sykora J, Valeckova K, Amlerova J, et al. Effects of a specially designed fermented milk product containing probiotic Lactobacillus casei DN-114 001 and the eradication of H. pylori in children: a prospective randomized double-blind study. J Clin Gastroenterol. 2005;39(8):692–698. | ||
Nista EC, Candelli M, Cremonini F, et al. Bacillus clausii therapy to reduce side-effects of anti-Helicobacter pylori treatment: randomized, double-blind, placebo controlled trial. Aliment Pharmacol Ther. 2004;20(10):1181–1188. | ||
Sheu BS, Wu JJ, Lo CY, et al. Impact of supplement with Lactobacillus- and Bifidobacterium-containing yogurt on triple therapy for Helicobacter pylori eradication. Aliment Pharmacol Ther. 2002;16(9):1669–1675. | ||
Armuzzi A, Cremonini F, Bartolozzi F, et al. The effect of oral administration of Lactobacillus GG on antibiotic-associated gastrointestinal side-effects during Helicobacter pylori eradication therapy. Aliment Pharmacol Ther. 2001;15(2):163–169. | ||
Canducci F, Armuzzi A, Cremonini F, et al. A lyophilized and inactivated culture of Lactobacillus acidophilus increases Helicobacter pylori eradication rates. Aliment Pharmacol Ther. 2000;14(12):1625–1629. |
Supplemenatary Materials
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.