Back to Journals » OncoTargets and Therapy » Volume 13
PRMT5 Promotes Aerobic Glycolysis and Invasion of Breast Cancer Cells by Regulating the LXRα/NF-κBp65 Pathway
Received 22 November 2019
Accepted for publication 27 February 2020
Published 21 April 2020 Volume 2020:13 Pages 3347—3357
DOI https://doi.org/10.2147/OTT.S239730
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sanjay Singh
Xiao Han,1 Linlin Wei,2 Bin Wu3
1Oncology Ward 5, The First Affiliated Hospital of Jinzhou Medical University, Jinzhou, Liaoning Province, People’s Republic of China; 2Medical Center, The First Affiliated Hospital of Jinzhou Medical University, Jinzhou, Liaoning Province, People’s Republic of China; 3Biobank, The First Affiliated Hospital of Jinzhou Medical University, Jinzhou, Liaoning Province, People’s Republic of China
Correspondence: Xiao Han
Oncology Ward 5, The First Affiliated Hospital of Jinzhou Medical University, No. 2, Fifth Section, Renmin Road, Guta District, Jinzhou 121001, Jinzhou 121001, Liaoning Province, People’s Republic of China
Email [email protected]
Objective: To explore the effects of protein arginine methyltransferase 5 (PRMT5) on the biological function of breast cancer cells (BCCs) by regulating the liver X receptor α (LXRα)/NF-κBp65 pathway.
Methods: A total of 80 patients with breast cancer (BC) admitted to our hospital were collected, and 80 breast cancer tissue specimens and 80 corresponding tumor-adjacent tissue specimens were sampled from them for analysis. The reverse transcription-polymerase chain reaction (RT-PCR) was employed to determine the expression of PRMT5 mRNA in the sampled tissues, and the Western blot to determine the expression of LXRα and NF-κBp65 proteins in the tissues and cells. The patients were followed up to analyze their 3-year survival rate. Stable and transient overexpression vectors and inhibition vectors were constructed and transfected into BCCs. The cell counting kit-8 (CCK8), transwell, and flow cytometry were adopted to analyze the proliferation, invasion, and apoptosis of transfected cells, on which the effects of PRMT5 on LXRα and NF-κBp65 proteins were analyzed.
Results: PRMT5 was highly expressed in BC patients, and LXRα was lowly expressed in them, which had a high diagnostic value. Patients with high expression of PRMT5 showed a poor prognosis, and the expression of PRMT5 was related to the tumor size, pathological stage, differentiation, and metastatic in BC patients. Overexpressed PRMT5 enhanced the cell proliferation, invasion, and glycolysis abilities, weakened apoptosis ability, further lowered expression of LXRα and increased expression of NF-κBp65, while inhibited PRMT5 caused opposite results in those aspects. Up-regulating the expression of LXRα suppressed the proliferation, invasion, and aerobic glycolysis of BCCs and promoted their apoptosis, while inhibiting it posed opposite effects. The rescue experiment revealed that down-regulating the expression of PRMT5 could counteract the promotion of down-regulation of LXRα on proliferation, invasion and glycolysis of BCCs, and the nude mouse tumorigenesis test revealed that PRMT5 induced tumor on nude mice by mediating LXRα/NF-κBp65.
Conclusion: Inhibition of the PRMT5 expression can accelerate apoptosis of BCCs and weaken their proliferation, invasion, and aerobic glycolysis through the LXRα/NF-κBp65 pathway.
Keywords: PRMT5, LXRα, NF-κ BP65, breast cancer cell, biological function
Introduction
Breast cancer (BC) is a prevalent malignant tumor among women, whose incidence is gradually increasing in recent years with the changes of social environment and living habits.1 With the progress of medical technology in recent years, the diagnosis and treatment of BC have been greatly improved. However, due to the proliferation and metastasis of breast cancer cells (BCCs) remaining after chemotherapy, BC patients still face a relatively high recurrence rate and metastasis rate after being treated, which is the main death cause of them.2,3 Therefore, it is still a great clinical challenge to deal with the recurrence and metastasis of BC, and it is of great clinical significance to find alternative therapies to replace traditional chemotherapy methods in solving the recurrence and metastasis caused by chemotherapy resistance.4
A previous study reported that the disorder of histone lysine methylation intensified the invasiveness of BCCs.5 Protein arginine methyltransferase 5 (PRMT5), a member of the PRMT family, has been proved to have high expression in various tumors such as lung cancer and bladder cancer.6,7 A previous study concluded that PRMT5 could promote the growth of tumor cells and inhibit apoptosis of them by regulating carcinogenesis and apoptosis signal transduction.8 At present, PRMT5 is under investigation as a potential therapeutic target for cancer in clinical practice, so the mechanism of it in tumor also becomes the focus of basic research. In the procession of tumors, due to the nutritional supply disorder caused by abnormal oxygen and blood vessels, tumor cells have to undergo metabolic reprogramming to maintain their proliferation, and the main characterization of metabolic reprogramming is aerobic glycolysis.9 A previous study revealed that PRMT5 could intensify the glycolysis of pancreatic cancer,10 but there was no study on the effect of PRMT5 on the metabolism of BC and its mechanism. Liver X receptor α (LXRα), as a member of the nuclear receptor family, plays an important role in glucose metabolism and inflammatory response,11 and in recent years, a study has found its important role in the development and progression of tumors. For example, one study found that regulation of the LXRα pathway could suppress the proliferation of colon cancer cells,12 and one other study pointed out that the LXRα pathway may be related to apoptosis of BCCs promoted by 7-ketocholesterol.11 However, there is no study on the relationship between PRMT5 and LXRα yet.
In this study, we explored the influence of PRMT5 on the biological function and aerobic glycolysis of BCCs and its possible mechanism, so as to provide a more theoretical basis for targeted therapy of BC.
Materials and Methods
A total of 80 BC patients undergoing mastectomy from July 2014 to March 2016 in The First Affiliated Hospital of Jinzhou Medical University were collected, and 80 BC tissue specimens and 80 corresponding tumor-adjacent tissue specimens were sampled from them with their consent. The specimens were stored in liquid nitrogen jars, and information about the patients is shown in Table 1. The inclusion criteria of the patients were as follows: Patients diagnosed with BC based on pathology for the first time. The exclusion criteria of them were as follows: Patients who had received radiotherapy and chemotherapy, patients comorbid with one of the malignant tumors, patients with severe liver or kidney dysfunction or a severe infectious disease, and those who refused to provide experimental specimens. All patients and their families agreed to participate in the experiment and signed informed consent forms. This experiment has been approved by the Ethics Committee of The First Affiliated Hospital of Jinzhou Medical University, and in line with the Declaration of Helsinki.
 |
Table 1 Primer Sequences |
All animal experiments were conducted in accordance with policies of the NIH Guide for the Care and Use of Laboratory Animals and the Institutional Animal Care and Use Committee (IACUC) of the First Affiliated Hospital of Jinzhou Medical University. Specific protocols used in this study were approved by the First Affiliated Hospital of Jinzhou Medical University IACUC.
Experimental Materials and Reagents
BCC lines (CF-7, SKBR-3, MDA-MB-231, and BT-20) and human normal breast cells (Hs 578Bst) (American Type Culture Collection (ATCC), Rockville. Maryland, the United States), dulbecco’s modified eagle medium (DMEM) (Gibco, Waltham, Massachusetts, the United States), quantitative real-time polymerase chain reaction (qRT-PCR) and reverse transcription kits (Beijing TransGen Biotech Co., Ltd., China), cell counting kit-8 (CCK8) (Beyotime Biotechnology, Shanghai, China), Transwell kit (Shanghai Fanke Biotechnology Co., Ltd.), phosphate buffer saline (PBS) and fetal bovine serum (FBS) (Gibco Company, the United State), Trizol reagent (Beijing Biolab Technology Co., Ltd.), dual luciferase reporter assay kit (Beijing Biolab Technology Co., Ltd.), radio Immunoprecipitation assay (RIPA) and bicinchoninic acid (BCA) kits (Thermo Scientific Company, the United States), Annexin V-FITC/PI apoptosis kit (Beijing Jiamay Nuno Biotechnology Co., Ltd.), and glucose assay kit and lactic acid assay kit (Sigma-Aldrich), PRMT5, LXRα, NF-κBp65, Glut1, HK2, LDH-A, Caspase-3, Bax, Bcl-2, and β-Actin antibodies (Abcam Company, the United States, 109451, 41902, 16502, 115730, 257118, 101562, 197202, 32503, 185002, and 8226), goat anti-rabbit immunoglobulin G (IgG) secondary antibody (Wuhan BOSTER Biological Technology Co., Ltd.), electrochemiluminescence (ECL) developer (the Thermo Company), and polymerase chain reaction (PCR) instrument (ABI company, the United States). All primers were designed and synthesized by Shanghai Sangon Biotech Co., Ltd.
Cell Culture and Transfection
The human BCC lines (MCF-7, SKBR-3, MDA-MB-231, and BT-20) and human normal breast cells (Hs 578Bst) were cultured in DMEM containing 10% FBS, and then the expression of PRMT5 in each cell line was detected. Subsequently, the targetedly inhibited RMT5 RNA (si-PRMT5), targetedly overexpressed PRMT5 RNA (sh-PRMT5), Si-LXRα, Sh-LXRα, and negative control RNA (Control) were used to transfect MCF-7 and SKBR-3 cells, respectively, with a Lipofectamine™ 2000 kit in strict accordance with the kit instructions.
Real-Time Quantitative PCR
The total RNA was extracted from 100 mg of tissues and 3x106 cells using the Trizol reagent according to the manufacturer’s instructions, and then 5 μg of total RNA was sampled from the extracted total RNAs, respectively, and reversely transcribed into cDNA according to the kit instructions. Then, 1 μL of synthetic cDNA was taken for amplification after reverse transcription. The amplification system consisted of 20 µL of the total volume containing 1μL of cDNA, 0.4 μL of 2 umol/L upstream primers, 0.4 μL of 2 umol/L downstream primers, 10 μL of 2X TransScript® Tip Green qPCR SuperMix, 0.4 μL of Passive Reference Dye (50X), and nuclease-free water to adjust the volume. Data in this study were analyzed using 2−ΔΔct with β-Actin as internal reference. The primer sequences are shown in Table 1.
Proliferation Assay (CCK-8)
The transfected cells were collected to prepare cell suspension, and the suspension was seeded into a 96-well plate at 4×104 cells per well with 100μL of suspension in each well. The plate was cultured under 5% CO2 at 37°C. CCK-8 reagent (10 μL) for cell counting was added into the plate at 24 hrs, 38, 72, and 96 hrs after culturing. Subsequently, the cells were cultured for another 4 hrs, and the optical density was measured at 450 nm using a microplate reader.
Apoptosis Assay
The transfected cells were digested with 0.25% trypsin. After digestion, the cells were washed with PBS two times, and then added with 100μL of binding buffer to prepare 1*106 cells /mL suspension. The suspension was added with AnnexinV-FITC and PI in order, incubated in the dark at room temperature for 5 min, and finally detected using the FACSVerse flow cytometer system.
Cell Invasion Assay
The invasion ability of cells was evaluated using the Transwell assay. DMEM culture solution (200μL) containing 1x105 cells was added into the upper chamber, and 500 mL of DMEM containing 20% FBS was added into the lower chamber. The plate was cultured at 37°C for 48 hrs, and the substrates and cells not passing through the membrane surface in the upper chamber were wiped off. The plate was washed three times, immobilized with paraformaldehyde for 10 mins, washed with double-distilled water three times, and stained with 0.1% crystal violet after being dry, and then detected with regards to cell invasion with a microscope.
Glucose Consumption and Lactic Acid Content
Collected cells were seeded into a 6-well plate at 3x105 cells per well, and the plate was cultured under 5% CO2 at 37°C for 48 hrs. The culture medium of the cells was collected for the determination of glucose consumption and lactic acid production. The glucose and lactic acid levels were determined using corresponding assay kits in strict accordance with the kit instructions.
Western Blot Assay
The RIPA lysis method was employed to lyse cells and extract the total protein. The BCA method was used to determine the protein concentration, and the concentration was adjusted to 4μg/μL. The protein was separated by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and then transferred to polyvinylidene fluoride (PVDF) membrane. The membrane was blocked with 5% skim milk powder for 2 h, and then added with PRMT5 (1:500), LXRα (1:500), NF-κBp65 (1:500), Caspase-3 (1:500), Bax (1:500), Bcl-2 (1:500), Glut1 (1:500), HK2 (1:500), LDH-A (1:500), and β-Actin (1:1000) primary antibodies, and blocked at 4°C overnight. The membrane was washed to remove the primary antibody, added with horseradish peroxidase-labeled goat anti-rabbit secondary antibody (1:1000), incubated at 37°C for 1 hr, rinsed with PBS three times, 5 mins each time. Then, the membrane was developed in a darkroom, absorbed with filter paper to remove excess liquid on the membrane, and made to be luminescent with ECL and developed.
Xenotransplantation Tumor Model
Female BALB/c nude mice (5 weeks old) were raised in sterile conditions, and 100μL of phosphate buffer with 3x106 MCF-7 cells transfected with stable Si-PRMT5 and their control plasmids were injected subcutaneously into the dorsal subcutaneous site of them. The mice were divided into groups (n=5), and their tumor growth was analyzed every 7 days. The tumor volume was calculated by the formula (volume=length × width2×0.52). After 28 days, the nude mice were euthanized, and their tumor size and mass were accurately measured.
Statistical Analysis
In this study, the collected data were analyzed statistically using SPSS19.0 and visualized into required figures using GraphPad 7. Comparison between groups was analyzed using the independent t test, and comparison among multiple groups was analyzed using the one-way ANOVA. Post hoc pairwise comparison was subject to the LSD-t test, and comparison in expression at multiple time points was performed using the repeated measures analysis of variance, and Bonferroni post hoc test was applied. P < 0.05 indicated a significant difference.
Results
High Expression of PRMT5 in BC
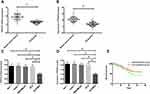
RT-PCR and Western blot assay results showed that PRMT5 mRNA and protein levels in BC tissues and cells were significantly up-regulated. The patients were divided into a high PRMT5 mRNA expression group (45 patients) and a low PRMT5 mRNA expression group (35 patients) according to the average expression of PRMT5 mRNA (1.83). The Kaplan-Meier survival curves revealed that the 3-year survival rate of the high expression group was significantly lower than that of the low expression group, suggesting that high expression of PRMT5 may predict poor prognosis of BC patients. Figure 1. Analysis on clinicopathological features revealed that PRMT5 was linked to tumor size, pathological stage, differentiation, and lymph node metastasis of BC patients (all P<0.05) in Table 2 and Figure 1.
 |
Table 2 Relationship Between PRMT5 and Pathological Data of BC Patients |
Effects of PRMT5 on the Biological Function of BCCs
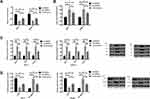
RT-PCR assay revealed that compared with MCF-7 and SKBR-3 cells transfected with Si-PRMT5, those transfected with miR-NC showed significantly decreased expression of PRMT5. The detection of the biological function of cells in the two groups revealed that compared with the miR-NC group, the cells transfected with Si-PRMT5 showed significantly weakened proliferation and invasion abilities and significantly increased apoptosis rate, while those transfected with Sh-PRMT5 showed significantly strengthened proliferation and invasion abilities and significantly decreased apoptosis rate. In addition, compared with the miR-NC group, the cells transfected with Si-PRMT5 showed significantly down-regulated expression of Bcl-2 and significantly up-regulated expression of Caspase-3 and Bax protein, while the cells transfected with Sh-PRMT5 showed significantly up-regulated expression of Bcl-2 and significantly down-regulated expression of Caspase-3 and Bax proteins Figure 2.
Effects of PRMT5 on Aerobic Glycolysis of BCCs and LXRα/NF-κBp65 Pathway
Compared with the Si-NC group, MCF-7 and SKBR-3 cells transfected with Si-PRMT5 showed significantly inhibited glycolysis, down-regulated expression of Glut1, HK2, and LDH-A, up-regulated expression of LXRα, as well as significantly decreased expression of NF-κBp65 (all P<0.05). Compared with the Control group, the MCF-7 and SKBR-3 cells transfected with Sh-PRMT5 showed significantly enhanced glycolysis, significantly up-regulated expression of Glut1, HK2, and LDH-A proteins, significantly decreased expression of LXRα, as well as significantly up-regulated expression of NF-κBp65 (all P<0.05) Figure 3.
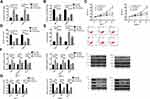
Effects of LXRα on the Biological Function of BCCs and Aerobic Glycolysis
In order to further explore whether PRMT5 affects BCCs by regulating the LXRα/NF-κBp65 pathway, we transfected Si-LXRα and Sh-LXRα into MCF-7 and SKBR-3 cells, finding that compared with the Control group, the Sh-LXRα group showed significantly increased expression of LXRα protein, significantly decreased expression of NF-κBp65 protein, weakened abilities of cell proliferation, invasion, and aerobic glycolysis, significantly increased apoptosis rate, significantly down-regulated expression of Bcl-2, and significantly increased expression of Glut1, HK2, LDH-A, Caspase-3, and Bax proteins (all P<0.05), while the Si-LXRα group showed opposite cell phenotype Figure 4.
Effects of Reversing the Low Expression of LXRα by Down Regulating PRMT5 on BCCs
We co-transfected Si-PRMT5 and Si-LXRα into MCF-7 and SKBR-3 cells. It was turned out that compared with the Si-LXRα group, cells in the Si-PRMT5+Si-LXRα group showed significantly weakened abilities of proliferation, invasion, and glycolysis, enhanced apoptosis rate, significantly decreased expression of Bcl-2, and significantly increased expression of Glut1, HK2, LDH-A, Caspase-3, and Bax proteins (all P<0.05), but those cells were not different from the Control group in those aspects (all P>0.05) Figure 5.
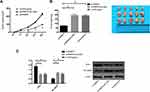
The Role of PRMT5 in Promoting the Tumor Formation in Nude Mice by Mediating LXRα/NF-κBp65
In order to find out whether PRMT5 affects solid tumors through LXRα/NF-κBp65, we conducted a nude mouse tumorigenesis test. We subcutaneously injected Si-NC, si-PRMT5, and si-PRMT5+Si-LXRα into the dorsal subcutaneous site of mice, finding that the tumor volume and mass of nude mice injected with si-PRMT5 obviously reduced, and the mice injected with si-PRMT5+si-LXRα were not greatly different from those in the Control group in tumor volume and mass. We additionally detected the expression of NF-κBp65 protein in tumor tissues of the nude mice, finding that the expression of LXRα protein in the tumor tissues of the mice injected with si-PRMT5 was obviously up-regulated, while the expression of NF-κBp65 protein in them was obviously inhibited, and the expression of LXRα protein and NF-κBp65 protein in tumor tissues of nude mice co-transfected with si-PRMT5+Si-LXRα was reversed (Figure 6).
Discussion
In recent years, the incidence of BC is rising all over the world, and it has become the tumor with the highest incidence among women in some areas.13 The pathogenesis of BC is relatively complicated, which involves factors such as dysregulation and apoptosis regulation of oncogenes and tumor suppressor genes.14 A good progress has been made in the diagnosis and treatment of BC and life quality of the patients after operation, but the high recurrence and high metastasis of BC still pose a serious threat to the long-term survival rate of the patients. Therefore, it is of great clinical significance to explore the pathological mechanism of BC and find new diagnosis and treatment targets.15,16
In recent years, PRMT5 has attracted more and more attention for its role in cancer, and there is evidence indicating that PRMT5 can silence tumor suppressor genes and act as an oncogene based on epigenetics, thus accelerating tumor growth and metastasis.17 For example, PRMT5 can specifically catalyze symmetric dimethylation of histone H4R3 in the promoter region of miR-99 family member to promote the growth and metastasis of lung cancer cells.18 In our study, we found that PRMT5 was also highly expressed in BC. We regulated the expression of PRMT5 in BCCs, finding that silencing the expression of it could suppress the proliferation and invasion of BCCs and promote their apoptosis. A previous study revealed that PRMT5 was highly expressed in triple-negative BC, and it believed that PRMT5 was necessary for cell proliferation,19 and some studies about other tumors also reported that PRMT5 had certain regulatory effects on proliferation, invasion and apoptosis of tumor cells,20,21 which was consistent with our results. The abnormality of aerobic glycolysis is considered as one of the tumor markers, and in BC, aerobic glycolysis can not only provide nutrition for cell proliferation but also help to promote cell proliferation and metastasis.22 A previous study revealed that histone methyltransferase Set8 could regulate aerobic glycolysis by stabilizing HIF1α.23 In our study, we also found that the inhibition of PRMT5 expression significantly suppressed the aerobic glycolysis of BCCs, which was the first time we found that PRMT5 could affect it of BCCs. One previous study reported that PRMT5 could regulate aerobic glycolysis by regulating the expression of PTEN in glioma.24 However, the mechanism of regulating biological function and aerobic glycolysis in BC by PRMT5 is still under investigation.
As a subtype of LXR, LXRα acts as a tumor suppressor gene in a variety of tumors, and it plays a vital role as a nuclear receptor in lipid replacement and transcription control.25 In our study, we found that LXRα was lowly expressed in BC, so we overexpressed LXRα, finding that the overexpression of it significantly suppressed proliferation, invasion, and aerobic glycolysis of BCCs, and dramatically accelerated apoptosis rate, which suggested that LXRα played the role as a tumor suppressor gene in BCCs. We also found that the up-regulation of LXRα significantly inhibited the expression of NF-κBp65 protein. The results from a cancer research center in Britain revealed that compared with normal breast tissues, breast cancer tissues showed significantly inhibited expression of LXRα.26 One study also revealed that LXRα could not only suppress cancer cells related to metabolism but also control many other genes in the tumorigenesis process,27 and one other study clearly pointed out that the inverse agonist of LXR, SR9243, could strongly inhibit the aerobic glycolysis of tumor cells,28 which was consistent with our results. A previous study pointed out that PRMT5 could regulate the inflammatory reaction of tumors and tumorigenesis through methylated modification on NF-κBp65.29 Moreover, one previous study also pointed out that activation of NF-kB could effectively stimulate aerobic glycolysis of BCCs,30 which also confirmed our conclusion. Our study found that LXRα could inhibit the expression of NF-κBp65. Therefore, we suspected that PRMT5 could regulate the expression of NF-κBp65 through LXRα, and ultimately affect the progression and development of tumors. First of all, we found that when the expression of PRMT5 in BCCs was down-regulated, the expression of LXRα was up-regulated accordingly. Then, we performed a co-transfection experiment to cells, finding that down-regulation of PRMT5 can reverse the influence of low expression of LXRα on BCCs. Subsequently, in order to further confirm the regulatory effect of PRMT5 on LXRα, we performed a nude mouse tumorigenesis test, finding that the tumor volume and mass of nude mice injected with si-PRMT5 were much smaller than those of nude mice injected with sh-PRMT5+Sh-LXRα. We further detected the expression of NF-κBp65 protein in tumor tissues of nude mice, finding that injecting si-PRMT5+Si-LXRα for co-transfection could reverse the expression of LXRα protein and NF-κBp65 protein in tumor tissues of nude mice injected with si-PRMT5. A previous study also suggested that inhibition of PRMT5 could suppress the growth of tumors in vivo,31 which further confirmed our conjecture.
To sum up, PRMT5 plays the role of oncogene in BC, which can promote aerobic glycolysis and invasion of BCCs by regulating LXRα/NF-κBp65pathway. However, there are still some deficiencies in this study. For example, firstly, the regulatory mechanism of PRMT5 on LXRα remains unclear. Secondly, the upstream regulation mechanism of PRMT5 in BC is also under investigation. In the future, we will further carry out more basic research to provide more data support for our experimental conclusion.
Acknowledgment
This study was conducted for the Mechanism of Arginine Metabolism and its effect on Biological behavior of Breast Cancer regulated by LXR, JYTFUDF201746, Department of Education of Liaoning Province, Youth Project of Basic Scientific Research Project of Colleges and Universities in Liaoning Province.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Thirumal Kumar D, Jain N, Evangeline J, et al. A computational approach for investigating the mutational landscape of RAC-alpha serine/threonine-protein kinase (AKT1) and screening inhibitors against the oncogenic E17K mutation causing breast cancer. Comput Biol Med. 2019;115:103513. doi:10.1016/j.compbiomed.2019.103513
2. Rosch Justin G, Winter H, DuRoss Allison N, et al. Inverse-micelle synthesis of doxorubicin-loaded alginate/chitosan nanoparticles and assessment of breast cancer cytotoxicity. Colloid Interface Sci Commun. 2019;28:69–74. doi:10.1016/j.colcom.2018.12.002
3. Lea L, Xu S, Czerniecki BJ. CD4 Th1 to the rescue in HER-2+ breast cancer. Oncoimmunology. 2019;8:e1078062. doi:10.1080/2162402X.2015.1078062
4. Zivaljevic V, Jovanovic M, Perunicic V, et al. Surgical treatment of metastasis to the thyroid gland: a single center experience and literature review. Hippokratia. 2018;22(3):137–140.
5. Chang C-J, Yang J-Y, Xia W, et al. EZH2 promotes the expansion of breast tumor-initiating cells by activating RAF1-β-catenin signaling. Cancer Cell. 2011;19:86–100. doi:10.1016/j.ccr.2010.10.035
6. Jing P, Xie N, Zhu X, et al. The methylation induced by protein arginine methyltransferase 5 promotes tumorigenesis and progression of lung cancer. J Thorac Dis. 2018;10(12):7014–7019. doi:10.21037/jtd
7. Hu G, Wang X, Han Y, et al. Protein arginine methyltransferase 5 promotes bladder cancer growth through inhibiting NF-kB dependent apoptosis. EXCLI J. 2018;17:1157–1166. doi:10.17179/excli2018-1719
8. Wu Z, Lin Y. Long noncoding RNA LINC00515 promotes cell proliferation and inhibits apoptosis by sponging miR-16 and activating PRMT5 expression in human glioma. Oncol Targets Ther. 2019;12:2595–2604. doi:10.2147/OTT
9. Deberardinis RJ, Sayed N, Ditsworth D, et al. One brick and one watt: metabolism and growth of tumor cells. Curr Opinion Genet Dev. 2008;18(1):54–61. doi:10.1016/j.gde.2008.02.003
10. Qin Y, Hu Q, Xu J, et al. PRMT5 enhances the tumorigenicity and glycolysis of pancreatic cancer via the FBW7/cMyc axis. Cell Commun Sig. 2019;17(1):30. doi:10.1186/s12964-019-0344-4
11. Levy D, de Melo TC, Oliveira BA, et al. 7-Ketocholesterol and cholestane-triol increase expression of SMO and LXRα signaling pathways in a human breast cancer cell line. Biochem Biophys Rep. 2019;19:100604.
12. Wang G, Li Z, Li X, et al. RASAL1 induces to downregulate the SCD1, leading to suppression of cell proliferation in colon cancer via LXRα/SREBP1c pathway. Biol Res. 2019;52(1):60. doi:10.1186/s40659-019-0268-x
13. Bastos DCDA, Maldaun MVC, Sawaya R, et al. Biological subtypes and survival outcomes in breast cancer patients with brain metastases in the targeted therapy era. Neurooncol Pract. 2018;5:161–169. doi:10.1093/nop/npx033
14. Parvin T, Das C, Choudhury M, et al. Prognostic utility of cyclin D1 in invasive breast carcinoma. Indian J Surg Oncol. 2019;10:167–173. doi:10.1007/s13193-018-0839-2
15. Wang Y, Lewin N, Qaoud Y, et al. The oncologic impact of hormone replacement therapy in premenopausal breast cancer survivors: A systematic review. Breast. 2018;40:123–130. doi:10.1016/j.breast.2018.05.002
16. Inoue H, Horii R, Ito Y, et al. Tumor-infiltrating lymphocytes affect the efficacy of trastuzumab-based treatment in human epidermal growth factor receptor 2-positive breast cancer. Breast Cancer. 2018;25(3):268–274. doi:10.1007/s12282-017-0822-8
17. Jing P, Zhao N, Ye M, et al. Protein arginine methyltransferase 5 promotes lung cancer metastasis through epigenetic regulation of the miR-99 family/FGFR3 signaling. Cancer. 2018;427:38–48.
18. Wang L, Pal S, Sif S. Protein arginine methyltransferase 5 inhibits transcription of the tumor suppressor RB family in leukemia and lymphoma cells. Mol Cell Biol. 2008;28(20):6262–6277. doi:10.1128/MCB.00923-08
19. Morettin A, Baldwin RM, Cote J. Arginine methyltransferases as novel therapeutic targets for breast cancer. Mutagenesis. 2015;30(2):177–189. doi:10.1093/mutage/geu039
20. Wang Y, Li Y, He H, et al. Circular RNA circ-PRMT5 facilitates non-small cell lung cancer proliferation through upregulating EZH2 via sponging miR-377/382/498. Gene. 2019;720:144099. doi:10.1016/j.gene.2019.144099
21. Li Y, Yang Y, Liu X, et al. PRMT5 promotes human lung cancer cell apoptosis via Akt/Gsk3β signaling induced by resveratrol. Cell Transplant. 2019;28(12):1664–1673. doi:10.1177/0963689719885083
22. Al-Shammari AM, Abdullah AH, Allami ZM, et al. 2-Deoxyglucose and Newcastle disease virus synergize to kill breast cancer cells by inhibition of glycolysis pathway through glyceraldehyde3-phosphate downregulation. Front Mol Biosci. 2019;6:90. doi:10.3389/fmolb.2019.00090
23. Huang R, Yu Y, Zong X, et al. The monomethyltransferase SETD8 regulates breast cancer metabolism by stabilizing hypoxia-inducible factor 1alpha. Cancer. 2017;390:1–10.
24. Banasavadi-Siddegowda YK, Russell L, Frair E, et al. The PRMT5-PTEN molecular pathway regulates senescence and self-renewal of primary glioblastoma neurosphere cells. Oncogene. 2017;36(2):263–274. doi:10.1038/onc.2016.199
25. Xiong T, Li Z, Huang X, et al. TO901317 inhibits the development of hepatocellular carcinoma by LXRα/Glut1 decreasing glycometabolism. Am J Physiol Gastrointest Liver Physiol. 2019;316(5):G598–G607. doi:10.1152/ajpgi.00061.2018
26. Vigushin DM, Dong Y, Inman L, et al. The nuclear oxysterol receptor LXRalpha is expressed in the normal human breast and in breast cancer. Med Oncol. 2004;21(2):123–131. doi:10.1385/MO:21:2:123
27. Mutemberezi V, Guillemot-Legris O, Muccioli GG. Oxysterol: from cholesterol metabolites to key mediators. Prog Lipid Res. 2016;64:152–169. doi:10.1016/j.plipres.2016.09.002
28. Flaveny Colin A, Kristine G, El-Gendy Bahaa El-Dien M, et al. Broad anti-tumor activity of a small molecule that selectively targets the warburg effect and lipogenesis. Cancer Cell. 2015;28:42–56. doi:10.1016/j.ccell.2015.05.007
29. Wei H, Wang B, Miyagi M, et al. PRMT5 dimethylates the R30 of the p65 subunit to activate NF-κB. Proc Natl Acad Sci USA. 2013;110(33):13516–13521. doi:10.1073/pnas.1311784110
30. Nasir Kansestani A, Mansouri K, Hemmati S, et al. High glucose-reduced apoptosis in human breast cancer cells is mediated by activation of NF-κB. Iran J Allergy Asthma Immunol. 2019;18:153–162.
31. Saha K, Fisher Matthew L, Adhikary G, et al. Sulforaphane suppresses PRMT5/MEP50 function in epidermal squamous cell carcinoma leading to reduced tumor formation. Carcinogenesis. 2017;38(8):827–836. doi:10.1093/carcin/bgx044
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.