Back to Journals » OncoTargets and Therapy » Volume 14
Primary Tumor Radiotherapy During EGFR-TKI Disease Control Improves Survival of Treatment Naïve Advanced EGFR-Mutant Lung Adenocarcinoma Patients
Authors Hsu KH , Huang JW , Tseng JS , Chen KW, Weng YC, Yu SL, Yang TY, Huang YH, Chen JJW, Chen KC, Chang GC
Received 3 January 2021
Accepted for publication 10 March 2021
Published 25 March 2021 Volume 2021:14 Pages 2139—2148
DOI https://doi.org/10.2147/OTT.S300267
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Gaetano Romano
Kuo-Hsuan Hsu,1,2,* Jing-Wen Huang,2,3,* Jeng-Sen Tseng,2,4,5 Kuan-Wen Chen,6 Yih-Chyang Weng,7 Sung-Liang Yu,8– 12 Tsung-Ying Yang,4,5 Yen-Hsiang Huang,2,4 Jeremy JW Chen,2 Kun-Chieh Chen,4,13– 15 Gee-Chen Chang2,4,5,13– 15
1Division of Critical Care and Respiratory Therapy, Department of Internal Medicine, Taichung Veterans General Hospital, Taichung, Taiwan; 2Institute of Biomedical Sciences, College of Life Sciences, National Chung Hsing University, Taichung, Taiwan; 3Department of Radiation Oncology, Taichung Veterans General Hospital, Taichung, Taiwan; 4Division of Chest Medicine, Department of Internal Medicine, Taichung Veterans General Hospital, Taichung, Taiwan; 5Faculty of Medicine, School of Medicine, National Yang-Ming University, Taipei, Taiwan; 6Department of Radiation Oncology, Taichung Tzu-Chi Hospital, Buddhist Tzu-Chi Medical Foundation, Taichung, Taiwan; 7Radiation Oncology, Nantou Hospital of Ministry of Health and Welfare, Nantou City, Taiwan; 8Department of Clinical Laboratory Sciences and Medical Biotechnology, College of Medicine, National Taiwan University, Taipei, Taiwan; 9Department of Laboratory Medicine, National Taiwan University Hospital, Taipei, Taiwan; 10Center of Genomic Medicine, National Taiwan University College of Medicine, Taipei, Taiwan; 11Department of Pathology and Graduate Institute of Pathology, College of Medicine, National Taiwan University, Taipei, Taiwan; 12Center for Optoelectronic Biomedicine, College of Medicine, National Taiwan University, Taipei, Taiwan; 13Division of Pulmonary Medicine, Department of Internal Medicine, Chung Shan Medical University Hospital, Taichung, Taiwan; 14Institute of Medicine, Chung Shan Medical University, Taichung, Taiwan; 15School of Medicine, Chung Shan Medical University, Taichung, Taiwan
*These authors contributed equally to this work
Correspondence: Gee-Chen Chang; Kun-Chieh Chen
Division of Pulmonary Medicine, Department of Internal Medicine, Chung Shan Medical University Hospital, No. 110, Sec. 1, Jianguo N. Road, Taichung, 402, Taiwan, Republic of China
Tel +886-4-24739595 ext. 34412
Fax +886-4-24739595 #34710
Email [email protected]; [email protected]
Background: Whether radiotherapy only for primary lung tumor (RTPLT) after epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitor (TKI) therapy improves survival of treatment naïve advanced EGFR-mutant lung adenocarcinoma (LAD) patients with/without polymetastasis.
Materials and Methods: This was a retrospective, single-center, observational study. Patients with stage IIIB-IV EGFR-mutant LAD with disease control by EGFR-TKI therapy were divided into curative RTPLT, and control, without radiotherapy (WRTPLT) groups.
Results: A total of 138 patients were enrolled; 46 in the RTPLT group and 92 in the WRTPLT group. Amongst them, 37% had oligometastasis, and 26.1% brain metastasis. The RTPLT group had both significantly longer progression-free survival (PFS) (27.5 months [95% CI 18.1– 36.9] vs 10.9 months [95% CI 6.3– 15.5], P< 0.001) and overall survivor (OS) (NR [95% CI NR-NR] vs 38.0 months [95% CI 31.2– 44.8], P< 0.001), respectively, when compared to the WRTPLT group. In multivariate analysis, the adjusted HR of radiotherapy on PFS was 0.30 (0.19– 0.47) and on OS, 0.11 (0.04– 0.30). Patients with oligometastasis had significantly longer PFS than those with polymetastasis with an HR of 0.35 (0.14– 0.85), P=0.02. Patients with either oligometastasis or polymetastasis had significant longer PFS when undergoing radiotherapy than those without (both P< 0.05). An EGFR-TKI to radiotherapy interval < 24 weeks seemed more beneficial (P=0.097). Radiation pneumonitis comprised 32 (69.6%), 12 (26.1%), and two (4.3%) cases of common terminology criteria grade I, II, and III, respectively.
Conclusion: Curative RTPLT can prolong survival in patients with LAD following EGFR-TKI disease control, both involving oligometastasis and polymetastasis. RTPLT within 24 weeks after EGFR-TKI initiation appeared to be more beneficial with tolerable radiation pneumonitis.
Keywords: radiotherapy to primary lung tumor, RTPLT, EGFR-TKI, lung adenocarcinoma, oligometastasis, polymetastasis
Introduction
Lung cancer is the leading cause of cancer-related mortality worldwide.1 In patients with non-small cell lung cancer (NSCLC), particularly lung adenocarcinoma, activating epidermal growth factor receptor (EGFR) mutation could be found in approximately 10% of Caucasian, and more than 50% of Asian patients.2–4 In patients with EGFR-mutant NSCLC, EGFR-tyrosine kinase inhibitor (TKI) had been considered as the first-line of treatment due to its better response rates and less adverse effects than platinum-based chemotherapy, with a median progression-free survival (PFS) of 9.2 to 13.1 months.5–9 A recent study however has revealed that the third-generation EGFR-TKI, osimertinib, offers longer PFS than first-generation EGFR-TKI.10
Nearly all patients with EGFR-mutated NSCLC eventually develop resistance after their initial response to EGFR-TKIs. Various strategies, which involve combining EGFR-TKI with anti-angiogenesis agents,11,12 cytotoxic chemotherapy,13,14 immunotherapy,15,16 or radiotherapy,17 have all been attempted to decrease the emergence of resistance. In preclinical studies, EGFR-TKI could increase radiosensitivity, while radiotherapy could reduce EGFR-TKI resistance.18,19 A previous study has shown that EGFR-TKIs plus local therapy, including radiotherapy, offered prolonged survival benefits compared to EGFR-TKIs alone in patients experiencing EGFR-mutant NSCLC with synchronous oligometastatic disease.17 This indicates that the addition of local therapy would be valuable even in patients with advanced EGFR-mutant lung cancer. Additionally, it may be beneficial to use local radiotherapy at oligometastatic sites to prolong the duration of EGFR-TKI treatment; however, this may cause adverse effects in patients with polymetastatic lesions in whom more sites should be irradiated. From observational studies, in approximately one-third to one-half of EGFR-mutant patients, the initial progression of TKI-treated cancers occurred predominantly in the original disease sites.20,21 In a retrospective study from the Surveillance, Epidemiology, and End Results (SEER) database, primary tumor resection was associated with improved survival in patients with extrathoracic metastatic NSCLC.22
Here, we aimed to investigate whether radiotherapy at a curative dose only to primary lung tumor can improve survival in treatment naïve patients with EGFR-mutant lung adenocarcinoma following EGFR-TKI disease control for either oligometastasis or polymetastasis, while also delaying or preventing subsequent metastasis. Furthermore, we tried to understand which patients may benefit from local radiotherapy while experiencing tolerable side effects such as radiation pneumonitis.
Materials and Methods
Patients and Methods
This was a single-center, retrospective, observational study, which was approved by the Institutional Review Board of Taichung Veterans General Hospital, Taiwan, and it was also conducted in accordance with the Declaration of Helsinki. Patients diagnosed with lung adenocarcinoma between 2010 and 2018 were identified. Those possessing the following criteria were enrolled: lung adenocarcinoma with sensitizing EGFR mutation, stage IIIB-IV disease according to the 7th Edition of the American Joint Committee for Cancer staging system,23 treatment naïve to EGFR-TKI, and having disease control by first- or second-generation EGFR-TKI treatment. The exclusion criteria were: EGFR mutations with T790M and exon 20 insertion, multiple primary lung cancers, postoperative recurrence, undergoing osimertinib treatment, or having another active malignancy. We also excluded patients from the radiotherapy group if EGFR-TKI had been used for more than 14 months (over the expected median EGFR-TKI efficacy duration) prior the start of local radiotherapy. We defined oligometastasis as being up to five lesions. During the selection process to include subjects for the non-radiotherapy group (control group), we attempted to match the Eastern Cooperative Oncology Group (ECOG) performance status, and oligometastasis with those of the radiotherapy group. Patients were selected randomly from a database that consisted of a total of 527 advanced EGFR-mutant NSCLC patients with a history of EGFR-TKI treatment at Taichung Veterans General Hospital. The selection process would end when the patient numbers achieved the preplanned 1:2 fraction. In total, 46 patients were included in the radiotherapy group; hence, 92 patients without radiotherapy, who met the matching criteria, were selected as the control group after the screening of 129 subjects. We evaluated the treatment response of EGFR-TKIs by the Response Evaluation Criteria in Solid Tumors (Version 1.1).24 We enrolled disease control patients with stable disease and partial response to EGFR-TKI. Tumor response was assessed through systemic imaging including a contrast-enhanced chest computed tomography (CT) with coverage of the whole liver, adrenal glands, and kidneys, as well as abdominal ultrasonography, bone scan, and contrast-enhanced magnetic resonance imaging of the brain. The patients were divided into two groups; with one including those who underwent either radiotherapy at a curative dose only for primary lung tumor using stereotactic ablative radiotherapy (SABR) or conventional radiotherapy after confirmed disease control by EGFR-TKIs, and the other, the control group, involving those without radiotherapy for primary tumor but with disease control through EGFR-TKIs. The decision for using either SABR or conventional radiotherapy depended upon the location and size of the primary tumor. In our practice, we used the definition of central lesion for tumors within 2 cm in all directions of any mediastinal critical structure, including the bronchial tree, esophagus, heart, brachial plexus, major vessels, spinal cord, phrenic nerve, and recurrent laryngeal nerve.25
We used conventional radiotherapy for central lesions. SABR was usually used for non-central tumors sized up to 5 cm, with no limitation on tumor size for patients who had received conventional radiotherapy. In the SABR group, gross tumor volume (GTV) was delineated on axial CT images in the mediastinal setting before the target volume was expanded to include the spiculated margin in the lung window setting. Clinical target volume (CTV) was identical to GTV because the latter encompassed enough microscopic tumors in the lung window setting. Internal target volume (ITV) encompasses the entire intrafraction motion during a breathing cycle on the tumor volumes segmented in each of the 10 phases. Planning target volume (PTV) was determined by adding an identical 3–5 mm margin to the axial plane and longitudinal direction of the ITV. Image-guided radiotherapy was used for daily setup accuracy and real-time tumor mobility and intrafractional shift after the application of an on-board imager with KV cone-beam CT. Volumetric modulated arc therapy involving two coplanar arcs was used in this group. Treatment dose was prescribed to the PTV margin such that 85% of the isodose curve of the isocenter dose volume would cover 95% of the PTV. SABR was performed for 4–10 consecutive days, with each fractionated dose being 6–12 Gy. Respiratory tumor movement was assessed using four-dimensional CT (4D CT) in the SABR group.
The immobilized model and the setup of the conventional radiotherapy patient were the same as SABR although 4D CT was not routinely performed. Thus, ITV was not determined by adding an identical 8–10 mm margin to the axial plane and longitudinal direction of the CTV to become PTV. Intensity-modulated radiation therapy with 4–7 fixed angles was selected in the coplanar fields for this group. Treatment dose was 50–70 Gy in 25–35 fractions with the same restriction in coverage of PTV.
Demographic characteristics and clinical data, including age, gender, smoking status, baseline EGFR mutation status, type of EGFR-TKIs treatment, PFS of EGFR-TKIs, and overall survival (OS) were all collected for analysis. Written informed consent for genetic testing, as well as the use of clinical data was obtained from all patients.
All tests were performed at the ISO15189-certified TR6 Pharmacogenomics Lab in the National Center of Excellence for Clinical Trial and Research of National Taiwan University Hospital. EGFR mutations were assessed using matrix-assisted laser desorption ionization-time of flight mass spectrometry.2,3
Statistical Analyses
Regarding the difference in patient characteristics and demographic data between the radiotherapy and control (without radiotherapy) groups, we used the Fisher’s exact test for assessing age, gender, smoking status, metastatic sites and numbers, baseline EGFR mutation status, response to EGFR-TKIs, and the type of EGFR-TKI treatment. The association between the use and non-use of local radiotherapy, as well as the patterns of failure of EGFR-TKIs was analyzed by the Fisher’s exact test. Survival curves were estimated using the Kaplan–Meier method, whereas the between-group differences in PFS and OS were assessed using a stratified Log rank test. A Cox proportional hazard model for multivariate analyses was used to evaluate both PFS and OS. All statistical tests were performed using SPSS 23.0 (SPSS Inc., Chicago, IL, USA). Two-tailed tests were used and P values < 0.05 were considered statistically significant.
Results
Patient Characteristics and Demographic Data
In total, 138 patients were enrolled, 46 in the radiotherapy group and 92 in the control group. The baseline characteristics are shown in Table 1. There were no differences between the two groups regarding oligometastasis status, ECOG performance status, age, gender, smoking status, tumor stage, brain metastasis, EGFR mutation subtypes, EGFR-TKI drugs, or response to EGFR-TKIs. The PFS and OS of all patients was 15.2 months (95% confidence interval [CI] 13.2–17.2) and 57.5 months (95% CI 43.5–71.4), respectively (Table 2). Amongst the 138 patients, 77.5% (107/138) of them had progressed to first-line EGFR-TKI treatment, with 52.2% (24/46) being in the radiotherapy and 90.2% (83/92) in the control group (P < 0.001). Median follow-up time for the radiotherapy group was 35.1 months (95% CI 27.9–42.4) vs the control group time of 32.9 months (95% CI 27.4–38.3); P = 0.154.
 |
Table 1 Patient Characteristics |
 |
Table 2 Progression-Free Survival, Overall Survival, and Disease Progression Pattern Within the Two Groups |
Amongst those patients experiencing progression, 48.6% (52/107) had progressive disease of primary tumor, with significantly more patients being in the control group than in the radiotherapy group (55.4% vs 25.0%, P = 0.011) (Table 2).
As for other recurrence sites, the incidences were similar for the brain, liver, and bones in both groups (Table 2).
Comparison of Survival Outcomes Between Patients with and without Radiotherapy
Patients who received radiotherapy to primary tumor had both significantly longer PFS (27.6 months [95% CI 18.8–36.5] vs 10.9 months [95% CI 6.3–15.5], P < 0.001) and OS (not reached (NR)) ([95% CI NR-NR] vs 38.0 months [95% CI 31.2–44.8], P < 0.001), respectively, when compared to those who did not (Table 2 and Figure 1).
We conducted multivariate analysis to clarify the prognostic impact of radiotherapy.
The adjusted hazard ratio (HR) of radiotherapy on PFS was 0.27 (0.17–0.44) and on OS 0.11 (0.04–0.30), both P < 0.001 (Table 3).
 |
Table 3 Multivariate Analysis of the Influence of Radiotherapy to Primary Lung Tumor on the Outcome of EGFR-TKI Treatment |
Further analysis on the influence of clinical characteristics and radiotherapy protocol on the outcome of primary tumor radiotherapy showed that oligometastasis had significantly longer PFS than non-oligometastasis (polymetastasis) with an HR of 0.35 (0.14–0.87), P = 0.024 (Table 4).
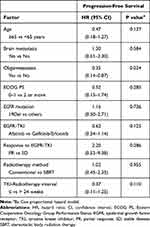 |
Table 4 Univariate Analysis of the Influence of Clinical Characteristics and Radiotherapy Protocol on the Outcome of Primary Tumor Radiotherapy (n = 46) |
Detailed analyses regarding the impact of primary lung tumor radiotherapy on PFS after EGFR-TKI treatment in patients with oligometastatic and polymetastasis diseases were performed. Patients with both oligometastasis and polymetastasis experienced significant PFS benefits due to the addition of local radiotherapy to primary tumor as compared to those without radiotherapy; for patients with oligometastasis NR (95% CI: NR-NR) vs 14.3 months (95% CI 4.0–24.6), P < 0.001, HR 0.25 (95% CI 0.11–0.57), P = 0.001 (Figure 2A); for patients with polymetastasis 23.1 months (95% CI 15.1–31.2) vs 9.1 months (95% CI 7.9–10.2), P < 0.001, HR 0.30 (95% CI 0.18–0.53), P < 0.001 (Figure 2B).
An EGFR-TKI to radiotherapy interval of no more than 24 weeks displayed a better trend of PFS than did a more than 24-week interval in the Log rank test; NR (95% CI: NR-NR) vs 26.4 months (95% CI 20.5–32.4), (P = 0.097) (Supplemental Figure).
There were no significant differences regarding PFS in patients older than 65, those with brain metastasis, ECOG PS 0–1 vs 2, EGFR mutation subtypes, first- vs second-generation EGFR-TKI, partial response vs stable disease, or radiotherapy methods (conventional vs SABR) (Table 4).
OS data were not evaluated because there were only four events amongst the 46 patients. Regarding side effects, cases of radiation pneumonitis in particular resulted as follows: 32 (69.6%), 12 (26.1%), and 2 (4.3%) cases of common terminology criteria (CTC) grade I, grade II, and grade III, respectively. Two patients with CTC grade II and two with grade III required admission. One patient with grade III radiation pneumonitis was admitted to the intensive care unit and expired; however, concurrent influenza B with pneumonia was noted.
Regarding the different radiotherapy methods, the percentage of grade II or more pneumonitis was 47.1% in conventional radiotherapy and 20.7% in SABR, P = 0.097 (OR 3.41 [95% CI 0.92–12.62]; P = 0.066). Additionally, there was no difference in radiation pneumonitis between the first- and second-generation of EGFR-TKIs (gefitinib or erlotinib 31.4% vs afatinib 27.3%; P = 1.000).
Discussion
In this study, we have determined that radiotherapy at a curative dose to the primary lung cancer site is not only feasible, but also allows for prolonged survival in a subset of patients following EGFR-TKI disease control, irrespective of oligometastasis or polymetastasis. Local radiotherapy seems to be more beneficial if it is begun within 24 weeks after EGFR-TKI initiation. The side effect of radiation pneumonitis is tolerable for most patients.
Our study differed from other studies in several aspects. First, local radiotherapy was applied only after disease control through EGFR-TKIs, and not in patients with local progression after EGFR-TKI therapy26 or when concurrent EGFR-TKI and local radiotherapy were performed initially.27 Second, we applied additional local radiotherapy to only primary and not all oligometastatic tumors.17 Third, the patients included were not limited to only those with oligometastasis; there were also patients with polymetastasis who could benefit from the additional local radiotherapy to the primary tumor site after disease control by EGFR-TKIs.
Not all patients with EGFR-mutant lung cancer responded to EGFR-TKIs. The median response time and time to maximal tumor shrinkage for disease control in patients were approximately 2 and 4 months, respectively.28 The timing in combination with radiotherapy deserves discussion. Local radiotherapy was performed at the primary lung tumor with either SABR or conventional radiotherapy after confirming disease control by EGFR-TKIs, approximately 4–6 months after the initial EGFR-TKI treatment. This could obviate the patients with rapid progression after the initial control. Does it make a difference when applying local radiotherapy to only the primary tumor opposed to primary lung and all metastatic tumors? As seen in a previous study, consolidative local ablative therapy (LAT) to primary lung tumor and all metastatic sites in patients with EGFR-mutant oligometastatic NSCLC during first-line EGFR-TKI treatment, offered significantly improved PFS and OS when compared with consolidative LAT to only partial sites or observation alone.17 The types of consolidative LAT included either surgery or radiotherapy, or both.17 Most of our patients (63%) had multiple metastases with more than five lesions, which would cause many adverse effects if all lesions were radiated.
Regarding the effect of local radiotherapy to only primary lung tumor, is this approach reasonable particularly for patients with polymetastatic lung cancer? Resection of the primary tumor is not usually recommended for stage IV cancer. However, an increasing number of publications have revealed that patients may benefit from primary tumor resection (PTR) in various solid organ cancers, including stomach, colon, ovary, breast, and kidney.29–33 Regarding lung cancer, one study from the SEER database showed that the 1-year survival rate of stage IV NSCLC was 15.9%,34 with another revealing that the 5-year survival rate was less than 10% in stage IV NSCLC.35
However, several studies which examined the effects of PTR on stage IV lung cancer found 33.1% of patients experiencing 3-year survival,36 21.1%,37 4-year survival and 26.5% 5-year survival.38 In two retrospective studies taken from the SEER database, PTR was associated with an improved survival in patients with extrathoracic metastatic NSCLC.22,39 These results imply that there is a potential role for PTR in stage IV NSCLC, and may also infer that local radiotherapy with a curative dose to the primary lung tumor could have similar effects to those resulting from primary tumor resection.
The possible mechanisms explaining why primary tumor treatment benefits advanced NSCLC patients were based upon several hypotheses. Primary tumor treatment could reduce the total tumor burden, leading to a better effect from systemic therapy.40 Furthermore, the primary tumor has the potential to spread tumor cells continuously, which are capable of causing metastases.41 Moreover, patients with primary tumors were frequently immunosuppressed. In one report, the presence of primary tumor suppressed both T-cell and antibody responses. After the removal of the primary tumor, immune-competence was restored despite the presence of metastatic tumors.42 In our study, we discovered that more patients in the radiotherapy group were still in disease control, which indirectly proves that local radiotherapy to the primary tumor after disease control could subsequently inhibit metastasis in EGFR-mutant lung adenocarcinoma.
Next, whether adding local radiotherapy after initial response would make a difference in comparison to cases of local treatment failure or concurrent treatment can be argued. There have been few studies performed comparing the different timings of local treatment before, concurrent with, and during EGFR-TKI treatment. In the study by Xu et al, 39 patients who did not receive LAT during first-line EGFR-TKI therapy experienced inferior PFS and OS when compared to those had with 25 patients (64.1%) who had developed disease progression receiving salvage LAT. This result implies that deferral of LAT may be associated with inferior survival.17
In another trial involving concurrent EGFR-TKI and thoracic radiotherapy as first-line treatment for stage IV NSCLC harboring EGFR mutations, only 10 patients were enrolled who experienced a median PFS of 13 months (95% CI: 4.9–21.1 months).27 In another study, 33 patients received local radiotherapy during EGFR-TKI treatment, with the median duration of TKI administration being 14.2 months. The median duration regarding the administration of EGFR-TKI prior to radiotherapy was 5.4 months (range: 0.3–47.3 months).43 In our study, we found that there was a tendency for patients to receive more benefits if local radiotherapy began within 24 weeks after the start of EGFR-TKI, as compared to those who began after more than 24 weeks. This could be the synergistic effect, as EGFR-TKI displays its maximum activity after 4 months of drug use.28
There were several limitations in this study. First, this was a retrospective, single-institution study. Selection bias and imbalances in baseline characters would be inevitably present despite our attempt to match them at every aspect. Herein, we have matched the performance status and metastatic burden, while also having no significant differences in other baseline characteristics among both groups. Second, not all patients with disease control through EGFR-TKIs received local radiotherapy to the primary tumor. In the control group, we excluded patients with primary resistance to EGFR-TKI and the PFS within them was in line with expectations. In the radiotherapy group, the median time to prescribe radiotherapy was 6.6 months, with the majority of them receiving radiotherapy within 10 months of EGFR-TKI treatment. Although selection bias cannot be completely excluded in this retrospective study, we suggest that local therapy to primary tumor may account for, at least in part, the superior outcome of the radiotherapy group. Third, the sample size was not large enough even when we used the control cohort to make the effect of local radiotherapy clearer.
In conclusion, radiotherapy at a curative dose to primary lung tumors after EGFR-TKI disease control is a feasible option for treatment naïve patients with advanced EGFR-mutant lung adenocarcinoma in both oligometastatic and polymetastatic cases, with significantly improved PFS and OS rates when compared with the control group. There is a trend towards achieving benefit if radiotherapy begins within 24 weeks after EGFR-TKI initiation. The side effect of radiation pneumonitis is considered tolerable for most patients. Future prospective clinical trials remain necessary in order to further evaluate these findings.
Disclosure
Kuo-Hsuan Hsu and Jing-Wen Huang are co-first authors for this study. All authors declare no conflicts of interest.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. doi:10.3322/caac.21590
2. Hsu KH, Ho CC, Hsia TC, et al. Identification of five driver gene mutations in patients with treatment-naive lung adenocarcinoma in Taiwan. PLoS One. 2015;10:e0120852. doi:10.1371/journal.pone.0120852
3. Shi Y, Au JS, Thongprasert S, et al. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER). J Thorac Oncol. 2014;9:154–162. doi:10.1097/JTO.0000000000000033
4. Hirsch FR, Bunn PA
5. Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–2388. doi:10.1016/S1470-2045(09)70110-X
6. Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised Phase 3 trial. Lancet Oncol. 2010;11:121–128. doi:10.1016/S1470-2045(09)70364-X
7. Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239–246.
8. Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31:3327–3334. doi:10.1200/JCO.2012.44.2806
9. Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12:735–742. doi:10.1016/S1470-2045(11)70184-X
10. Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med. 2018;378:113–125. doi:10.1056/NEJMoa1713137
11. Saito H, Fukuhara T, Furuya N, et al. Erlotinib plus bevacizumab versus erlotinib alone in patients with EGFR-positive advanced non-squamous non-small-cell lung cancer (NEJ026): interim analysis of an open-label, randomised, multicentre, phase 3 trial. Lancet Oncol. 2019;20:625–635. doi:10.1016/S1470-2045(19)30035-X
12. Nakagawa K, Garon EB, Seto T, et al. Ramucirumab plus erlotinib in patients with untreated, EGFR-mutated, advanced non-small-cell lung cancer (RELAY): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20:1655–1669. doi:10.1016/S1470-2045(19)30634-5
13. Hosomi Y, Morita S, Sugawara S, et al. Gefitinib alone versus gefitinib plus chemotherapy for non-small-cell lung cancer with mutated epidermal growth factor receptor: NEJ009 Study. J Clin Oncol. 2020;38:115–123. doi:10.1200/JCO.19.01488
14. Noronha V, Patil VM, Joshi A, et al. Gefitinib versus gefitinib plus pemetrexed and carboplatin chemotherapy in EGFR-mutated lung cancer. J Clin Oncol. 2020;38:124–136. doi:10.1200/JCO.19.01154
15. Yang JC, Shepherd FA, Kim DW, et al. Osimertinib plus durvalumab versus osimertinib monotherapy in EGFR T790M-positive NSCLC following previous EGFR TKI therapy: CAURAL brief report. J Thorac Oncol. 2019;14:933–939. doi:10.1016/j.jtho.2019.02.001
16. Oxnard GR, Yang JC, Yu H, et al. TATTON: a multi-arm, phase Ib trial of osimertinib combined with selumetinib, savolitinib, or durvalumab in EGFR-mutant lung cancer. Ann Oncol. 2020;31:507–516. doi:10.1016/j.annonc.2020.01.013
17. Xu Q, Zhou F, Liu H, et al. Consolidative local ablative therapy improves the survival of patients with synchronous oligometastatic NSCLC harboring EGFR activating mutation treated with first-line EGFR-TKIs. J Thorac Oncol. 2018;13:1383–1392. doi:10.1016/j.jtho.2018.05.019
18. Shintani S, Li C, Mihara M, et al. Enhancement of tumor radioresponse by combined treatment with gefitinib (Iressa, ZD1839), an epidermal growth factor receptor tyrosine kinase inhibitor, is accompanied by inhibition of DNA damage repair and cell growth in oral cancer. Int J Cancer. 2003;107:1030–1037. doi:10.1002/ijc.11437
19. Tsai YC, Ho PY, Tzen KY, et al. Synergistic blockade of EGFR and HER2 by new-generation EGFR tyrosine kinase inhibitor enhances radiation effect in bladder cancer cells. Mol Cancer Ther. 2015;14:810–820. doi:10.1158/1535-7163.MCT-13-0951
20. Al-Halabi H, Sayegh K, Digamurthy SR, et al. Pattern of failure analysis in metastatic EGFR-mutant lung cancer treated with tyrosine kinase inhibitors to identify candidates for consolidation stereotactic body radiation therapy. J Thorac Oncol. 2015;10:1601–1607. doi:10.1097/JTO.0000000000000648
21. Patel SH, Rimner A, Foster A, et al. Patterns of initial and intracranial failure in metastatic EGFR-mutant non-small cell lung cancer treated with erlotinib. Lung Cancer. 2017;108:109–114. doi:10.1016/j.lungcan.2017.03.010
22. Sun Z, Sui X, Yang F, Wang J. Effects of primary tumor resection on the survival of patients with stage IV extrathoracic metastatic non-small cell lung cancer: a population-based study. Lung Cancer. 2019;129:98–106. doi:10.1016/j.lungcan.2018.11.012
23. Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–1474. doi:10.1245/s10434-010-0985-4
24. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–247. doi:10.1016/j.ejca.2008.10.026
25. Chang JY, Bezjak A, Mornex F. IASLC Advanced Radiation Technology Committee: stereotactic ablative radiotherapy for centrally located early stage non-small-cell lung cancer: what we have learned. J Thorac Oncol. 2015;10:577–585. doi:10.1097/JTO.0000000000000453
26. Yu HA, Sima CS, Huang J, et al. Local therapy with continued EGFR tyrosine kinase inhibitor therapy as a treatment strategy in EGFR-mutant advanced lung cancers that have developed acquired resistance to EGFR tyrosine kinase inhibitors. J Thorac Oncol. 2013;8:346–351. doi:10.1097/JTO.0b013e31827e1f83
27. Zheng L, Wang Y, Xu Z, et al. Concurrent EGFR-TKI and thoracic radiotherapy as first-line treatment for stage IV non-small cell lung cancer harboring EGFR active mutations. Oncologist. 2019;24:1031–e612. doi:10.1634/theoncologist.2019-0285
28. Wu TH, Hsiue EH, Lee JH, et al. Best response according to RECIST during first-line EGFR-TKI treatment predicts survival in EGFR mutation-positive non-small-cell lung cancer patients. Clin Lung Cancer. 2018;19:e361–372. doi:10.1016/j.cllc.2018.01.005
29. Sun J, Song Y, Wang Z, et al. Clinical significance of palliative gastrectomy on the survival of patients with incurable advanced gastric cancer: a systematic review and meta-analysis. BMC Cancer. 2013;13:577. doi:10.1186/1471-2407-13-577
30. Wancata LM, Banerjee M, Muenz DG, Haymart MR, Wong SL. Conditional survival in advanced colorectal cancer and surgery. J Surg Res. 2016;201:196–201. doi:10.1016/j.jss.2015.10.021
31. Eisenkop SM, Friedman RL, Wang HJ. Complete cytoreductive surgery is feasible and maximizes survival in patients with advanced epithelial ovarian cancer: a prospective study. Gynecol Oncol. 1998;69:103–108. doi:10.1006/gyno.1998.4955
32. Gnerlich J, Jeffe DB, Deshpande AD, Beers C, Zander C, Margenthaler JA. Surgical removal of the primary tumor increases overall survival in patients with metastatic breast cancer: analysis of the 1988–2003 SEER data. Ann Surg Oncol. 2007;14:2187–2194. doi:10.1245/s10434-007-9438-0
33. Graham J, Heng DY. Real-world evidence in metastatic renal cell carcinoma. Tumori. 2018;104:76–82. doi:10.1177/0300891618761004
34. Cetin K, Ettinger DS, Hei YJ, O’Malley CD. Survival by histologic subtype in stage IV nonsmall cell lung cancer based on data from the surveillance, epidemiology and end results program. Clin Epidemiol. 2011;3:139–148. doi:10.2147/CLEP.S17191
35. Goldstraw P, Chansky K, Crowley J, et al. The IASLC lung cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol. 2016;11:39–51. doi:10.1016/j.jtho.2015.09.009
36. Pfannschmidt J, Muley T, Bülzebruck H, et al. Prognostic assessment after surgical resection for non-small cell lung cancer: experiences in 2083 patients. Lung Cancer. 2007;55:371–377. doi:10.1016/j.lungcan.2006.10.017
37. Strand TE, Rostad H, Møller B, Norstein J. Survival after resection for primary lung cancer: a population based study of 3211 resected patients. Thorax. 2006;61:710–715. doi:10.1136/thx.2005.056481
38. Asamura H, Goya T, Koshiishi Y, et al. A Japanese Lung Cancer Registry study: prognosis of 13,010 resected lung cancers. J Thorac Oncol. 2008;3:46–52. doi:10.1097/JTO.0b013e31815e8577
39. Xu J, Fan L, Yu H, Lu D, Peng W, Sun G. Survival value of primary tumor resection for stage IV non-small-cell lung cancer: a population based study of 6466 patients. Clin Respir J. 2020. doi:10.1111/crj.13194
40. Griffiths CT, Parker LM, Lee S, Finkler NJ. The effect of residual mass size on response to chemotherapy after surgical cytoreduction for advanced ovarian cancer: long-term results. Int J Gynecol Cancer. 2002;12:323–331. doi:10.1046/j.1525-1438.2002.01150.x
41. Khan SA, Stewart AK, Morrow M. Does aggressive local therapy improve survival in metastatic breast cancer? Surgery. 2002;132:
42. Danna EA, Sinha P, Gilbert M, Clements VK, Pulaski BA, Ostrand-Rosenberg S. Surgical removal of primary tumor reverses tumor-induced immunosuppression despite the presence of metastatic disease. Cancer Res. 2004;64:2205–2211. doi:10.1158/0008-5472.can-03-2646
43. Borghetti P, Bonù ML, Roca E, et al. Radiotherapy and tyrosine kinase inhibitors in stage IV non-small cell lung cancer: real-life experience. Vivo. 2018;32:159–164. doi:10.21873/invivo.11219
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


