Back to Journals » Cancer Management and Research » Volume 13
Primary Testicular Lymphoma with Central Nervous System Relapse Was Successfully Treated by a Chemo-Free Regimen: A Case Report and Literature Review
Authors Yan Z , Yao S, Wang Y, Liu Y , Yao Z
Received 4 October 2021
Accepted for publication 19 December 2021
Published 31 December 2021 Volume 2021:13 Pages 9489—9500
DOI https://doi.org/10.2147/CMAR.S341342
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Harikrishna Nakshatri
Zheng Yan,1 Shuna Yao,1 Yuanyuan Wang,2 Yanyan Liu,1 Zhihua Yao1
1Department of Internal Medicine, Affiliated Cancer Hospital of Zhengzhou University, Zhengzhou, Henan, People’s Republic of China; 2Department of Pathology, Affiliated Cancer Hospital of Zhengzhou University, Zhengzhou, Henan, People’s Republic of China
Correspondence: Zhihua Yao; Yanyan Liu
Department of Internal Medicine, Affiliated Cancer Hospital of Zhengzhou University, Henan Cancer Hospital, No. 127 Dongming Road, Zhengzhou, Henan, 450008, People’s Republic of China
Tel/Fax +86 371-65587791
Email [email protected]; [email protected]
Abstract: Primary testicular lymphoma (PTL) is a rare malignancy of testis. Although the multimodality treatment (including orchiectomy, systemic chemotherapy, scrotal radiotherapy, and preventive central nervous system (CNS)-targeted treatment) is widely used to treat PTL, recurrence, especially CNS recurrence, occurred frequently. Patients with relapsed PTL have a dismal prognosis and limited treatment options. In this report, we described the case of a 63-year-old man with early-stage PTL. The patient received the multimodality treatment, but CNS relapse occurred 3 months following the front-line therapy. We gave him a combined chemo-free regimen treatment, including rituximab, ibrutinib, and lenalidomide (RIL), based on the tumor’s gene mutation profile and the patient’s preference. A complete response was achieved after the first cycle of treatment. Whole-brain radiotherapy was delivered as consolidative treatment following three more cycles of RIL. Thereafter, ibrutinib and lenalidomide continued as maintenance treatment. As of the submission of this manuscript, the response has lasted for more than 16 months. Based on the case, we believe chemo-free regimen RIL might be a favorable approach for PTL patients with CNS relapse, especially those frail elderly patients, when alternative treatments are not available.
Keywords: primary testicular lymphoma, chemo-free regimen, central nervous system relapse, case report
Introduction
Primary testicular lymphoma (PTL) represents 1–7% of all testicular malignancies and is the most common testicular neoplasm in patients over 60 years of age. Histologically, the most common type of PTL is diffuse large B-cell lymphoma (DLBCL), which accounts for approximately 90% of all cases.1,2 PTL has a tendency to disseminate to the contralateral testis and central nervous system (CNS). Due to the rarity of this disease, no randomized prospective trials have been conducted so far to optimize the management of PTL. However, in practice, multimodality treatment, including orchiectomy, systemic chemotherapy, scrotal radiotherapy (RT), and preventive CNS-targeted treatment, is widely used to treat PTL. Although aggressive treatment was applied, recurrence occurred frequently. In patients with long-term follow-up, the 10-year cumulative risk of all relapses is as high as 55% and the 10-year cumulative risk of relapses in the CNS and contralateral testis is 21%.3 The prognosis of patients with relapsed PTL is dismal, with a median survival time of 4.5 to 10 months, and neither salvage chemotherapy nor stem cell transplant had impact on survival.3–5 The prognosis is even worse for patients with CNS relapse, as the treatment options for this extranodal relapse are more limited. So far, only a few PTL patients with CNS relapse have been reported to be successfully treated with salvage therapy. Therefore, novel treatment modalities are urgently needed for this type of patients. In this case report, we introduce a PTL patient, who developed CNS relapse but achieved a durable response following a chemo-free treatment including rituximab, lenalidomide, and ibrutinib. Given the lack of a comprehensive understanding of PTL, we have also reviewed the relevant literature of PTL here.
Case Presentation
In April 2019, a 63-year-old man was admitted to the Department of Urology in our hospital when he found a painless mass in his left testis. He had a history of hypertension for 20 years and diabetes for 2 years. Both diseases were well managed with oral drugs.
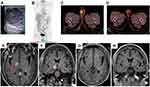
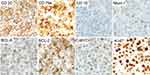
Ultrasound examination showed a low-density mass in his left testis with abundant blood supply (Figure 1A). Laboratory examination found noticeable abnormalities. The patient underwent a left testis orchiectomy on April 16, 2019. Following surgery, the mass was pathologically diagnosed as DLBCL. The tumor cells were positive for CD20, CD19, BCL-6, MUM-1 (dim), and C-MYC, and negative for CD10. BCL-2 was expressed in 90% of tumor cells and Ki-67 was positive in 90% of cells (Figure 2). In situ hybridization of Epstein–Barr encoding region (EBER) was negative. BCL-2, BCL-6, and MYC gene rearrangements using FISH were all negative (Supplementary materials Figure S1). The patient was then transferred to our department for further diagnosis and treatment. PET/CT showed an FDG-avid lesion in the right testis with SUVmax of 11.4, which was considered to be lymphoma involvement (Figure 1B and C). Laboratory parameters were normal, and there was no abnormality on head magnetic resonance imaging (MRI). Cerebrospinal fluid (CSF) examination was negative. The patient was diagnosed with PTL stage I.
The patient received six cycles of R-CHOP regimen (rituximab, cyclophosphamide, epirubicin, vincristine, and prednisone) immunochemotherapy. Intravenous high-dose methotrexate (HD-MTX) was added to the R-CHOP regimen in the first four cycles of immunochemotherapy to prevent CNS relapse. During the last cycle of immunochemotherapy with HD-MTX, the patient experienced reversible renal dysfunction. The HD-MTX was replaced by intrathecal chemotherapy (ITC) with MTX and cytarabine in the last two cycles of immunochemotherapy, to prevent CNS relapse. A complete response (CR) evaluated by PET/CT was achieved at the end of immunochemotherapy (Figure 1D). Following systemic immunochemotherapy, 40 Gy of radiotherapy (RT) were delivered to the whole scrotum in 20 fractions. The entire treatment ended in October 2019.
In March 2020, the patient returned for the first follow-up. He had no discomfort. Physical and laboratory examinations found no abnormalities, but head MRI showed a new lesion involving his right basal ganglia and pons, which was considered as a CNS relapse secondary to PTL (Figure 1E and F). Next-generation sequencing of the initially resected tumor tissue revealed gene mutations in CD79B, MYD88, PIM1, PRDM1, PCLO, TCF3, FOXO1, BCL6, RB1, and SMARCA4 (Supplementary materials Table S1). Salvage chemotherapy was recommended, but the patient detested chemotherapy. Concomitant CD79B and MYD88 mutations may be a genetic marker for the efficacy of BTK inhibitors.6,7 Taking into account the tumor’s gene mutation profile and the patient’s preference, we gave him an individualized treatment, a combined chemo-free regimen including rituximab (375 mg/m2 d1 iv q3w), lenalidomide (25 mg d1-10 po q3w), and ibrutinib (560 mg po daily) (RIL). After the first cycle of treatment, repeated head MRI showed that the intracranial lesion disappeared (Figure 1G and H), indicating that a CR was achieved. Three more cycles of RIL were given. No adverse events were observed. After that, autologous stem cell transplantation (ASCT) was recommended, but the patient was very satisfied with the chemo-free treatment and refused the ASCT. Whole-brain RT (23.4 Gy in 13 fractions) was delivered to the patient as consolidative treatment. Thereafter, lenalidomide (25 mg d1-10 po q3w) and ibrutinib (560 mg po daily) continued as maintenance treatment. As of the submission of this manuscript, the treatment is still ongoing, and the response has lasted for more than 16 months. During this treatment, there were still no adverse events observed. The treatment timelines are shown in Figure 3.
 |
Figure 3 Treatment timelines of the patient. |
Discussion and Literature Review
Clinical Characteristics of PTL
PTL was first reported by Malassez in 1877 and subsequently defined as a clinical entity by Curling in 1878.8 PTL is a rare and peculiar clinical subtype of hematological malignancy in an immune privileged site, which accounts for only 1–2% of extranodal lymphomas. The annual incidence of PTL is 0.09–0.26 per 100,000 individuals.2 The median age of patients at diagnosis is 52 to 70 years, depending on individual reports,4−5−10−25 but the onset age can be as young as 2 years old.2 Pathologically, approximately 90% of PTL patients are DLBCL type, and more than 80% of testicular DLBCL are ABC type based on gene expression profile signature.1–3 Other lymphoma subtypes, such as follicular lymphoma, Burkitt lymphoma, T/B-cell lymphoblastic lymphoma, NK/T-cell lymphoma, mantle cell lymphoma, marginal zone B-cell lymphoma, and plasmablastic lymphoma, have also been reported.2,26–30 At the time of diagnosis, most patients presented with unilateral painless testicular masses, and bilateral testicular involvement was seen in about 2–13.6% of cases.2–5,13,19,20,22 Also, 56–93.8% of the patients presented with Ann Arbor stage I/II disease (Table 1).
 |
Table 1 Reported PTL Studies |
Molecular Features
Genetically, PTL and primary CNS lymphoma (PCNSL) have similar gene mutation profiles, characterized by a high prevalence of MYD88 mutations with or without CD79B mutations. The frequency of mutated MYD88 in PTL ranges from 60% to 82% (Table 2). Coexistence of MYD88 and CD79B mutations, now referred to as the MCD type, was observed in 18.9% of the cases in one study and 91% in another study.31,32 Following MYD88, the frequently mutated genes are PIM1, TBL1XR1, KMT2D, KMT2C, BTG2, ETV6, FAT1, CREBBP, PRDM1, DUSP2, ARID1A, B2M, CIITA, ETS1, NOTCH1, ACTB, CD58, RELN, BRCA1, BRCA2, ATM, FOXO1, and KDM2B.33
 |
Table 2 Important Molecular Aberrations in PTL |
BCL6 rearrangement seems to be a common molecular event in PTL, observed in 16.2–48% of cases, while BCL2 or MYC rearrangement was observed in 4–7% of cases (Table 2). In phenotyping studies, 70% of cases were BCL-2 positive (cutoff 70%), and 13% were MYC positive (cutoff 40%),34 while BCL-2 and MYC double expressor was observed in 15.1–21.4% of cases (Table 2).
Similar to PCNSL, high frequencies of 9p24.1 or PDL gene aberrations were observed in PTL. PDL rearrangement occurred in 4–8% of cases, while PDL gain or amplification was observed in 35.5–54% of cases (Table 2). These aberrations can lead to increased PDL protein expression, which was observed in 50–66.7% of cases.32,35,36
In addition, a complete loss of HLA-A expression was observed in 66% of testicular DLBCL.37 This molecular abnormality may be associated with immune privilege and high propensity for CNS relapse.38
Conventional Treatment and Prognosis
Although the incidence of PTL has increased significantly in the past few decades, there is currently no consensus on a standard therapeutic regimen.
In the early days, the prognosis of PTL was very poor due to insufficient understanding of the biological behavior of the disease and suboptimal treatment. The 5-year overall survival (OS) was only 15.4% before 1977.13 In the early 1980s, it was recognized that patients with surgery alone or surgery plus locoregional radiation had a high rate of distant relapse, so systemic chemotherapy was introduced. However, the recurrence of the CNS or contralateral testis was still common. In 1995, a combined modality treatment was recommended for PTL to reduce the risk of CNS and contralateral testicular recurrence, including systemic anthracycline-based chemotherapy, prophylactic ITC, and scrotal RT. This multimodality treatment was referred to as the standard of care (SOC) by Caumont et al.24 The superiority of SOC was supported by a series of retrospective studies. In a study conducted by the International PTL Consortium on 280 PTL patients, only those who received chemotherapy in combination with both prophylactic ITC and RT had a better survival than those who received chemotherapy alone or chemotherapy plus either prophylactic ITC or RT.3 Similarly, Caumont et al reported that after analyzing the records of 1897 PTL patients from the National Cancer Database (NCDB), the 5-year survival rate of patients receiving SOC treatment was significantly better than that of patients receiving non-SOC treatment (74.1% vs 51.2%).24
PTL shows a continuously high risk of recurrence, and its survival curve following primary treatment does not have a plateau. In addition, once it recurs, PTL has a considerable propensity to involve other extranodal sites, especially the CNS and the contralateral testis. In the long-term follow-up series of 280 cases, the 10-year cumulative risk of relapse was 55%, the risk of extranodal relapse was 46%, and the risk of CNS relapse or contralateral testicular relapse was 21%.3 In another study with 373 cases, the 10-year risk of CNS relapse was 34% and 72% relapses involved extranodal organs or tissues.4 In addition to the CNS and contralateral testis, the frequently involved sites at relapse are bone, skin, bone marrow, lung, soft tissue, adrenal glands, liver, and gastrointestinal tract (Table 1). The prognosis of PTL patients after relapse is extremely poor.3,4
Orchidectomy
There is universal agreement that orchidectomy is the diagnostic and first therapeutic procedure for PTL. Practically, almost all PTL patients received orchidectomy because the urologist was their first consultant when they found a testicular mass. It is unknown if the orchidectomy can be omitted when the entire scrotum and bilateral testis are covered by radiation.
Chemotherapy
Systemic chemotherapy is the most important treatment for PTL, because systemic dissemination is almost inevitable in patients who only receive local-regional treatment.39 Although the optimal combination is unknown, CHOP or CHOP-like regimens are most commonly used worldwide (Table 1). By comparing the outcomes of PTL patients receiving anthracycline-containing chemotherapy with non-anthracycline-containing chemotherapy in a retrospective study of 280 cases, Deng et al concluded that anthracycline-containing chemotherapy is beneficial in improving survival.3
Retrospective studies with small sample sizes and heterogenous treatment have observed the contradictory effects of rituximab on PTL.19 To date, there have been only four small nonrandomized prospective trials in patients with PTL, two in the pre-rituximab era and two in the rituximab era. Linassier et al analyzed 16 patients with early-stage PTL as part of a prospective multicenter study that included 494 cases with stage I/II aggressive lymphoma in the pre-rituximab era. All 16 patients underwent orchiectomy, anthracycline-based chemotherapy, and prophylactic ITC. Also, contralateral radiation was delivered to 14 patients and prophylactic whole-brain radiation to 9 patients. After a median follow-up of 73.5 months, the OS was 65%.12 Another pre-rituximab era study included 34 cases with stage I/II PTL, who were uniformly treated with orchiectomy, CHOPB regimen (CHOP plus bleomycin) chemotherapy, contralateral testicular RT, and HD-MTX (except 1 patient). The 5-year survival rate was 30%.40 In the two prospective studies conducted in the rituximab era, the 5-year survival rates were 66% and 85%, respectively, which were higher than those in the pre-rituximab studies.14,16 Besides, in three retrospective studies that spanned the pre- and post-rituximab eras and included relatively large sample sizes, the 5-year survival rate of patients who received and did not receive rituximab-containing chemotherapy was 60–70.4% vs 34–54%.2,3,19 Collectively, these data show that PTL patients can benefit from rituximab-based treatment.
RT
Prophylactic scrotal RT plays an important role in the multimodality treatment of PTL to reduce the contralateral testicular relapse. The scrotal relapse rate in patients who did not receive scrotal radiation was as high as 35%, while the relapse rate of patients who received scrotal radiation was 0–10%.20 The IELSG study of 373 patients reported that in patients who did not undergo prophylactic RT, the continuous risk of contralateral testicular relapse was 15% at 3 years and 42% at 15 years.4 In another study involving 280 cases, 39% of the patients received RT to the contralateral testis, and the 10-year cumulative risk of relapse of the contralateral testis was 21%.3 In addition to reducing contralateral testicular relapse, prophylactic scrotal RT also reduces CNS relapse and improves the 5-year survival.2,3
For stage I disease, RT should cover the entire scrotum and the contralateral testis; for stage II disease, paraaortic, iliac and pelvic lymph nodes should be covered additionally. Although the radiation technique, delivered dose, and target volume vary from institute to institute, a prescribed dose of at least 30 Gy is necessary for better survival.39
Given the high rate of CNS relapse, a prospective study added prophylactic whole-brain RT into the multimodality treatment for PTL. Of the 16 patients, 9 cases considered to be at high risk of CNS relapse received a total dose of 24 Gy of radiation to the brain and meninges above the second cervical vertebra. After a median follow-up period of 73.5 months, only one patient had CNS relapse.12 It seems that this approach reduces the risk of CNS relapse, but neurotoxicity is a big concern.
Preventive CNS-Targeted Treatment
Compared with other lymphoma subtypes, the most striking feature of PTL might be the continuously high risk of CNS relapse. In most reported series, CNS was the most commonly involved organ in patients with relapsed PTL (Table 1). CNS relapse occurred continuously for up to 10 years after diagnosis.4 Compared to advanced stage disease, limited-stage disease has a longer median time to CNS recurrence (5.4 years vs 0.5 year). The addition of rituximab does not seem to reduce the risk of CNS relapse.19 In long-term follow-up retrospective studies, the 5-year cumulative incidence of CNS relapse was 19–25%, and the 10-year relapse rate was 21–34%.3,4,19,35 However, the incidence of CNS relapse was 0–6.3% in four prospective studies involving 16 to 53 cases.12,14,16,40 There may be two reasons for this discrepancy: first, these prospective studies only included cases of stage I/II disease with a relatively low risk of CNS relapse compared with advanced-stage disease; second, patients in prospective studies received homogenous treatment. In these prospective studies (two in the pre-rituximab era and two in the rituximab era), almost all patients uniformly received anthracycline-based chemotherapy, orchiectomy, contralateral testicular RT, and preventive CNS-targeted treatment.
Preventive CNS-targeted treatment is considered to be a critical part of multimodal treatment. Although the best strategy to prevent CNS relapse remains undetermined, ITC with cytarabine and MTX was the most commonly used method in the reported studies (Table 1). Some studies have shown the protective effect of ITC,3,11 but more studies have failed to support its efficacy in reducing the risk of CNS relapse.3,4,10,20,23,25 In addition, the brain parenchyma is a more common site of CNS involvement in relapsed PTL than the leptomeninges.4,13 A study analyzed the relationship between relapse pattern and disease stage. It found that patients with limited stage mainly had parenchymal relapse (90%), and patients with advanced stage had more recurrence in the leptomeninges (83%).19 These data highlight that ITC is a problematic approach for preventing CNS relapse.
Intravenous HD-MTX has been used as a preventive CNS-targeted treatment in a few studies. In two prospective studies, one in the pre-rituximab era and the other in the rituximab era, intravenous HD-MTX was employed to prevent CNS relapse. The results showed that there was no CNS recurrence after a median follow-up duration of 74 months (pre-rituximab era study) and 64.8 months (the rituximab era study).14,40 In two other prospective studies, one in the pre-rituximab era and the other in the rituximab era, ITC was used as a prophylactic method. After a median follow-up period of 73.5 months (pre-rituximab era study) and 5 years (the rituximab era study), the CNS relapse rates were 6.3% and 6%, respectively.12,16 It seems that intravenous HD-MTX is better than ITC in preventing CNS relapse. A retrospective study compared the effects of intravenous MTX/cytarabine and ITC on the outcomes of PTL patients. It found that intravenous MTX/cytarabine but not ITC improved patients’ OS, but the impact on CNS relapse was not statistically significant.25 It should be noted that these data are generated from studies with small sample sizes.
Prognostic Factors
The 5-year OS rate of PTL patients seems to be comparable to that of DLBCL-NOS patients (Table 1). However, DLBCL-NOS rarely relapses 5 years after primary treatment, while continuous relapses are observed in patients with PTL. So far, only a few studies have reported the 10-year OS of PTL. In two studies involving 280 and 373 cases, the 10-year OS rate was 24% and 27%, respectively.3,4 Note that a considerable number of patients in these studies did not receive SOC. In addition, Cao et al reported a 10-year OS of 68.3%, but they only observed 32 cases, of which 93.8% had early-stage disease.15
It has been reported that the commonly used prognostic factors for DLBCL-NOS, such as advanced stage, older age, poor performance status (PS), multiple extranodal site involvement, elevated lactase dehydrogenase (LDH), and non-anthracycline-based chemotherapy, also have prognostic values for PTL. Besides, prophylactic contralateral testicular RT and CNS-targeted treatment are PTL-specific prognosticators. In addition to these clinical parameters, it is reported that tumor microenvironment features (eg, macrophage and lymphocyte infiltration, CXCR4 and/or pCXCR4 expression) and specific gene (eg, B1XR1, TBL1XR1) mutations are also associated with patients’ survival (Table 3).23,33,34,41,42 However, the most commonly mutated genes MYD88 or CD79B do not affect the prognosis of PTL patients.43 The prognostic value of concomitant MYD88 and CD79B mutations has not been reported. Since most reports are retrospective studies with small sample sizes and heterogenous treatments, these prognostic factors need to be further evaluated in prospective studies.
 |
Table 3 Prognostic Factors for PTL |
Given the high propensity of CNS relapse in PTL patients, identifying the predictors for CNS relapse is of importance. In individual studies, several factors have been reported to be associated with an increased risk of CNS relapse, including advanced stage, elevated LDH levels, and involvement of multiple extranodal sites.19,25 In particular, renal or adrenal involvement is a robust indicator of CNS relapse.15,19 In the report by Twa et al, 4/5 patients with renal/adrenal involvement had CNS relapse. The authors also found that the presence of BCL6 and/or PDL gene rearrangements increased the risk of CNS relapse; in patients with BCL6 and/or PDL rearrangements, the 5-year cumulative incidence of CNS relapse was as high as 41%. However, MYC rearrangement, double hit and dual expressor, and expression of BCL-6, PD-L1, and PD-L2 are not associated with CNS recurrence.35
Moreover, the OS of patients with PTL is significantly influenced by the treatment era.2,13 In a study that included 1169 PTL patients in the SEER (Epidemiology, diagnosis, and End Results) database from 1973–2013, the 5-year survival rate increased from 44.0% in 1973–1997 to 70.4% in 2006–2013.2 It can be said that the significant improvement in survival is mainly due to the optimization of multimodal treatment.
Novel Treatment Strategies
Frequent CNS relapses and poor prognosis require novel treatment strategies for patients with PTL. It must be admitted that there are huge challenges in the treatment of PTL with CNS relapse: first, relapsed diseases are intrinsically resistant to chemotherapy. Second, due to the natural defense of the blood–brain barrier, most anti-cancer drugs are ineffective against CNS metastases. Third, most PTL patients are elderly, for whom intensive treatments are not always applicable.
In recent years, various chemo-free treatments for cancer patients have emerged. These new therapies may overcome chemoresistance through totally different anticancer mechanisms with mild toxicity. These non-cytotoxic drugs have shown excellent antitumor activities in specific lymphoma subtypes, such as BTK inhibitors in mantle-cell lymphoma and chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL), BCL-2 inhibitors in CLL/SLL, immunoregulatory agent lenalidomide in follicular lymphoma, and PD-1/PD-L1 inhibitors in classical Hodgkin lymphoma and NK/T-cell lymphoma. However, these drugs are largely disappointing in DLBCL. For DLBCL, current guidelines recommend the use of BTK inhibitors and lenalidomide for second-line treatment in certain cases, and the XPO1 inhibitor selinexor and anti-CD19 CAR T-cell therapy for third-line treatment. Nevertheless, these drugs have not yet been tried in PTL patients. So far, only two PTL cases with CNS relapse have been successfully treated with chemo-free therapy. Nayak et al reported that a PTL patient with CNS relapse achieved a CR and PFS > 14 months after receiving the PD-1 inhibitor nivolumab.45 As mentioned above, PTL harbors frequent 9p24.1 or PDL gene aberrations and increased PDL protein expression, which are regarded as a predictor of response to anti-PD-1/PD-L1 immunotherapy. Therefore, in the absence of alternative treatment, PD-1/PD-L1 inhibitors are a reasonable choice for relapsed/refractory PTL. Ping et al reported that a PTL patient with CNS relapse was successfully treated with CAR-T therapy.46 This case indicates that CAR T-cell therapy is applicable to selected cases with metastatic CNS cancer, although most clinical trials involving CAR T-cell therapy excluded patients with CNS metastases. In this case report, we present a PTL patient with CNS relapse who achieved durable remission after a combined treatment of ibrutinib, lenalidomide, and rituximab and was well tolerated. To our knowledge, this is the third case of CNS relapse of PTL that has been successfully treated by chemo-free therapy, and the first case of relapsed PTL in the brain that has been successfully treated by RIL regimen. The outstanding efficacy of the RIL regimen in this case can be explained by several reasons. CD79B with or without MYD88 mutations, which are common in PTL, might be a genetic marker for the efficacy of BTK inhibitors.6,7 Moreover, the NF-κB pathway is commonly activated by mutated MYD88 in PTL,43 while lenalidomide is a blocker of NF-κB pathway.9,34 More importantly, both ibrutinib and lenalidomide can cross the blood–brain barrier,47,48 thereby acting on CNS metastases. Nevertheless, the efficacy and the general applicability of the treatments in these reports need to be verified in more patients.
Conclusion
PTL has unique genetic and clinical characteristics different from DLBCL-NOS. Although the optimal treatment has not yet been determined, it is recommended that PTL patients undergo a combination of orchiectomy, anthracycline-based chemotherapy, scrotal radiation, and preventive CNS-targeted therapy. CNS relapse is common in PTL, but the preferable method to prevent CNS is unknown. Patients with relapsed PTL have a dismal prognosis and limited treatment options. Chemo-free regimen RIL might be a favorable approach for PTL patients with CNS relapse, especially those frail elderly patients, when alternative treatments are not available.
Abbreviations
PTL, primary testicular lymphoma; DLBCL, diffuse large B-cell lymphoma; CNS, central nervous system; RT, radiotherapy; CSF, cerebrospinal fluid; R-CHOP, rituximab, cyclophosphamide, epirubicin, vincristine, and prednisone; HD-MTX, high-dose methotrexate; ITC, intrathecal chemotherapy; CR, complete response; ASCT, autologous stem cell transplantation; PCNSL, primary central nervous system lymphoma; OS, overall survival; SOC, standard of care; PS, performance status; LDH, lactase dehydrogenase; PFS, progression-free survival.
Ethics Approval and Consent to Participate
This study was approved by the Research Ethics Committee of Affiliated Cancer Hospital of Zhengzhou University, and informed consent was obtained from the patient.
Consent for Publication
We have obtained consent for publication from the patient.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Cheah CY, Wirth A, Seymour JF. Primary testicular lymphoma. Blood. 2014;123(4):486–493. doi:10.1182/blood-2013-10-530659
2. Xu H, Yao F. Primary testicular lymphoma: a SEER analysis of 1,169 cases. Oncol Lett. 2019;17(3):3113–3124. doi:10.3892/ol.2019.9953
3. Deng L, Xu-Monette ZY, Loghavi S, et al. Primary testicular diffuse large B-cell lymphoma displays distinct clinical and biological features for treatment failure in rituximab era: a report from the International PTL Consortium. Leukemia. 2016;30(2):361–372. doi:10.1038/leu.2015.237
4. Zucca E, Conconi A, Mughal TI, et al. Patterns of outcome and prognostic factors in primary large-cell lymphoma of the testis in a survey by the International Extranodal Lymphoma Study Group. J Clin Oncol. 2003;21(1):20–27. doi:10.1200/JCO.2003.11.141
5. Ma RZ, Tian L, Tao LY, et al. The survival and prognostic factors of primary testicular lymphoma: two-decade single-center experience. Asian J Androl. 2018;20(6):615–620. doi:10.4103/aja.aja_73_18
6. Wilson WH, Young RM, Schmitz R, et al. Targeting B cell receptor signaling with ibrutinib in diffuse large B cell lymphoma. Nat Med. 2015;21(8):922–926. doi:10.1038/nm.3884
7. Grommes C, Pastore A, Palaskas N, et al. Ibrutinib unmasks critical role of Bruton tyrosine kinase in primary CNS lymphoma. Cancer Discov. 2017;7(9):1018–1029. doi:10.1158/2159-8290.CD-17-0613
8. Kraan W, Horlings HM, van Keimpema M, et al. High prevalence of oncogenic MYD88 and CD79B mutations in diffuse large B-cell lymphomas presenting at immune-privileged sites. Blood Cancer J. 2013;3:e139. doi:10.1038/bcj.2013.28
9. Oishi N, Kondo T, Nakazawa T, et al. High prevalence of the MYD88 mutation in testicular lymphoma: immunohistochemical and genetic analyses. Pathol Int. 2015;65(10):528–535. doi:10.1111/pin.12336
10. Zouhair A, Weber D, Belkacemi Y, et al. Outcome and patterns of failure in testicular lymphoma: a multicenter Rare Cancer Network study. Int J Radiat Oncol Biol Phys. 2002;52(3):652–656. doi:10.1016/s0360-3016(01)02647-5
11. Hasselblom S, Ridell B, Wedel H, Norrby K, Sender Baum M, Ekman T. Testicular lymphoma–a retrospective, population-based, clinical and immunohistochemical study. Acta Oncol. 2004;43(8):758–765. doi:10.1080/02841860410002851
12. Linassier C, Desablens B, Lefrancq T, et al. Stage I-IIE primary non-Hodgkin’s lymphoma of the testis: results of a prospective trial by the GOELAMS Study Group. Clin Lymphoma. 2002;3(3):167–172. doi:10.3816/clm.2002.n.023
13. Mazloom A, Fowler N, Medeiros LJ, Iyengar P, Horace P, Dabaja BS. Outcome of patients with diffuse large B-cell lymphoma of the testis by era of treatment: the M. D. Anderson Cancer Center experience. Leuk Lymphoma. 2010;51(7):1217–1224. doi:10.3109/10428191003793358
14. Aviles A, Nambo MJ, Cleto S, Neri N, Huerta-Guzman J. Rituximab and dose-dense chemotherapy in primary testicular lymphoma. Clin Lymphoma Myeloma. 2009;9(5):386–389. doi:10.3816/CLM.2009.n.075
15. Cao B, Ji DM, Zhou XY, et al. A clinical analysis of primary testicular diffuse large B-cell lymphoma in China. Hematology. 2011;16(5):291–297. doi:10.1179/102453311X13085644680221
16. Vitolo U, Chiappella A, Ferreri AJ, et al. First-line treatment for primary testicular diffuse large B-cell lymphoma with rituximab-CHOP, CNS prophylaxis, and contralateral testis irradiation: final results of an international Phase II trial. J Clin Oncol. 2011;29(20):2766–2772. doi:10.1200/JCO.2010.31.4187
17. Jordanova ES, Riemersma SA, Philippo K, Schuuring E, Kluin PM. Beta2-microglobulin aberrations in diffuse large B-cell lymphoma of the testis and the central nervous system. Int J Cancer. 2003;103(3):393–398. doi:10.1002/ijc.10824
18. Kim J, Yoon DH, Park I, et al. Treatment of primary testicular diffuse large B cell lymphoma without prophylactic intrathecal chemotherapy: a single center experience. Blood Res. 2014;49(3):170–176. doi:10.5045/br.2014.49.3.170
19. Kridel R, Telio D, Villa D, et al. Diffuse large B-cell lymphoma with testicular involvement: outcome and risk of CNS relapse in the rituximab era. Br J Haematol. 2017;176(2):210–221. doi:10.1111/bjh.14392
20. Zhou, Bao C, Ye X, et al. Clinical and histological features of primary testicular diffuse large B-cell lymphoma: a single center experience in China. Oncotarget. 2017;8(68):112384–112389. doi:10.18632/oncotarget.19736
21. Jovanovic MP, Mihaljevic B, Jovanovic P, et al. Clinicopathological and fluorescence in situ hibridisation analysis of primary testicular diffuse large B-cell lymphoma: a single-centre case series. Pol J Pathol. 2018;69(2):136–142. doi:10.5114/pjp.2018.76697
22. Liu YZ, Luo P, Liu C, et al. Prognostic significance of LDH ratio in serum/cerebral spinal fluid of patients with primary testicular diffuse large B-cell lymphoma. Onco Targets Ther. 2019;12:10469–10475. doi:10.2147/OTT.S228746
23. Chen B, Cao DH, Lai L, et al. Adult primary testicular lymphoma: clinical features and survival in a series of patients treated at a high-volume institution in China. BMC Cancer. 2020;20(1):220. doi:10.1186/s12885-020-6711-0
24. Caumont F, Porter C, DeBerg H, Burns J, Frankel J, Flores JP. Combined chemotherapy and radiotherapy improves survival in 1897 testicular Lymphoma patients from a contemporary cohort. Urol Oncol. 2020;38(7):641e1–641e8. doi:10.1016/j.urolonc.2020.02.027
25. Mannisto S, Vahamurto P, Pollari M, et al. Intravenous but not intrathecal central nervous system-directed chemotherapy improves survival in patients with testicular diffuse large B-cell lymphoma. Eur J Cancer. 2019;115:27–36. doi:10.1016/j.ejca.2019.04.004
26. Huang Y, Shi X, Zhong P, et al. De Novo testicular extranodal NK/T-cell lymphoma: a clinicopathologic study of 21 cases with review of additional 18 cases in the literature. Am J Surg Pathol. 2019;43(4):549–558. doi:10.1097/PAS.0000000000001210
27. Lones MA, Raphael M, McCarthy K, et al. Primary follicular lymphoma of the testis in children and adolescents. J Pediatr Hematol Oncol. 2012;34(1):68–71. doi:10.1097/MPH.0b013e31820e4636
28. Liang DN, Yang ZR, Wang WY, et al. Extranodal nasal type natural killer/T-cell lymphoma of testis: report of seven cases with review of literature. Leuk Lymphoma. 2012;53(6):1117–1123. doi:10.3109/10428194.2011.645209
29. Sugimoto K, Koike H, Esa A. Plasmablastic lymphoma of the right testis. Int J Urol. 2011;18(1):85–86. doi:10.1111/j.1442-2042.2010.02669.x
30. Licci S, Morelli L, Covello R. Primary mantle cell lymphoma of the testis. Ann Hematol. 2011;90(4):483–484. doi:10.1007/s00277-010-1049-3
31. Kraan W, van Keimpema M, Horlings HM, et al. High prevalence of oncogenic MYD88 and CD79B mutations in primary testicular diffuse large B-cell lymphoma. Leukemia. 2014;28(3):719–720. doi:10.1038/leu.2013.348
32. Chapuy B, Roemer MG, Stewart C, et al. Targetable genetic features of primary testicular and primary central nervous system lymphomas. Blood. 2016;127(7):869–881. doi:10.1182/blood-2015-10-673236
33. Wang X, Xu X, Cai W, et al. TBL1XR1 mutation predicts poor outcome in primary testicular diffuse large B-cell lymphoma patients. Biomark Res. 2020;8:10. doi:10.1186/s40364-020-00189-1
34. Menter T, Ernst M, Drachneris J, et al. Phenotype profiling of primary testicular diffuse large B-cell lymphomas. Hematol Oncol. 2014;32(2):72–81. doi:10.1002/hon.2090
35. Twa DDW, Lee DG, Tan KL, et al. Genomic predictors of central nervous system relapse in primary testicular diffuse large B-cell lymphoma. Blood. 2021;137(9):1256–1259. doi:10.1182/blood.2020006338
36. Zhu D, Zhu J, Yu W, et al. Expression of programmed cell death-ligand 1 in primary testicular diffuse large B cell lymphoma: a retrospective study. Oncol Lett. 2019;18(3):2670–2676. doi:10.3892/ol.2019.10595
37. Riemersma SA, Oudejans JJ, Vonk MJ, et al. High numbers of tumour-infiltrating activated cytotoxic T lymphocytes, and frequent loss of HLA class I and II expression, are features of aggressive B cell lymphomas of the brain and testis. J Pathol. 2005;206(3):328–336. doi:10.1002/path.1783
38. Booman M, Douwes J, Legdeur MC, van Baarlen J, Schuuring E, Kluin P. From brain to testis: immune escape and clonal selection in a B cell lymphoma with selective outgrowth in two immune sanctuaries [correction of sanctuariesy]. Haematologica. 2007;92(6):e69–71. doi:10.3324/haematol.11421
39. Brouwer CL, Wiesendanger EM, van der Hulst PC, van Imhoff GW, Langendijk JA, Beijert M. Scrotal irradiation in primary testicular lymphoma: review of the literature and in silico planning comparative study. Int J Radiat Oncol Biol Phys. 2013;85(2):298–308. doi:10.1016/j.ijrobp.2012.06.019
40. Aviles A, Neri N, Huerta-Guzman J, Perez F, Fernandez R. Testicular lymphoma: organ-specific treatment did not improve outcome. Oncology. 2004;67(3–4):211–214. doi:10.1159/000081319
41. Pollari M, Bruck O, Pellinen T, et al. PD-L1(+) tumor-associated macrophages and PD-1(+) tumor-infiltrating lymphocytes predict survival in primary testicular lymphoma. Haematologica. 2018;103(11):1908–1914. doi:10.3324/haematol.2018.197194
42. Leivonen SK, Pollari M, Bruck O, et al. T-cell inflamed tumor microenvironment predicts favorable prognosis in primary testicular lymphoma. Haematologica. 2019;104(2):338–346. doi:10.3324/haematol.2018.200105
43. Chen YP, Ke LF, Lu JP, et al. Prevalence and clinical significance of oncogenic CD79B and MYD88 mutations in primary testicular diffuse large b-cell lymphoma: a retrospective study in China. Onco Targets Ther. 2019;12:10165–10175. doi:10.2147/OTT.S222189
44. Bernasconi B, Uccella S, Martin V, et al. Gene translocations in testicular lymphomas. Leuk Lymphoma. 2014;55(6):1410–1412. doi:10.3109/10428194.2013.834055
45. Nayak L, Iwamoto FM, LaCasce A, et al. PD-1 blockade with nivolumab in relapsed/refractory primary central nervous system and testicular lymphoma. Blood. 2017;129(23):3071–3073. doi:10.1182/blood-2017-01-764209
46. Ping N, Qu C, Bai L, et al. Successful chimeric antigen receptor T cell therapy in a case of primary testicular diffuse large-B-cell lymphoma with central nervous system progression. Leuk Lymphoma. 2019;60(11):2814–2816. doi:10.1080/10428194.2019.1605507
47. Bernard S, Goldwirt L, Amorim S, et al. Activity of ibrutinib in mantle cell lymphoma patients with central nervous system relapse. Blood. 2015;126(14):1695–1698. doi:10.1182/blood-2015-05-647834
48. Rubenstein JL, Treseler PA, Stewart PJ. Regression of refractory intraocular large B-cell lymphoma with lenalidomide monotherapy. J Clin Oncol. 2011;29(20):e595–7. doi:10.1200/JCO.2011.34.7252
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.