Back to Journals » Cancer Management and Research » Volume 14
Primary Testicular and Cutaneous Philadelphia Chromosome Positive B-Cell Lymphoblastic Lymphoma: A Rare Case and Review
Authors Yu Q, Wang G, Wang J, Zhang W, Meng L, Cao Y
Received 8 December 2021
Accepted for publication 4 April 2022
Published 21 April 2022 Volume 2022:14 Pages 1507—1514
DOI https://doi.org/10.2147/CMAR.S353022
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Antonella D'Anneo
Video abstract of "Primary Testicular and Cutaneous Philadelphia Chromosome Ph+ B-LBL" [ID 353022].
Views: 181
Qiuxia Yu,* Gaoxiang Wang,* Jue Wang, Wei Zhang, Li Meng, Yang Cao
Department of Hematology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, 430030, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yang Cao, Department of Hematology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, No. 1095 Jiefang Avenue, Wuhan, Hubei, 430030, People’s Republic of China, Tel +86 2783662680, Fax +86 2783662680, Email [email protected]
Abstract: Philadelphia chromosome positive B cell lymphoblastic lymphoma (Ph+ B-LBL) is an extremely rare disease. We report a 27-year-old patient diagnosed with primary testicular and cutaneous Ph+ B-LBL without bone marrow involvement. The CCCG-LBL-2016 regimen (https://clinicaltrials.gov/ct2/show/NCT02845882) was initially administered due to the fast pathological diagnosis as B-LBL that was first obtained. To identify potential therapeutic targets, RNA sequencing (RNAseq) was also performed on lymph node specimens as a part of the routine diagnostic workup in our center. Unexpectedly, IKZF1 deletions and BCR-ABL1 fusion transcripts were detected. Based on these results, we retrospectively performed fluorescence in situ hybridization (FISH) for BCR/ABL1 rearrangements in the same lymph node specimen, and a 70% positive signal was detected. The patient subsequently received the CCCG-LBL-2016 protocol combined with the BCR-ABL tyrosine kinase inhibitor (TKI) dasatinib, along with prophylactic intrathecal infusion. Then, the patient underwent TBI-based haploidentical (haplo) allogeneic hematopoietic stem cell transplantation (haplo-allo-HSCT) as consolidation following the achievement of remission and continued taking dasatinib as maintenance therapy. The patient was still in complete remission 1 year after diagnosis. This case indicates that the detection of potential molecular targets, especially those targets that can be pharmacologically treated, such as BCR/ABL1 fusion transcripts, is of important value to both LBL diagnosis and therapeutic strategy choices. FISH, reverse transcriptase polymerase chain reaction (RT-PCR) and/or RNAseq should be routinely carried out in lymphoma specimens to depict its genetic landscape for the further execution of a precise therapy strategy.
Keywords: B-cell lymphoblastic lymphoma, Philadelphia chromosome, tyrosine kinase inhibitor, hematopoietic stem cell transplantation
Background
Lymphoblastic lymphoma (LBL) is a rare neoplasm of immature lymphoid progenitors that arises from either B cell or T cell lineages. Due to their similar biological features, LBL and acute lymphoblastic leukemia (ALL) are considered a single entity (lymphoblastic leukemia/lymphoma) in the recent World Health Organization (WHO) classification of precursor lymphoid neoplasms.1 Nevertheless, LBL is distinguished from ALL in clinical presentation. ALL mainly manifests as extensive bone marrow involvement. However, LBL is characterized by a predominantly nodal distribution of disease, and the percentage of bone marrow lymphoblast involvement is less than 25%. B-lineage LBL comprises approximately 10% of all LBLs, with a higher incidence in the younger age population.2 Extranodal bone lesions, including the skin, soft tissues and osteolytic bone lesions, are frequently involved, but primary involvement sites of the bone marrow, visceral sites, testicles or central nervous system are rare.3 The general characteristics of B-ALL/LBL and the different types of B-ALL/LBL with recurrent genetic abnormalities are detailed in the Table S1.4–7
The Philadelphia (Ph) chromosome is the most common cytogenetic abnormality associated with chronic myeloid leukemia (CML) and ALL in adults, especially in elderly patients.8 Therefore, Ph+ B-LBL is an extremely rare disease. To date, only 8 cases of Ph+ B-LBL have been reported in the published literature.9–16 Herein, we report a case of a male with Ph+ B-LBL with unilateral swollen testis and scattered skin erythema as the first clinical presentations who successfully achieved complete remission through the high-risk chemotherapy protocol of CCCG-LBL-2016 combined with dasatinib. He maintained remission after TBI-based haplo-allo-HSCT and was subsequently maintained on dasatinib. This case study was approved by the Ethics Committee of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology. The patient provided written informed consent according to the Declaration of Helsinki.
Case Presentation
A previously healthy 27-year-old male first presented to the outpatient department in Tongji Hospital due to right testis swelling and scattered skin erythema onset for approximately 1 month. There were no other concomitant symptoms, such as fever, night sweat, or loss of weight. On physical examination, painless enlargement of submandibular and cervical lymph nodes was palpable. Then, he was admitted to our department for further diagnosis and treatment.
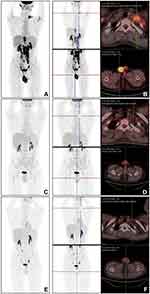
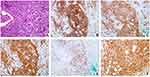
Routine laboratory evaluation demonstrated normal white blood cell (WBC) counts and hemoglobin and mildly increased platelet counts [376.0*109/L (normal: 150–350*109/L)], lactate dehydrogenase [234 U/L (normal:135–225 U/L)] and β2 microglobulin [2.76 mg/L (normal:0.8–2.2 mg/L)]. Serology tests were negative for HIV, HBV, CMV and EBV infection. An abdominal ultrasound did not indicate hepatomegaly or splenomegaly. Bone marrow smear and histology revealed normal morphological, immunological flow cytometric (FCM), cytogenetic and molecular (MICM) results. No central nervous system infiltration was detected by cerebrospinal fluid flow cytometry. However, positron emission tomography/computed tomography (PET/CT) scans demonstrated abnormally increased metabolic activity in multiple lymph nodes (SUV max: 9.4), bones (right clavicle, left scapula, left 2nd rib, right accessory of 11th thoracic vertebra, 1st-2nd lumbar vertebra, bilateral femurs and bilateral superior tibial segment; SUV max: 6.2), the posterior walls of the nasopharynx (SUV max: 6.5), the bilateral ethmoid sinuses (SUV max: 5.0), and the bilateral testicular and right spermatic cords (SUV max: 7.3). There was also mildly increased metabolic activity in the subcutaneous nodules on the bilateral cheeks (SUV max: 2.8) (Figure 1A and B). The patient underwent ultrasound-guided fine-needle aspiration in the left enlarged supraclavicular lymph node. The pathological biopsy specimen was extensively replaced with tumor cells characterized by distinct nucleoli and scant cytoplasm. Immunohistochemical staining showed that representative B lymphoblastic tumor cells were positive for CD20 and terminal deoxynucleotidyl transferase (TdT) and negative for CD3, and approximately 90% of the neoplastic cells displayed nuclear Ki67 staining (Figure S1). Meanwhile, the cutaneous biopsy, also revealed consistency of the phenotype of the B lymphoblasts, were positive for CD20, CD19, PAX5, TDT, CD10, CD38, C-MYC, CD99, BCL-2 and CD43, but negative for CD3, CD34, CD7, BCL-6, MPO and MUM1, and Ki67 staining about 90% (Figure 2). Therefore, the diagnosis of B-LBL (Ann Arbor stage IIIA, IPI 3 scores) was confirmed.
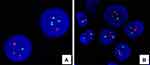
To control lymphoma in a timely manner, the patient received the high-risk chemotherapy protocol of CCCG-LBL-2016 (Induction I with prednisone, vincristine, daunorubicin, PEG-LASP, cyclophosphamide, cytarabine, 6-mercaptopurine plus prophylactic intrathecal infusion from Day 1 to Day 63). RNAseq, with reference to the GRCh37/hg19 genome and mainly to detect genetics disruption at the level of the human transcriptome, was performed on the left supraclavicular lymph node specimens as a part of routine diagnostic workup in our institution. Unexpectedly, IKZF1 deletions and BCR-ABL1 fusion transcripts were detected approximately 3 weeks after chemotherapy. Based on these results, we retrospectively validated BCR/ABL1 rearrangement in previous lymph node biopsy specimens by fluorescence in situ hybridization (FISH), which detected a 70% positive signal (Figure 3). Given that the modified diagnosis turned to Ph+ B-LBL, a combination of dasatinib (140mg, qd) was added to therapeutic regimen. PET/CT evaluation was carried out before Protocol M of CCCG-LBL-2016 began, and it showed complete remission (CR) (Figure 1C and D), Protocol M (5 g/m2 methotrexate and 6-mercaptopurine) combined with dasatinib proceeded as planned for 4 cycles. Then, the patient finally underwent allogeneic transplantation with granulocyte colony-stimulating factor-mobilized bone marrow cells plus peripheral blood hematopoietic stem cells from his father, who was mismatched for five loci in March 2021. He received a TBI/VP/CY/ATG conditioning regimen consisting of total body irradiation (Day −9), etoposide (15 mg/kg/day on Days −8 and −7), cyclophosphamide (1.8 g/m2/day on Days −6 and −5), and ATG (thymoglobulin, 2.0 mg/kg/day on Days −4 to −1). The procedure was well tolerated, and he achieved successful engraftment on Day 15 post-stem cell infusion (SCI). He did not undergo any grade of acute or chronic graft-versus-host disease (GVHD) but only had temporary and asymptomatic Epstein–Barr virus (EBV)-reactivated viremia. All immunosuppressants were tapered and stopped within 2 months after SCI, and he took dasatinib 50 mg daily for maintenance. Monthly follow-ups were regularly performed, including the monitoring of bone marrow chimerism and BCR/ABL1 copies by polymerase chain reaction amplification of short tandem repeats (PCR-STR) and RT-PCR, respectively. Sustained complete chimerism and negative BCR/ABL1 detection, along with recent PET/CT results (Figure 1E and F), indicated a satisfactory CR for more than 1 year.
Discussion and Conclusion
Philadelphia chromosome-positive (Ph+) B cell lymphoblastic lymphoma is an extremely uncommon entity. To date, only 8 cases of Ph+ B-LBL have been published (see Table 1). Although B-LBL predominantly occurs in the younger-aged population, the 8 cases of Ph+ B-LBL that are currently reported involve all ages. B-LBL typically involves lymph nodes and extranodal sites, such as the skin, bone and soft tissues. Testicular infiltration occurs more frequently in disease progression and relapse and rarely appears as an initial manifestation. To our knowledge, this is the first case report of primary involvement of the testis and skin without evidence of bone marrow infiltration in B-LBL.
 |
Table 1 Summary of Previous Case Reports |
The lack of positive findings in the bone marrow would cause physicians to discount further detection of lymphoma tissues. Our case revealed that it may be clinically warranted to assess the molecular and cytogenetic characteristics in lymphoma tissues at initial diagnosis, regardless of whether the bone marrow is involved. With the RNAseq results of BCR/ABL1 (p190) rearrangement and IKZF1 deletion, a precise diagnosis was ultimately made in our case. We noticed that a small number of B-LBL patients with KMT2A (MLL) rearrangements have also been reported previously.17,18 Chromosomal translocations in B-ALL, such as the BCR/ABL, t (9;22) (q34; q11.2) alteration and MLL rearrangements involving chromosome 11 at 11q23, have also been associated with adverse outcomes. These B-LBL cases with ALL-associated BCR/ABL and KMT2A gene rearrangements support the interpretation of B-LBL and B-ALL as a spectrum of the same disease by the WHO classification. Routine assessment of molecular and cytogenetic characteristics in B-LBL specimens as in B-ALL bone marrow at initial diagnosis may be useful for risk stratification.
The advent of tyrosine kinase inhibitors (TKIs) has changed the prognosis of Ph+ ALL.19,20 Although Ph+ B-LBL is a rare entity without standard treatment strategies, the current therapy program could utilize the Ph+ ALL protocol that predominantly contains tyrosine kinase inhibitors (TKIs), which target BCR/ABL1. Notably, Case 2, listed in Table 1, failed to perform a molecular abnormality screening at his initial diagnosis, leading to a loss of opportunity for targeted therapy with rapid relapse and very poor outcomes.10 Therefore, this study clearly shows the importance and necessity of potential target analysis in B-LBL at first diagnosis.
The choice to use TKIs is another key point of concern. Because the testis is an immune-privileged site, primary testicular malignancies provide a sanctuary for cancer cells. They have a uniformly poor prognosis with a high risk of relapse in the CNS and the contralateral testis.21 Studies have indicated that some patients with Ph+ ALL on prolonged imatinib therapy have progressed to isolated CNS leukemia due to the poor permeability of imatinib.22 In contrast, dasatinib, a dual-specific SRC/BCR-ABL1 kinase inhibitor, can cross the blood–brain barrier and be present at detectable levels in the CSF to clear blasts.23 Remarkably, in the previous reports of patients with testicular infiltration (shown in Table) and in our case, the patients were administered dasatinib, and they achieved CR without relapses in any immune-privileged sites.11 Thus, Ph+ B-LBL with testis involvement could also benefit from dasatinib, which might effectively penetrate the blood-testis barrier as well as the blood–brain barrier, clear blasts and prevent relapse in these privileged sites.
Given its rapid proliferative features and the expansion of a BCR/ABL1 mutated dasatinib and imatinib-resistant clone, the treatment strategy for Ph+ ALL should be consolidated with allo-HSCT following achievement of remission. However, a recent study of TKI-based chemotherapy-free induction and consolidation as a first-line treatment in GIMEMA LAL2217 poses a challenge to the allo-HSCT approach at CR1 for the consolidation of Ph+ ALL.24 The role of allo-HSCT in Ph+ B-LBL is also not clear. We observed that 4 of the 8 cases in Table were treated with a TKI followed by allo-HSCT at CR1, with disease-free survival (DFS) ranging from 4 to 30 months.9,12,13,15 The outcomes of the other 4 cases without allo-HSCT varied widely. One patient without a TKI upfront relapsed 4 months after chemotherapy.10 Another patient with a TKI upfront but without allo-HSCT relapsed 4 years after the initial diagnosis.11 Interestingly, the 2 patients without allo-HSCT were probably due to age as contraindications and were finally sustained in a CR status for 4 and 5 years, respectively, using TKIs only for maintenance.11,14 In light of the younger age and longer survival expectations of the patient in our study, we speculated that he would have benefit from allo-HSCT in CR1, although this should be confirmed in large-scale studies in the future.
In conclusion, we describe a rare case of primary testicular and cutaneous Ph+ B-LBL without bone marrow involvement. Therefore, it was very easy to neglect the screening of potential molecular targets, which would lead to a missed diagnosis. However, we successfully made an accurate diagnosis and implemented an appropriate therapeutic strategy at the initial hospitalization through the proper detection of lymphoma specimens. We suggest that FISH, reverse transcriptase polymerase chain reaction (RT–PCR) and/or RNAseq should be routinely carried out in lymphoma specimens. It is not only valuable for hematologists to tailor treatment but also for patients to improve their outcomes. Nevertheless, optimized therapeutic strategies for Ph+ B-LBL need further study.
Data Sharing Statement
The materials and methods supporting the conclusion of this study have been included within the manuscript and supplementary materials.
Ethics Approval and Consent to Participate
This case study was approved by the Ethics Committee of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology. Institutional approval was not required to publish the case details. The patient provided written informed consent according to the Declaration of Helsinki.
Consent for Publication
The patient provided written informed consent for the case details to be published.
Acknowledgments
We are grateful to the pathologist Dong Kuang, the radiologist Zhiping Shu, and the laboratory staff Dr. Min Xiao, Qinlu Li, Kefeng Shen, Jin Wang and Jie Xiong for their efforts regarding diagnosis and follow-up tests for the patient.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
This work was supported by the National Natural Science Foundation of China (Grant Number: 82000176, to Gaoxiang Wang).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Campo E, Swerdlow SH, Harris NL, et al. The 2008 WHO classification of lymphoid neoplasms and beyond: evolving concepts and practical applications. Blood. 2011;117(19):5019–5032. doi:10.1182/blood-2011-01-293050
2. Cortelazzo S, Ferreri A, Hoelzer D, et al. Lymphoblastic lymphoma. Crit Rev Oncol Hematol. 2017;113:304–317. doi:10.1016/j.critrevonc.2017.03.020
3. Lin P, Jones D, Dorfman DM, et al. Precursor B-cell lymphoblastic lymphoma: a predominantly extranodal tumor with low propensity for leukemic involvement. Am J Surg Pathol. 2000;24(11):1480–1490. doi:10.1097/00000478-200011000-00003
4. Brown PA, Shah B, Advani A, et al. Acute lymphoblastic leukemia, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2021;19(9):1079–1109. doi:10.6004/jnccn.2021.0042
5. Yasuda T, Tsuzuki S, Kawazu M, et al. Recurrent DUX4 fusions in B cell acute lymphoblastic leukemia of adolescents and young adults. Nat Genet. 2016;48(5):569–574. doi:10.1038/ng.3535
6. Lilljebjörn H, Henningsson R, Hyrenius-Wittsten A, et al. Identification of ETV6-RUNX1-like and DUX4-rearranged subtypes in paediatric B-cell precursor acute lymphoblastic leukaemia. Nat Commun. 2016;7(1):11790. doi:10.1038/ncomms11790
7. Clappier E, Auclerc MF, Rapion J, et al. An intragenic ERG deletion is a marker of an oncogenic subtype of B-cell precursor acute lymphoblastic leukemia with a favorable outcome despite frequent IKZF1 deletions. Leukemia. 2014;28(1):70–77. doi:10.1038/leu.2013.277
8. Soverini S, Bassan R, Lion T. Treatment and monitoring of Philadelphia chromosome-positive leukemia patients: recent advances and remaining challenges. J Hematol Oncol. 2019;12(1):39. doi:10.1186/s13045-019-0729-2
9. Sadrzadeh H, Huck AE, Chen YB, et al. Philadelphia chromosome positive B-cell lymphoblastic lymphoma isolated to bone. Leuk Lymphoma. 2013;54(9):2052–2054. doi:10.3109/10428194.2012.760735
10. Zhu J, Zhang S, Zhu L, et al. Primary testicular Ph-positive B lymphoblastic lymphoma: an unusual presentation and review. Cancer Biol Ther. 2015;16(8):1122–1127. doi:10.1080/15384047.2015.1056412
11. Boddu P, Yin CC, Kanagal-Shamanna R, et al. An unsuspected finding of t (9;22): a rare case of Philadelphia chromosome-positive B-lymphoblastic lymphoma. Case Rep Hematol. 2017;2017:2413587. doi:10.1155/2017/2413587
12. Alshomar A, El Fakih R. Philadelphia chromosome-positive lymphoblastic lymphoma-Is it rare or underdiagnosed? Hematol Oncol Stem Cell Ther. 2020;13(4):242–243. doi:10.1016/j.hemonc.2018.05.007
13. Dragani M, Andreani G, Fava C, et al. Philadelphia-positive lymphoblastic lymphoma: a case report and review of the literature. Stem Cell Investig. 2019;6:17. doi:10.21037/sci.2019.06.06
14. Takahashi T, Ichikawa S, Ichinohasama R, et al. BCR-ABL1 positive lymphoblastic lymphoma - should it be treated like a B-lymphoblastic leukemia with t (9;22); BCR-ABL1? Leuk Lymphoma. 2020;61(5):1265–1267. doi:10.1080/10428194.2019.1706736
15. Yamada C, Shimomura Y, Kamijyo K, et al. BCR/ABL1-positive B-lymphoblastic lymphoma successfully treated with dasatinib-combined chemotherapy. Intern Med. 2021;60(19):3149–3153. doi:10.2169/internalmedicine.7066-21
16. Li X, Cao W, Zhang S, et al. Philadelphia chromosome-positive B-lymphoblastic lymphoma successfully treated with chemotherapy regimen containing imatinib: a rare case report and literature review. Medicine. 2021;100(23):e26323. doi:10.1097/MD.0000000000026323
17. Heerema NA, Sather HN, Ge J, et al. Cytogenetic studies of infant acute lymphoblastic leukemia: poor prognosis of infants with t (4;11) - a report of the Children’s Cancer Group. Leukemia. 1999;13(5):679–686. doi:10.1038/sj.leu.2401413
18. Mater DV, Goodman BK, Wang E, et al. MLL duplication in a pediatric patient with B-cell lymphoblastic lymphoma. J Pediatr Hematol Oncol. 2012;34(3):e120–e123. doi:10.1097/MPH.0b013e3182273b57
19. Shen S, Chen X, Cai J, et al. Effect of dasatinib vs imatinib in the treatment of pediatric Philadelphia chromosome-positive acute lymphoblastic leukemia: a randomized clinical trial. JAMA Oncol. 2020;6(3):358–366. doi:10.1001/jamaoncol.2019.5868
20. Tanasi I, Ba I, Sirvent N, et al. Efficacy of tyrosine kinase inhibitors in Ph-like acute lymphoblastic leukemia harboring ABL-class rearrangements. Blood. 2019;134(16):1351–1355. doi:10.1182/blood.2019001244
21. Møller MB, d’Amore F, Christensen BE. Testicular lymphoma: a population-based study of incidence, clinicopathological correlations and prognosis. The Danish Lymphoma Study Group, LYFO. Eur J Cancer. 1994;30(12):1760–1764. doi:10.1016/0959-8049(94)00311-r
22. Abdelhalim A, Barcos M, Block AW, et al. Remission of Philadelphia chromosome-positive central nervous system leukemia after dasatinib therapy. Leuk Lymphoma. 2007;48(5):1053–1056. doi:10.1080/10428190701258370
23. Porkka K, Koskenvesa P, Lundán T, et al. Dasatinib crosses the blood-brain barrier and is an efficient therapy for central nervous system Philadelphia chromosome-positive leukemia. Blood. 2008;112(4):1005–1012. doi:10.1182/blood-2008-02-140665
24. Foà R, Bassan R, Vitale A, et al. Dasatinib-blinatumomab for Ph-Positive acute lymphoblastic leukemia in adults. N Engl J Med. 2020;383(17):1613–1623. doi:10.1056/NEJMoa2016272
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.