Back to Journals » Breast Cancer: Targets and Therapy » Volume 11
Primary systemic therapy in HER2-positive operable breast cancer using trastuzumab and chemotherapy: efficacy data, cardiotoxicity and long-term follow-up in 142 patients diagnosed from 2005 to 2016 at a single institution
Authors Antolín S, Acea B, Albaina L, Concha Á, Santiago P, García-Caballero T, Mosquera JJ, Varela JR, Soler R, Calvo L
Received 10 July 2018
Accepted for publication 7 November 2018
Published 27 December 2018 Volume 2019:11 Pages 29—42
DOI https://doi.org/10.2147/BCTT.S179750
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Pranela Rameshwar
Silvia Antolín,1 Benigno Acea,2 Luis Albaina,2 Ángel Concha,3 Paz Santiago,3 Tomás García-Caballero,4 Joaquín J Mosquera,5 José Ramón Varela,5 Rafaela Soler,5 Lourdes Calvo1
1Medical Oncology Department, Breast Unit, A Coruña University Hospital, A Coruña, Spain; 2Surgery Department, Breast Unit, A Coruña University Hospital, A Coruña, Spain; 3Anatomic Pathology Department, Breast Unit, A Coruña University Hospital, A Coruña, Spain; 4Department of Morphological Sciences, University of Santiago de Compostela, Santiago de Compostela, A Coruña, Spain; 5Radiology Department, Breast Unit, A Coruña University Hospital, A Coruña, Spain
Objective: The aim of this study was to evaluate the efficacy, cardiotoxicity profile and long-term benefits of neoadjuvant therapy in human epidermal growth factor receptor 2-positive operable breast cancer patients.
Patients and methods: A total of 142 patients diagnosed from 2005 to 2016 were included in the study. The treatment consisted of a sequential regimen of taxanes and anthracyclines plus trastuzumab. The clinical and pathological responses were evaluated and correlated with clinical and biological factors. The cardiotoxicity profile and long-term benefits were analyzed.
Results: The median age was 49 years, and 4%, 69% and 27% of patients had stage I, II and III breast cancer, respectively, while 10% had inflammatory breast cancer at diagnosis. Hormone receptor (HR) status was negative in 43%, and 62% had grade III breast cancer. The clinical complete response rate was 49% and 63% as assessed using ultrasound and magnetic resonance imaging, respectively, and this allowed a high rate of conservative surgery (66%). The pathological complete response (pCR) rate was 52%, and it was higher in HR-negative (64%) patients than in HR-positive (41%) patients and in grade III breast cancer (53%) patients than in grade I–II breast cancer (45%) patients. Patients who achieved pCR had longer disease-free survival and a trend toward improved overall survival. A total of 2% of patients showed a 10% decrease in left ventricular ejection fraction to <50% during treatment. All patients except one recovered after discontinuation of trastuzumab.
Conclusion: A sequential regimen of taxanes and anthracyclines plus trastuzumab was effective, with high pCR rates and long-term benefit, and had a very good cardiotoxicity profile.
Keywords: neoadjuvant therapy, HER2-positive breast cancer, pathological complete response, cardiotoxicity, survival
Introduction
Neoadjuvant therapy (NT) is the standard of care for locally advanced and inflammatory breast cancer.1–4
It is now known that it offers similar benefits like adjuvant therapy in terms of disease-free survival (DFS) and overall survival (OS),5,6 and so NT is currently implemented for operable disease.
Currently, NT is generally used to improve surgical options, to determine the response to therapy, and it is expected to produce long-term benefits.
Aggressive breast cancer subtypes benefit the most from primary systemic therapy: these include human epidermal growth factor receptor 2 (HER2)-positive and triple-negative tumors.7
In HER2-positive tumors, a combination of taxanes and anthracyclines plus trastuzumab has been the standard treatment.8–13
Trastuzumab interacts with the extracellular domain of HER2 and inhibits signaling from HER2 homodimers more effectively than from HER2 heterodimers with EGF receptor 1 (HER1) or EGF receptor 3 (HER3). This results in the downregulation of the phosphatidylinositol-3 kinase (PI3K) pathway and cell apoptosis. Trastuzumab can also induce antibody-dependent cellular cytotoxicity.14–16
Despite the advance in the knowledge of HER2-positive tumors and improvements in treatments, a small percentage of patients still suffer from recurrence and die.
Many mechanisms underlying resistance to anti-HER2 therapies have been studied. They include three major groups. The first group involves the concept of “redundancy”. Examples include the inhibition of the incomplete receptor family by a truncated form of the HER2 receptor (p95-HER2) and the lack of exon 16 in the extracellular domain of the HER2 receptor (D16 isoform).
The second major group involves “reactivation”. Examples include the deregulation of the PI3K pathway because of mutations in PI3K catalytic subunit or reduced levels of phosphatidylinositol-3,4,5-triphosphate 3-phosphatase. The third group involves the concept of “scape”. This involves the use of other pathways that can preexist or be acquired at the time of resistance. An example is the cross talk between the estrogen receptor and the HER2 pathways.
Other mechanisms of resistance to anti-HER2 therapies are being studied and include regulators of cell cycle and apoptosis, the tumor microenvironment and immune system, mucins and various receptors and tyrosine kinases.17,18
However, combinations of targeted agents represent a way to reduce resistance to trastuzumab.
In recent years, we have observed that dual blockade of the HER2 pathway produces better results with an increase in pathological complete response (pCR) rates. In the Neosphere trial, pertuzumab plus trastuzumab and chemotherapy demonstrated higher pCR rates compared with the trastuzumab or pertuzumab arms, and these pCR rates were associated with better DFS.19–24
Lapatinib was studied in this context in combination with trastuzumab and chemotherapy in several clinical trials. Although some trials of the combination showed higher pCR rates, they did not demonstrate statistically significant long-term benefits. In addition, lapatinib was associated with a worse toxicity profile.25–29
It is known that the response to treatment depends on clinical factors such as lower stage at diagnosis (small tumors and few or no metastatic axillary nodes) and biological factors such as negative hormone receptor (HR) status, high ki-67 percentage and grade II–III tumors.30–32
The association between clinical and pathological response has been studied in the neoadjuvant setting. The best imaging methods, such as ultrasound or magnetic resonance imaging (MRI), to monitor tumor shrinkage during treatment33–38 and their correlation with pathological response have also been analyzed.39
pCR has been associated with long-term benefits, DFS and OS, in several clinical trials and meta-analyses,7,40 but controversies exist on this point. Some authors consider pCR a surrogate end point that has yet to demonstrate a real long-term benefit.41,42
The sequential administration of a taxane and an anthracycline plus trastuzumab provides a significant benefit to patients with a high pCR rate.43 The cardiotoxicity associated with this anthracycline plus trastuzumab combination is a drawback,44–46 but formulations such as epirubicin or liposomal anthracyclines have reduced this negative impact on heart function.47,48 Although it is still important to monitor left ventricular ejection fraction (LVEF) during treatment, improved knowledge of long-term cardiotoxicity years after the end of treatment is needed.21
We analyzed the efficacy data, cardiotoxicity and long-term follow-up in 142 patients with HER2-positive breast cancer tumors diagnosed from 2005 to 2016 at a single institution who were treated homogenously through sequential administration of a taxane and an anthracycline plus trastuzumab.
Patients and methods
This is a retrospective observational study that included 142 patients with stage I–III HER2-positive breast cancer tumors diagnosed from 2005 to 2016 at a single institution.
The study, as well as all procedures performed in the study, was approved by the ethics committee of the Galician region (Comité Autonómico de Ética de Investigación de Galicia, Spain) in 2015 with the code: SAN-TRA-2015-01, and all the patients who were alive at the time the retrospective study was started provided written informed consent for participation.
The study procedures were carried out in accordance with the 1975 Declaration of Helsinki, as revised in 2000, and Good Clinical Practice guidelines.
Patients
All patients included were ≥18 years of age and had Eastern Cooperative Oncology Group Performance Status of ≤1.
Histological type, tumor grade, ki-67 index, estrogen and progesterone receptor and HER2 status were determined locally using pretreatment core biopsies.
HER2-positive breast cancer was considered if the tumors exhibited threefold overexpression of the HER2 receptor as assessed using immunohistochemical techniques (HerceptestTM Dako until 2013 and Roche until 2016) or a threefold overexpression of the HER2 gene as assessed using fluorescent (until 2013) or silver (2013–2016) in situ hybridization (in the same laboratory) in accordance with 2007 and 2013 American Society of Clinical Oncology/College of American Pathologist guidelines.49,50
An ultrasound-guided fine-needle puncture aspiration was also performed on suspected malignant axillary lymph nodes at diagnosis.
The tumor site was marked using a stainless steel marker placed using ultrasound guidance in the majority of patients. Multicentric or inflammatory tumors were not marked because of the indication for mastectomy despite the response to treatment.
From 2005 to 2012, a sentinel lymph node biopsy (SLNB) was performed prior to treatment in patients with clinically negative axilla, and from 2012 to 2016 this procedure was performed after treatment.51,52
At diagnosis, patients were free from cardiovascular disease and demonstrated adequate cardiac function with an LVEF >50%, as measured using echocardiography.
This echocardiography was repeated after three to four cycles, at the end of chemotherapy and during the follow-up period, at least 6 months after the end of adjuvant trastuzumab.
Patients also had adequate hematological, renal and hepatic function.
The different treatment regimens administered are shown in Figure 1. The vast majority of patients received a total of eight cycles consisting of a sequence of anthracyclines and taxanes with trastuzumab.
Physical examinations were performed every 3 weeks during chemotherapy treatment. Mammograms, ultrasound and MRI were performed before and after neoadjuvant treatment.
The clinical response (CR) was measured through physical examination and MRI in accordance with the response evaluation criteria in solid tumors.53,54
Patients underwent surgery between 3 and 5 weeks from the end of chemotherapy. There was an increase in conservative breast surgery in patients that were candidates for mastectomy at diagnosis.
pCR was defined as the total absence of invasive tumor in both breast and axillary nodes (ypT0/is ypN0).
The pathological response was measured in accordance with the Miller and Payne system in most patients.55
We used the revised American Joint Committee on Cancer TNM system in patients included in the first 2 years of the study.56
This pathological response was correlated with clinical and biological factors (tumor size, axillary nodes, HR status and ki-67 index).
All patients treated using conservative breast surgery received whole-breast irradiation at a standard dose. Some patients with T3 tumors at diagnosis were also irradiated after mastectomy. Regional nodal irradiation of the supraclavicular fossa-axillary apex was used in patients with clinical stage III disease, in patients with four or more positive lymph nodes and in selected patients with one to three positive lymph nodes.57,58
After completion of systemic and local therapy, patients with HR-positive tumors received tamoxifen or an aromatase inhibitor if the patient was menopausal.
We report DFS (defined as the time from surgery to the first documented disease progression) and OS (time from surgery to death).
Statistical analyses
We performed a descriptive analysis for all variables. Continuous variables were reported using the median as the central value and the SD. For dichotomous or categorical variables, absolute numbers and percentages were computed. The chi-squared two-tailed test was used for comparative analyses between categorical variables.
We estimated DFS and OS rates for each group using the Kaplan–Meier method. A comparison of survival curves was performed using the log-rank test. Differences in survival between groups were compared using the Cox regression test. All statistical tests were two sided, and a significance level of 0.05 was applied.
Results
A total of 142 patients with stage I–III HER2-positive breast cancer tumors, who were candidates for primary systemic therapy and were diagnosed from 2005 to 2016 at our institution, were included in the study. The pretreatment characteristics of the patients are listed in Table 1.
The median patient age was 49 (30–79) years, with stage I, II or III breast cancer at diagnosis in 4%, 69% and 27%, respectively. A total of 10% of patients had inflammatory breast cancer, 43% of tumors were HR negative and 62% were grade III, while 90% of tumors were confirmed to be HER2 positive through immunohistochemistry (threefold increase in protein expression) and the rest through fluorescent in situ hybridization or silver in situ hybridization. A total of 84% of patients had an indication for mastectomy at diagnosis.
A total of 98% of patients completed the planned cycles of chemotherapy. The remaining 2% (three patients) could not complete chemotherapy because of hepatotoxicity in one patient, sustained grade III asthenia and grade II neutropenia in an elderly patient and a massive pulmonary thromboembolism in the third patient after four cycles of chemotherapy.
Clinical response
The CR was assessed through mammography, ultrasound imaging and MRI before and after systemic therapy and immediately before surgery in most patients.
In the first years of the study, breast MRI was not a routine imaging technique, so it was not performed in the first 18 patients.
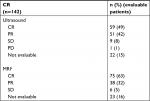
The CR data are listed in Table 2.
A total of 49% and 63% of patients demonstrated a clinical complete response, as measured through ultrasound and MRI, respectively.
Pathological response
The pCR rate, defined as the total absence of invasive tumor in both breast and axillary nodes (ypT0/is ypN0), was 52%. The pCR rates in breast, breast and axilla and in different subgroups are summarized in Tables 3 and 4.
  | Table 3 pCR in breast and breast/axilla Abbreviation: pCR, pathological complete response. |
  | Table 4 pCR by subgroups Abbreviations: 5-FEC, 5-fluorouracil–epirubicin–cyclophosphamide; HR, hormone receptor; Pac, paclitaxel; pCR, pathological complete response; T, trastuzumab. |
The pCR rate was higher in ductal (53%) than in lobular (14%) cancer, in HR-negative (64%) than in HR-positive (41%) cancer, in grade III (53%) than in grade I–II (45%) cancer and in tumors with a ki-67 value >20 (85%) than in those with a ki-67 value <20 (15%).
A total of 61% and 68% of patients with CR, as assessed using ultrasound and MRI, respectively, also demonstrated pCR.
pCR was higher in patients treated using paclitaxel/trastuzumab (PT) followed by Myocet/cyclophosphamide/trastuzumab (54%) than those treated using PT followed by fluorouracil/epirubicin/cyclophosphamide/trastuzumab (38%), but the numbers of patients assigned to each group were too low to result in statistically significant differences.
Surgery
At diagnosis, 84% of patients were indicated for mastectomy. After treatment, tumorectomy was performed in 66%.
Axillary dissection, SLNB and no axillary procedure were performed in 69%, 22% and 9% of patients, respectively. Those patients with a negative SLNB before treatment did not undergo an axillary procedure.
Long-term efficacy data
At the time of the analysis in March 2018, and with a median follow-up of 55 months, 27 patients suffered from breast cancer recurrence and 15 patients died as a result of any cause.
DFS and OS curves are shown in Figures 2 and 3, respectively. We were able to demonstrate an association between pCR and better DFS with statistical significance (P=0.19) and a trend toward improved OS (P=0.068; Figures 4 and 5).
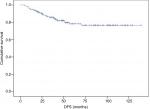  | Figure 2 DFS assessed using the Kaplan–Meier method. Abbreviation: DFS, disease-free survival. |
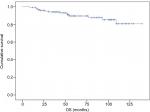  | Figure 3 OS assessed using the Kaplan–Meier method. Abbreviation: OS, overall survival. |
  | Figure 4 DFS based on pCR. Abbreviations: DFS, disease-free survival; pCR, pathological complete response. |
  | Figure 5 OS based on pCR. Abbreviations: OS, overall survival; pCR, pathological complete response. |
Cardiotoxicity
Because of the potential cardiotoxicity associated with anthracyclines and trastuzumab, knowledge of cardiovascular risk factors and patient monitoring were very important during the study.
Age at diagnosis, body mass index (BMI), arterial hypertension, dyslipidemia, preexisting cardiovascular disease and previous radiotherapy are recognized as risk factors for heart disease in breast cancer patients treated using anthracyclines and trastuzumab.59–65
The risk factors for developing a cardiac event in the current cohort of patients are listed in Table 5.
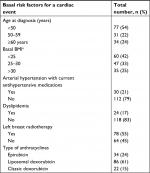  | Table 5 Risk factors for cardiac events Note: aBMI: 25–30, overweight; >30, obese. Abbreviation: BMI, body mass index. |
At diagnosis, all patients had adequate cardiac function with an LVEF of >50%, as measured using echocardiography.
The echocardiography was repeated after chemotherapy treatment and during follow-up at least 6–12 months after the end of adjuvant trastuzumab in 70% of patients.
At the end of chemotherapy treatment, five patients suffered a decline in LVEF <50% (3.5% of patients). The cardiovascular risk factors for these patients are listed in Table 6.
  | Table 6 Cardiovascular risk factors in patients who suffered a decline in LVEF <50% Abbreviations: BMI, body mass index; LVEF, left ventricular ejection fraction. |
Of these patients, four did not experience cardiac symptoms and demonstrated a rapid recovery after temporarily stopping trastuzumab. One did not receive adjuvant trastuzumab. Three received left breast radiotherapy and two had a history of high blood pressure.
The fifth patient who suffered a decline in LVEF below 50% did not receive adjuvant trastuzumab and developed severe ventricular dysfunction over subsequent years.
This patient had a high BMI and received left breast radiotherapy, which are risk factors for heart disease.
LVEF at baseline, end of chemotherapy and beyond 6 months from the end of trastuzumab treatment is shown in Figure 6.
  | Figure 6 Basal LVEF at the end of chemotherapy and follow-up. Abbreviation: LVEF, left ventricular ejection fraction. |
Discussion
Although clear clinical and preclinical evidence for dual blockade of the HER2 pathway in neoadjuvant treatment of HER2-positive breast cancer has been reported and this has become a standard approach in combination with chemotherapy, some issues remain controversial. Do all patients benefit from the addition of the second biological inhibitor to their chemotherapy regimen, and what is the role of anthracyclines and cardiotoxicity?
Considering that we retrospectively analyzed a homogeneous population treated using a taxane and anthracycline concomitantly with trastuzumab and all treatment was administered before surgery, the median pCR (total absence of invasive tumor in both breast and axillary nodes) achieved in our cohort was high (52%) and is comparable to other treatment series employing a similar sequence. In a previous study, Buzdar et al11 used sequential 5-fluorouracil–epirubicin–cyclophosphamide (FEC) and paclitaxel with trastuzumab and achieved a higher pCR (56%), but only evaluated pCR in the breast.
The median pCR achieved in studies employing dual blockade of the HER2 pathway ranges from 45% to 62%. In the most important of these studies (Neosphere and Tryphaena), only pCR in the breast was evaluated.
It is known that the combination of anthracyclines and trastuzumab is very active, but it can increase cardiotoxicity. There are anthracyclines such as epirubicin or liposomal formulations that can reduce this toxicity.
A Phase III trial reported that adding trastuzumab to epirubicin does not improve efficacy in terms of pCR in a sequence with taxane and trastuzumab vs epirubicin alone using the same sequence. However, long-term efficacy and cardiotoxicity data are needed to draw robust conclusions.10
In the current study, 37 patients were treated with epirubicin and 85 with liposomal doxorubicin (Myocet®; Teva B.V, Haarlem, the Netherlands), achieving a higher pCR rate in the Myocet group (38% and 51%, respectively). The number of patients assigned to each group was too low to produce statistically significant differences.
These rates are comparable to those in other published series using FEC or classic anthracyclines. There is little evidence to support the use of liposomal anthracyclines in the neoadjuvant setting. Most published series uses concomitant administration of chemotherapeutics instead of sequential administration and a higher number of cycles.48,66–68
We identified three biological factors associated with a better response to therapy: HR-negative status, ki-67 >20% and grade III breast cancer.
The high pCR achieved in the HR-negative patients vs the HR-positive patients was also seen in previous clinical trials in the HER2 neoadjuvant setting,19,26,69 and this could be explained by the cross talk between the HER2 and endocrine pathway conferring resistance to agents targeting either pathway.
Many studies have demonstrated the prognostic and predictive value of ki-67, whereby it is used to identify patients who will benefit most from systemic therapy. These studies used different methods for scoring this proliferation antigen and included a heterogeneous population with different biological subtypes.70,71
A 20% cutoff point for the ki-67 index appears to be a statistically significant prognostic factor in luminal B HER2-negative tumors. The median value of ki-67 is usually higher in triple-negative and HER2-positive tumors. Median ki-67 values of 35%–40% in HER2-positive and 50% in triple-negative breast cancer have been previously reported.72
In the current study, we examined three different cutoff points for the ki-67 index and their relationship with pCR, DFS and OS.
Tumors with ki-67 >20% (especially those >35%) achieved higher pCR rates, but no group was correlated with long-term benefits.
The correlation between histological grade and prognosis has been confirmed in multiple reports and currently helps clinicians to make treatment decisions.73–78 Its value as an independent factor to predict the response to NT is unclear.30,31 In the current series, grade III tumors achieved a higher pCR rate compared with grade I–II tumors.
There was a high rate of indication for mastectomy at diagnosis in the current series. This could be explained by the incidence of T3 and T4 tumors and the relationship between breast/tumor size in T2 tumors.
After systemic treatment, the rates of breast conservation were also high and greater than in other previously reported series.79–81 We attributed this to the high pCR achieved in this selected HER2 population.
The potential for an increase in local recurrence after NT followed by breast conservation was analyzed in several studies and meta-analyses.5,82 The MD Anderson Prognostic Index concludes that the local recurrence rate could depend on other biological factors such as clinical N2 or N3 disease, lymphovascular space invasion, a multifocal pattern of residual disease and a residual pathological primary tumor >2 cm, rather than the timing of chemotherapy delivery.83,84
Our locoregional recurrence rate of 3.9% is low at the time of data cutoff.
A high percentage (61%–68%) of tumors that achieved CR, assessed using ultrasound and MRI, also demonstrated pCR. MRI has been reported as superior to ultrasound and mammography in the assessment of tumor extent and is highly sensitive in identifying residual disease following neoadjuvant treatment.85,86 We perceived a slight superiority of MRI in the current series.
pCR in HER2-positive breast cancer is associated with a substantially longer time to recurrence and death, as reported in a meta-analysis published by Broglio et al,40 which included a total of 5,500 HER2-positive breast cancer patients.
We demonstrated an association between pCR and improved DFS (P=0.19) in the current patients and a trend toward improved OS (P=0.068). This absence of a statistically significant benefit in OS could be explained by the low number of deaths at the time of analyses and to improvements in treatments for metastatic disease. None of the patients in the current series received pertuzumab in the neoadjuvant setting.
Concomitant administration of trastuzumab and classic anthracyclines is cardiotoxic and not permitted in clinical use.
In recent years, the incidence of congestive heart failure associated with trastuzumab and anthracyclines has become very low, since close monitoring of cardiac function and liposomal and less cardiotoxic anthracyclines have been used.11,43,48
We used two-dimensional echocardiography to monitor trastuzumab-related cardiotoxicity following the current clinical guidelines. This is a readily available cardiac imaging modality with low cost and is radiation free. However, the current knowledge indicates that this technique does not accurately predict the development of cardiotoxicity. New imaging modalities can provide more information in the early stages and can quantify myocardial deformation as a marker of contractility. These new echocardiographic techniques, such as global longitudinal strain, can provide information that is predictive of cardiac dysfunction.87,88
Cardiac biomarkers, such as troponins and amino-terminal fragment of brain natriuretic peptide, are also a useful tool to monitor cardiotoxicity. They have been studied as early predictors of cardiac damage in patients receiving anthracyclines and anti-HER2 therapy with disparate results.89,90
After a long follow-up period, our data showed a low incidence of cardiac events: 2.95% of patients demonstrated an asymptomatic decrease in LVEF and 0.64% demonstrated symptomatic ventricular dysfunction. Both cardiac events were related to other cardiovascular risk factors such as high BMI or high blood pressure and left breast radiotherapy.
This low incidence of cardiac events did not allow us to achieve statistically significant differences between the cardiotoxicity profiles of the chemotherapy treatments. The five patients who suffered a decline in LVEF <50% at the end of chemotherapy treatment had received concomitant anthracyclines and trastuzumab instead of the sequential treatments.
Although the incidence of cardiotoxicity was low, it is desirable to avoid it through better selection of patients and the correction of cardiovascular risk factors (high BMI, arterial hypertension, dyslipidemia) from the beginning, not only through diet and exercise but also using medication. The early administration of angiotensin–convertin–enzyme inhibitors (enalapril type) or beta-blockers (carvedilol or bisoprolol type) or a combination of both has produced improvements in cardiac function in breast cancer patients treated using anthracyclines. This highlights the need for collaboration between cardiologists and oncologists from the beginning of treatment.91–93
Conclusion
The sequence of a taxane and a less cardiotoxic anthracycline concomitant to trastuzumab was effective in the HER2 neoadjuvant setting with high pCR and conservative breast surgery rates. Grade III and HR-negative tumors demonstrated the greatest benefit.
We observed a good correlation between the clinical complete response, as measured using ultrasound and MRI, and pCR.
We were able to associate pCR with improved DFS. Although there was a trend toward improved OS, it was not statistically significant.
The treatment was safe with an excellent long-term cardiotoxicity profile.
Acknowledgments
We would like to thank all the patients who participated in the study and the following people: Javier Prato, Aurea Molina, Joaquin Mosquera, Cristina Reboredo, Begoña Graña, María Quindós (Medical Oncology Department, A Coruña University Hospital), Manuel Juaneda (Surgery Department, Breast Unit, A Coruña University Hospital), Alberto Bouzón, Carmen Conde (Gynecology Department, Breast Unit, A Coruña University Hospital), Carlota Diaz (Gynecology Department, Breast Unit, A Coruña University Hospital), Carmen Cereijo (oncology nurse, Breast Unit, A Coruña University Hospital). This study did not receive any specific grants from funding agencies in the public, commercial or nonprofit sectors.
Disclosure
The authors report no conflicts of interest in this work.
References
Hortobagyi GN, Blumenschein GR, Spanos W, et al. Multimodal treatment of locoregionally advanced breast cancer. Cancer. 1983;51(5):763–768. | ||
Hortobagyi GN, Ames FC, Buzdar AU, et al. Management of stage III primary breast cancer with primary chemotherapy, surgery, and radiation therapy. Cancer. 1988;62(12):2507–2516. | ||
Bear HD. Indications for neoadjuvant chemotherapy for breast cancer. Semin Oncol. 1998;25(2 Suppl 3):3–12. | ||
Rastogi P, Anderson SJ, Bear HD, et al. Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol. 2008;26(5):778–785. | ||
Mauri D, Pavlidis N, Ioannidis JP. Neoadjuvant versus adjuvant systemic treatment in breast cancer: a meta-analysis. J Natl Cancer Inst. 2005;97(3):188–194. | ||
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Long-term outcomes for neoadjuvant versus adjuvant chemotherapy in early breast cancer: meta-analysis of individual patient data from ten randomised trials. Lancet Oncol. 2018;19(1):27–39. | ||
Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384(9938):164–172. | ||
Burris H, Yardley D, Jones S, et al. Phase II trial of trastuzumab followed by weekly paclitaxel/carboplatin as first-line treatment for patients with metastatic breast cancer. J Clin Oncol. 2004;22(9):1621–1629. | ||
Buzdar AU, Ibrahim NK, Francis D, et al. Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: results of a randomized trial in human epidermal growth factor receptor 2-positive operable breast cancer. J Clin Oncol. 2005;23(16):3676–3685. | ||
Buzdar AU, Valero V, Ibrahim NK, et al. Neoadjuvant therapy with paclitaxel followed by 5-fluorouracil, epirubicin, and cyclophosphamide chemotherapy and concurrent trastuzumab in human epidermal growth factor receptor 2-positive operable breast cancer: an update of the initial randomized study population and data of additional patients treated with the same regimen. Clin Cancer Res. 2007;13(1):228–233. | ||
Buzdar AU, Suman VJ, Meric-Bernstam F, et al; American College of Surgeons Oncology Group investigators. Fluorouracil, epirubicin, and cyclophosphamide (FEC-75) followed by paclitaxel plus trastuzumab versus paclitaxel plus trastuzumab followed by FEC-75 plus trastuzumab as neoadjuvant treatment for patients with HER2-positive breast cancer (Z1041): a randomised, controlled, phase 3 trial. Lancet Oncol. 2013;14(13):1317–1325. | ||
Untch M, Rezai M, Loibl S, et al. Neoadjuvant treatment with trastuzumab in HER2-positive breast cancer: results from the GeparQuattro study. J Clin Oncol. 2010;28(12):2024–2031. | ||
Untch M, Fasching PA, Konecny GE, et al. Pathologic complete response after neoadjuvant chemotherapy plus trastuzumab predicts favorable survival in human epidermal growth factor receptor 2-overexpressing breast cancer: results from the TECHNO trial of the AGO and GBG study groups. J Clin Oncol. 2011;29(25):3351–3357. | ||
Yarden Y. Biology of HER2 and its importance in breast cancer. Oncology. 2001;61(Suppl 2):1–13. | ||
Ghosh R, Narasanna A, Wang SE, et al. Trastuzumab has preferential activity against breast cancers driven by HER2 homodimers. Cancer Res. 2011;71(5):1871–1882. | ||
Rimawi MF, Schiff R, Osborne CK. Targeting HER2 for the treatment of breast cancer. Annu Rev Med. 2015;66:111–128. | ||
Rexer BN, Arteaga CL. Intrinsic and acquired resistance to HER2-targeted therapies in HER2 gene-amplified breast cancer: mechanisms and clinical implications. Crit Rev Oncog. 2012;17(1):1–16. | ||
Rimawi MF, de Angelis C, Schiff R. Resistance to anti-HER2 therapies in breast cancer. Am Soc Clin Oncol Educ Book. 2015;35:e157–e164. | ||
Gianni L, Pienkowski T, Im YH, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13(1):25–32. | ||
Gianni L, Pienkowski T, Im YH, et al. 5-Year analysis of neoadjuvant pertuzumab and trastuzumab in patients with locally advanced, inflammatory, or early-stage HER2-positive breast cancer (NeoSphere): a multicentre, open-label, phase 2 randomised trial. Lancet Oncol. 2016;17(6):791–800. | ||
Schneeweiss A, Chia S, Hickish T, et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA). Ann Oncol. 2013;24(9):2278–2284. | ||
Untch M, Jackisch C, Schneeweiss A, et al; German Breast Group (GBG); Arbeitsgemeinschaft Gynäkologische Onkologie—Breast (AGO-B) Investigators. Nab-paclitaxel versus solvent-based paclitaxel in neoadjuvant chemotherapy for early breast cancer (GeparSepto-GBG 69): a randomised, phase 3 trial. Lancet Oncol. 2016;17(3):345–356. | ||
Hurvitz SA, Symmans WF, Jung KH, Huang C-S, Thompson AM. Pathologic complete response (pCR) rates after neoadjuvant trastuzumab emtansine (T-DM1 [K]) + pertuzumab (P) vs docetaxel + carboplatin + trastuzumab + P (TCHP) treatment in patients with HER2-positive (HER2+) early breast cancer (EBC) (KRISTINE). J Clin Oncol. 2016; 500–500. | ||
Ulrike Nitz OG, Christgen M, Grischke E-M, Augustin D, Kümmel S. Final analysis of WSG-ADAPT HER2+/HR- trial: efficacy, safety, and predictive markers for 12-weeks of neoadjuvant dual blockade with trastuzumab + pertuzumab ± weekly paclitaxel in HER2+/HR- early breast cancer (EBC). J Clin Oncol. 2016;518–518. | ||
Baselga J, Bradbury I, Eidtmann H, et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomised, open-label, multicentre, phase 3 trial. Lancet. 2012;379(9816):633–640. | ||
de Azambuja E, Holmes AP, Piccart-Gebhart M, et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): survival outcomes of a randomised, open-label, multicentre, phase 3 trial and their association with pathological complete response. Lancet Oncol. 2014;15(10):1137–1146. | ||
Guarneri V, Frassoldati A, Bottini A, et al. Preoperative chemotherapy plus trastuzumab, lapatinib, or both in human epidermal growth factor receptor 2-positive operable breast cancer: results of the randomized phase II CHER-LOB study. J Clin Oncol. 2012;30(16):1989–1995. | ||
Untch M, Loibl S, Bischoff J, et al; German Breast Group (GBG); Arbeitsgemeinschaft Gynäkologische Onkologie-Breast (AGO-B) Study Group. Lapatinib versus trastuzumab in combination with neoadjuvant anthracycline-taxane-based chemotherapy (GeparQuinto, GBG 44): a randomised phase 3 trial. Lancet Oncol. 2012;13(2):135–144. | ||
Carey LA. Clinical and translational results of CALGB 40601: A neoadjuvant phase III trial of weekly paclitaxel and trastuzumab with or without lapatinib for HER2-positive breast cancer. J Clin Oncol. 2013; 500–500. | ||
Ellis P, Smith I, Ashley S, et al. Clinical prognostic and predictive factors for primary chemotherapy in operable breast cancer. J Clin Oncol. 1998;16(1):107–114. | ||
Chang J, Powles TJ, Allred DC, et al. Biologic markers as predictors of clinical outcome from systemic therapy for primary operable breast cancer. J Clin Oncol. 1999;17(10):3058–3063. | ||
Pusztai L, Gianni L. Technology insight: emerging techniques to predict response to preoperative chemotherapy in breast cancer. Nat Clin Pract Oncol. 2004;1(1):44–50. | ||
Vinnicombe SJ, MacVicar AD, Guy RL, et al. Primary breast cancer: mammographic changes after neoadjuvant chemotherapy, with pathologic correlation. Radiology. 1996;198(2):333–340. | ||
Herrada J, Iyer RB, Atkinson EN, Sneige N, Buzdar AU, Hortobagyi GN. Relative value of physical examination, mammography, and breast sonography in evaluating the size of the primary tumor and regional lymph node metastases in women receiving neoadjuvant chemotherapy for locally advanced breast carcinoma. Clin Cancer Res. 1997;3(9):1565–1569. | ||
Martincich L, Montemurro F, de Rosa G, et al. Monitoring response to primary chemotherapy in breast cancer using dynamic contrast-enhanced magnetic resonance imaging. Breast Cancer Res Treat. 2004;83(1):67–76. | ||
Chen JH, Bahri S, Mehta RS, et al. Breast cancer: evaluation of response to neoadjuvant chemotherapy with 3.0-T MR imaging. Radiology. 2011;261(3):735–743. | ||
Chen JH, Su MY. Clinical application of magnetic resonance imaging in management of breast cancer patients receiving neoadjuvant chemotherapy. Biomed Res Int. 2013;2013(2):348167. | ||
Woolf DK, Padhani AR, Makris A. Magnetic resonance imaging, digital mammography, and sonography: tumor characteristics and tumor biology in primary setting. J Natl Cancer Inst Monogr. 2015;2015(51):15–20. | ||
Chen JH, Feig B, Agrawal G, et al. MRI evaluation of pathologically complete response and residual tumors in breast cancer after neoadjuvant chemotherapy. Cancer. 2008;112(1):17–26. | ||
Broglio KR, Quintana M, Foster M. Association of pathologic complete response to neoadjuvant therapy in HER2-positive breast cancer with long-term outcomes: a meta-analysis. JAMA Oncol. 2016;2(6):E1–E10. | ||
von Minckwitz G, Fontanella C. Comprehensive review on the surrogate endpoints of efficacy proposed or hypothesized in the scientific community today. J Natl Cancer Inst Monogr. 2015;2015(51):29–31. | ||
Wang-Lopez Q, Chalabi N, Abrial C, et al. Can pathologic complete response (pCR) be used as a surrogate marker of survival after neoadjuvant therapy for breast cancer? Crit Rev Oncol Hematol. 2015;95(1):88–104. | ||
Gianni L, Eiermann W, Semiglazov V, et al. Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet. 2010;375(9712):377–384. | ||
Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344(11):783–792. | ||
Tan-Chiu E, Yothers G, Romond E, et al. Assessment of cardiac dysfunction in a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel, with or without trastuzumab as adjuvant therapy in node-positive, human epidermal growth factor receptor 2-overexpressing breast cancer: NSABP B-31. J Clin Oncol. 2005;23(31):7811–7819. | ||
Perez EA, Suman VJ, Davidson NE, et al. Cardiac safety analysis of doxorubicin and cyclophosphamide followed by paclitaxel with or without trastuzumab in the North Central Cancer Treatment Group N9831 adjuvant breast cancer trial. J Clin Oncol. 2008;26(8):1231–1238. | ||
Batist G, Barton J, Chaikin P, Swenson C, Welles L. Myocet (liposome-encapsulated doxorubicin citrate): a new approach in breast cancer therapy. Expert Opin Pharmacother. 2002;3(12):1739–1751. | ||
Gavilá J, Guerrero Á, Climent MÁ, et al. Efficacy and safety of neoadjuvant chemotherapy with concurrent liposomal-encapsulated doxorubicin, paclitaxel and trastuzumab for human epidermal growth factor receptor 2-positive breast cancer in clinical practice. Int J Clin Oncol. 2015;20(3):480–489. | ||
Wolff AC, Hammond ME, Schwartz JN, et al; American Society of Clinical Oncology; College of American Pathologists. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007;25(1):118–145. | ||
Wolff AC, Hammond ME, Hicks DG, et al; American Society of Clinical Oncology; College of American Pathologists. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. Arch Pathol Lab Med. 2014;138(2):241–256. | ||
Kuehn T, Bauerfeind I, Fehm T, et al. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicentre cohort study. Lancet Oncol. 2013;14(7):609–618. | ||
Boughey JC, Suman VJ, Mittendorf EA, et al; Alliance for Clinical Trials in Oncology. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the American College of Surgeons Oncology Group (ACOSOG) Z1071 clinical trial. JAMA. 2013;310(14):1455–1461. | ||
Therasse P, Arbuck SG, Eisenhauer EA, et al. Gwyther new guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000;92(3):205–216. | ||
Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247. | ||
Ogston KN, Miller ID, Payne S, et al. A new histological grading system to assess response of breast cancers to primary chemotherapy: prognostic significance and survival. Breast. 2003;12(5):320–327. | ||
Carey LA, Metzger R, Dees EC, et al. American joint committee on cancer tumor-node-metastasis stage after neoadjuvant chemotherapy and breast cancer outcome. J Natl Cancer Inst. 2005;97(15):1137–1142. | ||
Garg AK, Strom EA, McNeese MD, et al. T3 disease at presentation or pathologic involvement of four or more lymph nodes predict for locoregional recurrence in stage II breast cancer treated with neoadjuvant chemotherapy and mastectomy without radiotherapy. Int J Radiat Oncol Biol Phys. 2004;59(1):138–145. | ||
Huang EH, Tucker SL, Strom EA, et al. Postmastectomy radiation improves local-regional control and survival for selected patients with locally advanced breast cancer treated with neoadjuvant chemotherapy and mastectomy. J Clin Oncol. 2004;22(23):4691–4699. | ||
Swain SM, Whaley FS, Ewer MS. Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials. Cancer. 2003;97(11):2869–2879. | ||
Tsai HT, Isaacs C, Fu AZ, et al. Risk of cardiovascular adverse events from trastuzumab (Herceptin(®)) in elderly persons with breast cancer: a population-based study. Breast Cancer Res Treat. 2014;144(1):163–170. | ||
Dranitsaris G, Rayson D, Vincent M, et al. The development of a predictive model to estimate cardiotoxic risk for patients with metastatic breast cancer receiving anthracyclines. Breast Cancer Res Treat. 2008;107(3):443–450. | ||
Fumoleau P, Roché H, Kerbrat P, et al; French Adjuvant Study Group. Long-term cardiac toxicity after adjuvant epirubicin-based chemotherapy in early breast cancer: French Adjuvant Study Group results. Ann Oncol. 2006;17(1):85–92. | ||
Sciarretta S, Palano F, Tocci G, Baldini R, Volpe M. Antihypertensive treatment and development of heart failure in hypertension: a Bayesian network meta-analysis of studies in patients with hypertension and high cardiovascular risk. Arch Intern Med. 2011;171(5):384–394. | ||
Carver JR, Shapiro CL, Ng A, et al; ASCO Cancer Survivorship Expert Panel. American Society of Clinical Oncology clinical evidence review on the ongoing care of adult cancer survivors: cardiac and pulmonary late effects. J Clin Oncol. 2007;25(25):3991–4008. | ||
Groarke JD, Nguyen PL, Nohria A, Ferrari R, Cheng S, Moslehi J. Cardiovascular complications of radiation therapy for thoracic malignancies: the role for non-invasive imaging for detection of cardiovascular disease. Eur Heart J. 2014;35(10):612–623. | ||
Antón A, Ruiz A, Plazaola A, et al. Phase II clinical trial of liposomal-encapsulated doxorubicin citrate and docetaxel, associated with trastuzumab, as neoadjuvant treatment in stages II and IIIA HER2-overexpressing breast cancer patients. GEICAM 2003-03 study. Ann Oncol. 2011;22(1):74–79. | ||
Tuxen MK, Cold S, Tange UB, Balslev E, Nielsen DL. Phase II study of neoadjuvant pegylated liposomal doxorubicin and cyclophosphamide ± trastuzumab followed by docetaxel in locally advanced breast cancer. Acta Oncol. 2014;53(10):1440–1445. | ||
Uriarte-Pinto M, Escolano-Pueyo Á, Gimeno-Ballester V, Pascual-Martínez O, Abad-Sazatornil MR, Agustín-Ferrández MJ. Trastuzumab, non-pegylated liposomal-encapsulated doxorubicin and paclitaxel in the neoadjuvant setting of HER-2 positive breast cancer. Int J Clin Pharm. 2016;36(2):446–453. | ||
Carey LA, Berry DA, Cirrincione CT, et al. Molecular heterogeneity and response to neoadjuvant human epidermal growth factor receptor 2 targeting in CALGB 40601, a randomized phase III trial of paclitaxel plus trastuzumab with or without lapatinib. J Clin Oncol. 2016;34(6):542–549. | ||
Yerushalmi R, Woods R, Ravdin PM, Hayes MM, Gelmon KA. Ki67 in breast cancer: prognostic and predictive potential. Lancet Oncol. 2010;11(2):174–183. | ||
Dowsett M, Nielsen TO, A’Hern R, et al; International Ki-67 in Breast Cancer Working Group. Assessment of Ki67 in breast cancer: recommendations from the International Ki67 in Breast Cancer working group. J Natl Cancer Inst. 2011;103(22):1656–1664. | ||
Tashima R, Nishimura R, Osako T, et al. Evaluation of an optimal cut-off point for the Ki-67 index as a prognostic factor in primary breast cancer: a retrospective study. PLoS One. 2015;15(7):e0119565. | ||
Wolff B. Histological grading in carcinoma of the breast. Br J Cancer. 1966;20(1):36–40. | ||
Fisher ER, Sass R, Fisher B. Pathologic findings from the National Surgical Adjuvant Project for Breast Cancers (protocol no. 4). X. Discriminants for tenth year treatment failure. Cancer. 1984;53(3 Suppl):712–723. | ||
Contesso G, Mouriesse H, Friedman S, Genin J, Sarrazin D, Rouesse J. The importance of histologic grade in long-term prognosis of breast cancer: a study of 1,010 patients, uniformly treated at the Institut Gustave-Roussy. J Clin Oncol. 1987;5(9):1378–1386. | ||
Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991;19(5):403–410. | ||
Galea MH, Blamey RW, Elston CE, Ellis IO. The Nottingham Prognostic Index in primary breast cancer. Breast Cancer Res Treat. 1992;22(3):207–219. | ||
Dabbs DJ. Ductal carcinoma of breast: nuclear grade as a predictor of S-phase fraction. Hum Pathol. 1993;24(6):652–656. | ||
Fisher B, Brown A, Mamounas E. Effect of preoperative chemotherapy on local-regional disease in women with operable breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-18. J Clin Oncol. 1997;15(7):2483–2493. | ||
Wolmark N, Wang J, Mamounas E, Bryant J, Fisher B. Preoperative chemotherapy in patients with operable breast cancer: nine-year results from National Surgical Adjuvant Breast and Bowel Project B-18. J Natl Cancer Inst Monogr. 2001;2001(30):96–102. | ||
van der Hage JA, van de Velde CJ, Julien JP, Tubiana-Hulin M, Vandervelden C, Duchateau L. Preoperative chemotherapy in primary operable breast cancer: results from the European Organization for Research and Treatment of Cancer trial 10902. J Clin Oncol. 2001;19(22):4224–4237. | ||
Mieog JS, van der Hage JA, van de Velde CJ. Neoadjuvant chemotherapy for operable breast cancer. Br J Surg. 2007;94(10):1189–1200. | ||
Chen AM, Meric-Bernstam F, Hunt KK, et al. Breast conservation after neoadjuvant chemotherapy: the MD Anderson cancer center experience. J Clin Oncol. 2004;22(12):2303–2312. | ||
Chen AM, Meric-Bernstam F, Hunt KK, et al. Breast conservation after neoadjuvant chemotherapy. Cancer. 2005;103(4):689–695. | ||
Lobbes MBI, Prevos R, Smidt M, et al. The role of magnetic resonance imaging in assessing residual disease and pathologic complete response in breast cancer patients receiving neoadjuvant chemotherapy: a systematic review. Insights Imaging. 2013;4(2):163–175. | ||
Marinovich ML, Houssami N, Macaskill P, et al. Meta-analysis of magnetic resonance imaging in detecting residual breast cancer after neoadjuvant therapy. J Natl Cancer Inst. 2013;105(5):321–333. | ||
Thavendiranathan P, Poulin F, Lim KD, Plana JC, Woo A, Marwick TH. Use of myocardial strain imaging by echocardiography for the early detection of cardiotoxicity in patients during and after cancer chemotherapy: a systematic review. J Am Coll Cardiol. 2014;63(25 Pt A):2751–2768. | ||
Mehta LS, Watson KE, Barac A, et al; American Heart Association Cardiovascular Disease in Women and Special Populations Committee of the Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; and Council on Quality of Care and Outcomes Research. Cardiovascular disease and breast cancer: where these entities intersect: a scientific statement from the american heart association. Circulation. 2018;137(8):e30–e66. | ||
Cardinale D, Colombo A, Torrisi R, et al. Trastuzumab-induced cardiotoxicity: clinical and prognostic implications of troponin I evaluation. J Clin Oncol. 2010;28(25):3910–3916. | ||
Ponde N, Bradbury I, Lambertini M, et al. Cardiac biomarkers for early detection and prediction of trastuzumab and/or lapatinib-induced cardiotoxicity in patients with HER2-positive early-stage breast cancer: a NeoALTTO sub-study (BIG 1-06). Breast Cancer Res Treat. 2018;168(3):631–638. | ||
Ammon M, Arenja N, Leibundgut G, et al. Cardiovascular management of cancer patients with chemotherapy-associated left ventricular systolic dysfunction in real-world clinical practice. J Card Fail. 2013;19(9):629–634. | ||
Oliveira GH, Mukerji S, Hernandez AV, et al. Incidence, predictors, and impact on survival of left ventricular systolic dysfunction and recovery in advanced cancer patients. Am J Cardiol. 2014;113(11):1893–1898. | ||
Cueva JF, Antolín S, Calvo L, et al. Galician consensus on management of cardiotoxicity in breast cancer: risk factors, prevention an early intervention. Clin Trasl Oncol. 2017;19(9):1067–1078. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.