Back to Journals » International Journal of General Medicine » Volume 14
Primary Sites of Uveal Melanoma Associated with Distinct Survival Outcomes and Clinicopathological Features: A SEER Population-Based Study of 4359 Cases
Authors Liang X, Rong Y, Wang J, Zhang H
Received 11 July 2021
Accepted for publication 11 August 2021
Published 4 September 2021 Volume 2021:14 Pages 5221—5232
DOI https://doi.org/10.2147/IJGM.S328910
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Xin Liang, Yan Rong, Junming Wang, Hong Zhang
Department of Ophthalmology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, 430030, People’s Republic of China
Correspondence: Junming Wang; Hong Zhang Email [email protected]; [email protected]
Objective: We sought to investigate clinicopathological characteristics correlated with the prognosis of uveal melanoma (UM) patients and find the driving factors of prognosis for ciliary/iris melanoma relative to choroid melanoma.
Materials and Methods: We collected patients with uveal melanoma between 1983 and 2012 from the Surveillance, Epidemiology, and End Results (SEER) database. Primary outcomes were evaluated as cancer-specific survival (CSS) and overall survival (OS). The Kaplan-Meier analysis was applied for the univariate analysis of CSS and OS and corresponding survival curves. Cox proportional hazards regression was used for multivariate analysis to value hazard ratio (HR) of ciliary body/iris melanoma subgroup versus choroid melanoma subgroup.
Results: A total of 4359 eligible patients were collected in our study. Novel potential prognostic factors for CSS and OS of UM were identified. Age at diagnosis, sex, primary tumor site, histologic subtype, tumor size, the extent of disease, and treatment were the independent prognostic factors for UM patients (P < 0.05). Interestingly, when concerned with the primary site of UM, we found that the ciliary body/iris melanoma subgroup showed significant differences in prognosis (both CSS and OS), sex, histologic type, the extent of disease, and treatment options relative to choroid melanoma subgroup (P < 0.05). Subsequently, stratification analyses suggested that the distinct survival outcomes between the ciliary body/iris melanoma and choroid melanoma subgroups mainly attributed to patient sex, age, tumor size, the extent of disease, and treatment options (P < 0.05).
Conclusion: Age, sex, primary tumor site, histologic subtype, tumor size, the extent of disease, and treatment options are independent prognostic indicators for UM patients. Besides, the ciliary body/iris melanoma subgroup shows worse survival outcomes than choroid melanoma. Our findings offer inspiration to the individual treatment for UM patients with different primary sites.
Keywords: uveal melanoma, overall survival, cancer-specific survival, ciliary body/iris melanoma, choroid melanoma
Introduction
Uveal melanoma (UM) is one of the most usual malignant ocular tumors and mostly occurs in adults aged 40 to 60 years. Referring to the Surveillance, Epidemiology, and End Results (SEER) database, the incidence of UM for the period 1973–2008 was approximately 5.1 per million.1
Treatments for patients with UM include surgery, radiotherapy, and other methods based on different disease condition aspects, such as tumor characteristics, fellow eye status, tumor metastasis, and the patient’s general condition. Surgical treatments include enucleation and local tumor resection.2 Increasing attempts have been put forward to decrease the rate of irreplaceable enucleation, which is expected to conserve vision and increase survival rates. Radiotherapy, which could reduce the local recurrence rate, has also been widely used in UM treatments in recent years.1 Several treatments have been administered to patients to eliminate not only the primary tumor but also occult metastases.
The reported 5-year relative UM survival rate is approximately 77–84%.3 Several prognostic factors are used to estimate individual future survival. To date, a few significant analyses have been conducted to identify prognostic factors for patients with UM. These analyses indicated that factors such as sex, histology, TNM classification (T: extent of primary tumor, N: condition of regional nodes, M: presence or absence of distant metastases), initial malignancy, race, and age at diagnosis are associated with the UM survival rate while only the factors histology, TNM classification, and age at diagnosis are linked with disease-specific survival.4 However, previous reports focused only on a single prognostic factor for UM patients and did not perform multivariate analyses. Moreover, other potential prognostic factors, such as sex, year of diagnosis, and treatment, have not yet been reported or have not reached a consensus.5–11 Previous reports were mostly based on single-center research, in which the sample size might be insufficient. In addition, relevant systematic studies have not been performed on the differences in demographic or clinicopathological variables that affect the prognosis of UM between the different primary sites, ie, ciliary body, iris, and choroid. Therefore, more comprehensive and systematic efforts in identifying UM prognostic factors are required.
In the present study, we conducted a SEER population-based study that included 4359 cases and identified significant survival differences between choroid melanoma and ciliary body/iris melanoma. In addition, we confirmed the independent prognostic roles of several clinicopathological factors of uveal melanoma, which were previously disputed because of limited sample sizes. Developing a deeper understanding of the differences between ciliary body/iris melanoma and choroid melanoma can facilitate clinical predictions and prognoses and offer immunotherapy guidance for UM patients.
Materials and Methods
Patient Cohort
All patients’ cohort information was obtained from the SEER database in 18 registries [www.seer.cancer.gov]. The database covers about 27.8% of the overall US population and is publicly available and widely used in authoritative tumor epidemiology research. The data extracted from the database were analyzed using SEER*Stat software, version 8.3.2.
Certain inclusion criteria were applied to the patient cohort in the present study: 1. Patients diagnosed with primary malignancy from 1983 to 2012 were reviewed; 2. All cohort cases were diagnosed when the patients were still alive, and the death certificates or autopsies were excluded; 3. On the basis of the morphology codes defined by the international classification of diseases for oncology (ICD-O-3), 8720 malignant melanomas [not otherwise specified (NOS)], 8770 mixed epithelioid and spindle cell melanomas, 8771 epithelioid cell melanomas, and 8772–8774 spindle-cell melanomas were used to identify UM; 4. Primary sites of the cases were restricted to the choroid and ciliary body/iris by site codes C69.3 and C69.4, respectively; 5. Localized, regional, or distant tumors were used to describe the disease extent according to the SEER staging system (SEER historic stage A); and 6. Only patients with known tumor size, survival time, and cause of death were included and actively followed up without missing data.
In the present study, 6330 records of choroid and ciliary body/iris melanoma were obtained from the SEER database from 1983 to 2012. Among these patients, 1971 patients were excluded because of unknown data. Finally, 4359 patient records remained for analysis in the present study.
Statistical Analysis
Certain demographic or clinicopathological variables, including age, race, sex, year of diagnosis, primary tumor site, histologic subtype, tumor size, extent of disease, and treatment, were brought into the analysis. The age at diagnosis was sorted based on 10-year intervals. The basal diameter size of 1 cm is used as a cut-off value which can distinguish the large melanoma to small or medium one in Collaborative Ocular Melanoma Study (COMS) staging, and 10 mm was also used as the cut-off value to large melanoma in several published articles; therefore, we used the 1 cm to separate patients into two groups, <1 cm and ≥1 cm.12–14
Overall survival (OS) and cancer-specific survival (CSS) were recognized as the primary outcome measures. CSS time was defined as the months from diagnosis to death attributed to UM, while OS time was measured as the time from diagnosis to death due to all possible causes. The Kaplan-Meier method and Log-rank tests were conducted for the univariate analysis of CSS and OS and corresponding survival curves. A Cox proportional hazards regression was used for multivariate analysis to control for potential confounding variables. The hazard ratio (HR) and matched 95% confidence interval (CI) of CSS and OS were calculated using the Cox proportional hazards regression model.
Patients were assigned into two subgroups referring to the primary site: ciliary body/iris melanoma subgroup and choroid melanoma subgroup. A Cox proportional hazard model was also adopted to estimate the HR of the ciliary body/iris melanoma subgroup versus the choroid melanoma subgroup, and a forest plot of the HR was created to better evaluate the association between potential prognostic factors and OS and CSS.
Statistical significance was defined once a two-sided p-value <0.05. The Kaplan-Meier method, Log-rank tests, and Cox proportional hazards regression were analyzed using IBM SPSS Statistics, version 23 (SPSS Inc/IBM Chicago, Illinois, USA) and R software, version 4.0.2.
Results
Patient Baseline Characteristics
Overall, 4359 patients were brought into the present study, of which 1743 (40.0%) died due to various reasons and 1055 (24.2%) died from UM. The clinicopathological characteristics of UM patients are shown in Table 1. Among the patients included, 2307 (52.9%) patients in the cohort were male. The median age for the UM patients at diagnosis was 60-year-old. The primary tumor sites of 3822 (87.7%) patients occurred at the choroid, with the remaining cases occurring at the ciliary body and iris. For the histologic subtype, 2948 (67.6%) cases were malignant melanoma (NOS), 593 (13.6%) were mixed epithelioid and spindle cell melanoma, 184 (4.2%) were epithelioid cell melanoma, and 634 (14.5%) were spindle-cell melanoma. Among the overall patients, 130 (3.0%) did not receive any surgery or radiotherapy, 1549 (35.5%) underwent surgery, 2355 (54.0%) received radiotherapy, and 325 (7.5%) underwent both surgery and radiotherapy. The median survival time for all enrolled patients was 62 months (Table 1).
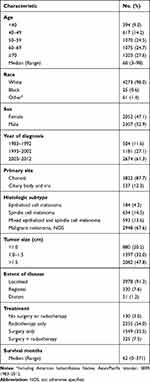 |
Table 1 Baseline Demographics and Clinical Characteristics of Patients (n=4359) |
Prognostic Factors Associated with the OS and CSS of UM
The factors age, sex, race, primary site, histologic subtype, tumor size, disease extent, and treatment were included in the univariate analysis. The Kaplan-Meier survival analysis identified different prognoses for patients of different ages (Tables 2 and 3). Older age was associated with worse outcomes. For the primary site, patients with choroid melanoma had significantly better OS and CSS rates than patients diagnosed at the ciliary/iris body (OS, HR 1.151, 95% CI 1.008–1.315, P = 0.038; CSS, HR 1.224, 95% CI 1.034–1.449, P = 0.019) (Figures 1 and 2). Different outcomes were observed with different histologic subtypes compared to the epithelioid subtype, and the other histologic types all presented better clinical outcomes (OS, epithelioid, reference; spindle, HR 0.386, 95% CI 0.312–0.478, P < 0.001; mixed, HR 0.698, 95% CI 0.569–0.856, P = 0.001; CSS, epithelioid, reference; spindle, HR 0.292, 95% CI 0.224–0.382, P < 0.001; mixed, HR 0.682, 95% CI 0.535–0.870, P = 0.002). We found that radiation treatment significantly improved the OS but not the CSS while surgery or surgery plus radiation therapy led to significantly unfavorable CSS in uveal melanoma (OS, No, reference; radiation, HR 0.710, 95% CI 0.538–0.937, P = 0.016; surgery, HR 1.225, 95% CI 0.930–1.612, P = 0.148; surgery + radiotherapy, HR 1.245, 95% CI 0.915–1.694, P = 0.164; CSS, No, reference; radiation, HR 0.887, 95% CI 0.582–1.352, P = 0.577; surgery, HR 1.841, 95% CI 1.213–2.794, P = 0.004; surgery + radiotherapy, HR 2.060, 95% CI 1.318–3.220, P = 0.002). Moreover, the extent of the disease might be an independent predictor of UM. Our results indicated that the sex or race of the patient had no impact on the OS or CSS (all, P > 0.05) (Tables 2 and 3).
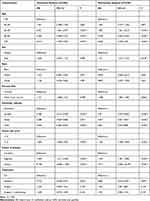 |
Table 2 Univariate and Multivariate Analysis of Overall Survival for Uveal Melanoma Patients |
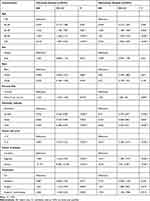 |
Table 3 Univariate and Multivariate Analysis of Cancer-Specific Survival for Uveal Melanoma Patients |
 |
Figure 1 The difference in overall survival between choroid melanoma and ciliary body/iris melanoma. |
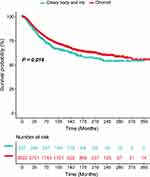 |
Figure 2 The difference in cancer-specific survival between choroid melanoma and ciliary body/iris melanoma. |
A multivariate Cox analysis was also used to prove the independent prognostic factors for OS and CSS (Tables 2 and 3). For OS, an increased mortality risk was observed for male (HR 1.121, 95% CI 1.019–1.233, P = 0.018) and older patients (Table 2). In particular, patients aged above 50 years presented dismal prognoses compared with those of patients aged below 40 years. White patients had similar outcomes compared to their black counterparts. However, patients with ciliary body/iris melanoma had worse outcomes than those with choroid melanoma (HR 1.208, 95% CI 1.056–1.382, P = 0.006). Concerning the histologic subtype, patients with epithelioid cell melanoma had worse OS than those with spindle-cell melanoma (HR 0.470, 95% CI 0.379–0.582, P < 0.001) and mixed epithelioid and spindle cell melanoma (HR 0.677, 95% CI 0.551–0.831, P < 0.001). As expected, a larger tumor size led to a worse prognosis. The distant extent or regional extent of the tumors led to a higher mortality risk in UM patients compared with localized cases. Radiotherapy only was associated with better OS than no surgery or radiation treatment (HR 0.682, 95% CI 0.516–0.901, P = 0.007), whereas both surgery only or radiotherapy plus surgery did not appear to provide a benefit (HR 1.264, 95% CI 0.950–1.683, P = 0.107; HR 1.291, 95% CI 0.946–1.761, P = 0.107) (Table 2). Similar conclusions were drawn based on the multivariate Cox analysis of CSS, except for sex and treatment. Briefly, age, primary site, histologic subtype, tumor size, disease extent, and treatment were independent prognostic factors of CSS (all, P < 0.05) (Table 3).
As mentioned above, the primary site represented an independent prognostic factor for OS and CSS. The relationship between different clinical features and prognosis in the different primary sites of UM has not been reported. As shown in Table 4, ciliary body/iris melanoma was related to worse OS and CSS than choroid melanoma (OS P < 0.001, CSS P = 0.002). Choroid melanoma occurred more frequently in male patients, while ciliary body/iris melanoma was more common in females (P = 0.029). The histologic type and disease extent showed significant differences between the two subgroups (P < 0.05). Patients with ciliary body/iris melanoma more frequently received surgery or surgery plus radiation, while patients with choroid melanoma more frequently received radiotherapy or did not receive surgery or radiation treatment (P < 0.001). In addition, significant differences in mean age, race, and tumor size were not observed between the two subgroups (P > 0.05).
 |
Table 4 Site Seer Summarization of Clinical Features |
The multivariate Cox analyses of the OS and CSS of the ciliary body/iris melanoma subgroup compared with the choroid melanoma subgroup were clearly demonstrated via a forest plot in Figures 3 and 4. The HRs for sex, age, histologic subtype, disease extent, and treatment were significantly different between choroid melanoma and ciliary body/iris melanoma in the subgroup analysis of OS and CSS (P < 0.05) (Figures 3 and 4). Ciliary body/iris melanomas in males, old patients, distant tumors, or treatment with both surgery and radiation were associated with worse OS prognoses than choroid melanomas (P < 0.05, Figure 3). Additionally, ciliary body/iris melanomas in older patients, patients with localized tumors, or patients in the surgery and radiation subgroups had worse CSS prognoses than choroid melanomas (P < 0.05, Figure 4).
Discussion
In this study, a large population of 4359 patients with different characteristics and outcomes from the SEER database was systematically analyzed for potential prognostic factors of UM. Age at diagnosis, sex, primary tumor site, histologic subtype, tumor size, extent of disease, and treatment were independent prognostic factors of UM. Sex, histologic type, disease extent, and treatment showed significant differences between the ciliary body/iris melanoma subgroup and choroid melanoma subgroup. Significant differences in age, race, or tumor size were not observed between the two groups. Patients with choroid melanoma showed distinct survival outcomes compared with patients with ciliary body/iris melanoma in the subgroup analyses of sex, age, tumor size, disease extent, and treatment options.
As mentioned above, although many clinical features are related to the prognosis of UM, detailed and comprehensive investigations on the correlationship between various primary sites and patient prognosis has not been reported. Here, we demonstrated that patients with choroid melanoma were related with significantly better OS and CSS than patients diagnosed with ciliary body/iris melanoma. This worse outcome of ciliary body melanoma was reported to be related to a larger tumor size, tumor microvascular patterns, and predilection for monosomy 3 and 8q gain.15–19 Consistent with previous studies, ciliary body/iris melanoma was reported to have a higher metastasis rate and worse prognosis than choroid melanoma.14,20 In a study of 8033 UM patients, the incidence of 5- and 10-year metastasis was 19% and 33% for ciliary body melanoma and only 15% and 25% for choroid melanoma, respectively.16 Li et al21 also showed that the degree of ciliary body involvement was significantly associated with melanoma-related metastasis, melanomas of estimated ciliary body origin had a 1.6–2.3 times higher risk of metastasis than those of choroid origin. However, Kaliki et al22 reported that iris melanoma contained a better prognosis as compared with ciliary and choroidal melanomas, which might be caused by the younger age at diagnosis, smaller tumor size and less biological activity. One limitation of the current study is that recorded from the SEER database put the iris UM and ciliary body UM together, which might be caused by the lower prevalence rate and adjacent location of these two types. Therefore, the association between tumor location to prognosis of melanomas need to be investigated in the future with more large number of cohorts, especially the difference of ciliary and iris melanoma.
In addition, there are several advantages in the current study. First, we revealed that age, sex, primary site, histologic subtype, tumor size, and whether extent are all the independent risk factors to the OS outcome for UM patients, but sex failed in the CSS outcome. Second, we focused on the different features and outcomes of choroid UM and iris/ciliary body type. The distribution of OS, CSS, sex, histologic type and whether extent are different, with the similar distribution of patient age, race and tumor size. Third, based on the 4359 UM patients recorded in the SEER database, we revealed that most patients with choroid type UM received radiation therapy (56.1%), while most iris/ciliary body UM patients received surgery therapy (50.5%); however, iris/ciliary body subgroup presented the poor prognosis after treatment; therefore, it is necessary to re-evaluate the clinical treatment of low prevalence UM type.
Several factors are associated with UM prognosis, although previous studies presented different results on the influence of sex on prognosis through retrospective cases.5–7,23,24 The multivariate analysis performed here revealed that sex was associated with OS, male patients contained a poor prognosis. In addition, an increasing number of UM patients are treated with radiation rather than enucleation. A previous study reported that UM prognosis is independent of the local treatment method, such as tumor resection, plaque radiotherapy, or proton beam irradiation, while the opposite results were observed for patients treated with enucleation.8–10 A recent review by Zimmerman et al25,26 suggested that UM enucleation increased tumor cell dissemination and likelihood of cancer metastasis. Surgical procedures for UM could unintentionally induce a high risk of extrascleral extension via the dissemination of the tumor emboli by transected orbital vessels and conjunctival lymphatics.27 The present study revealed that treatment with radiotherapy was associated with better OS than no surgery or radiation treatment, whereas patients who underwent surgery only or surgery plus radiotherapy did not show significantly different OS than patients without treatment.
Although great advances have been made in UM prognosis, exhaustive and comprehensive studies on the differences between different sites of UM are still lacking. We systematically investigate independent prognostic factors for UM patients based on a large-sample multicenter database. We systematically demonstrated the different influences of the factors sex, race, histologic type, tumor size, disease extent, treatment, and prognosis at different primary sites. It is also essential to think about the limitations of this study. First, the SEER database did not include details on several important therapies, such as chemotherapy, immunotherapy, and molecular targeted therapy, or a detailed characterization of the radiotherapy protocols (ie, dosage and location). Second, this database lacked certain clinical examinations, such as visual acuity. Furthermore, SEER did not include data from several large ocular oncology treatment centers in the US, including those in Boston and Philadelphia. Finally, because the dataset was retrospectively collected, further prospective studies are required.
Conclusion
In conclusion, our study identified independent prognostic factors for UM and different clinical features and factors that affect the prognosis of ciliary body/iris melanoma and choroid melanoma. These data can help provide a deeper understanding of UM, promote the predictive accuracy of the survival of individual UM patients.
Data Sharing Statement
The data from present study are available in Surveillance, Epidemiology, and End Results database, http://seer.cancer.gov.
Ethics Approval and Consent to Participate
Not applicable. Informed patient consent was not required for data released by the freely available SEER database.
Acknowledgments
We thank the contribution of the SEER database and the 18 registries supplying cancer research information.
Author Contributions
XL, YR, JM, and HZ conceived and designed this study. XL and YR performed the analyses and prepared all the tables and figures. XL wrote the main manuscript. JM and HZ directed the completion of the study and modified the manuscript. All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
All authors declare that they have no conflicts of interest for this work.
References
1. Singh AD, Turell ME, Topham AK. Uveal melanoma: trends in incidence, treatment, and survival. Ophthalmology. 2011;118(9):1881–1885. doi:10.1016/j.ophtha.2011.01.040
2. Jang BS, Chang JH, Oh S, Lim YJ, Kim IH. Chirurgie vs. strahlentherapie bei patienten mit uveamelanom: analyse der SEER-datenbank mithilfe von propensity-score-matching und -gewichtung. Strahlentherapie und Onkologie. 2017;193(11):931–942. doi:10.1007/s00066-017-1203-0
3. Singh AD, Topham A. Survival rates with uveal melanoma in the United States: 1973–1997. Ophthalmology. 2003;110(5):962–965. doi:10.1016/s0161-6420(03)00077-0
4. Andreoli MT, Mieler WF, Leiderman YI. Epidemiological trends in uveal melanoma. Br J Ophthalmol. 2015;99(11):1550–1553. doi:10.1136/bjophthalmol-2015-306810
5. Zloto O, Pe’er J, Frenkel S. Gender differences in clinical presentation and prognosis of uveal melanoma. Invest Ophthalmol Vis Sci. 2013;54(1):652–656. doi:10.1167/iovs.12-10365
6. Rietschel P, Panageas KS, Hanlon C, Patel A, Abramson DH, Chapman PB. Variates of survival in metastatic uveal melanoma. J Clin Oncol. 2005;23(31):8076–8080. doi:10.1200/jco.2005.02.6534
7. Shields CL, Kaliki S, Furuta M, Mashayekhi A, Shields JA. Clinical spectrum and prognosis of uveal melanoma based on age at presentation in 8033 cases. Retina. 2012;32(7):1363–1372. doi:10.1097/IAE.0b013e31824d09a8
8. Augsburger JJ, Gamel JW, Lauritzen K, Brady LW. Cobalt-60 plaque radiotherapy vs enucleation for posterior uveal melanoma. Am J Ophthalmol. 1990;109(5):585–592. doi:10.1016/S0002-9394(14)70691-9
9. Augsburger JJ, Schneider S, Freire J, Brady LW. Survival following enucleation versus plaque radiotherapy in statistically matched subgroups of patients with choroidal melanomas: results in patients treated between 1980 and 1987. Graefes Arch Clin Exp Ophthalmol. 1999;237(7):558–567. doi:10.1007/s004170050279
10. Seddon JM, Gragoudas ES, Egan KM, et al. Relative survival rates after alternative therapies for uveal melanoma. Ophthalmology. 1990;97(6):769–777. doi:10.1016/S0161-6420(90)32512-5
11. Querques G, Bux AV, Iaculli C, Delle Noci N. Local resection versus combined local resection and plaque radiotherapy in the treatment of choroidal melanoma. Eur J Ophthalmol. 2010;20(1):194–200. doi:10.1177/112067211002000127
12. Shields CL, Sioufi K, Robbins JS, et al. Large uveal melanoma (>/=10 mm thickness): clinical features and millimeter-by-millimeter risk of metastasis in 1311 cases. The 2018 Albert E. Finley lecture. Retina. 2018;38(10):2010–2022. doi:10.1097/IAE.0000000000002144
13. Diener-West M, Earle JD, Fine SL, et al. The COMS randomized trial of iodine 125 brachytherapy for choroidal melanoma, III: initial mortality findings. COMS Report No. 18. Arch Ophthalmol. 2001;119(7):969–982. doi:10.1001/archopht.119.7.969
14. Shields CL, Furuta M, Thangappan A, et al. Metastasis of uveal melanoma millimeter-by-millimeter in 8033 consecutive eyes. Arch Ophthalmol. 2009;127(8):989–998. doi:10.1001/archophthalmol.2009.208
15. Damato B, Coupland SE. A reappraisal of the significance of largest basal diameter of posterior uveal melanoma. Eye. 2009;23(12):2152–2160. doi:10.1038/eye.2009.235-cme
16. McLean IW, Foster WD, Zimmerman LE. Uveal melanoma: location, size, cell type, and enucleation as risk factors in metastasis. Hum Pathol. 1982;13(2):123–132. doi:10.1016/s0046-8177(82)80116-0
17. Prescher G, Bornfeld N, Hirche H, Horsthemke B, Jockel KH, Becher R. Prognostic implications of monosomy 3 in uveal melanoma. Lancet. 1996;347(9010):1222–1225. doi:10.1016/S0140-6736(96)90736-9
18. Damato B, Duke C, Coupland SE, et al. Cytogenetics of uveal melanoma: a 7-year clinical experience. Ophthalmology. 2007;114(10):1925–1931. doi:10.1016/j.ophtha.2007.06.012
19. Rummelt V, Folberg R, Woolson RF, Hwang T, Pe’er J. Relation between the microcirculation architecture and the aggressive behavior of ciliary body melanomas. Ophthalmology. 1995;102(5):844–851. doi:10.1016/S0161-6420(95)30947-5
20. Shields CL, Kaliki S, Shah SU, Luo W, Furuta M, Shields JA. Iris melanoma: features and prognosis in 317 children and adults. J AAPOS. 2012;16(1):10–16. doi:10.1016/j.jaapos.2011.10.012
21. Li W, Gragoudas ES, Egan KM. Metastatic melanoma death rates by anatomic site after proton beam irradiation for uveal melanoma. Arch Ophthalmol. 2000;118(8):1066–1070. doi:10.1001/archopht.118.8.1066
22. Kaliki S, Shields CL. Uveal melanoma: relatively rare but deadly cancer. Eye. 2017;31(2):241–257. doi:10.1038/eye.2016.275
23. Kujala E, Makitie T, Kivela T. Very long-term prognosis of patients with malignant uveal melanoma. Invest Ophthalmol Vis Sci. 2003;44(11):4651–4659. doi:10.1167/iovs.03-0538
24. Bergman L, Seregard S, Nilsson B, Lundell G, Ringborg U, Ragnarsson-Olding B. Uveal melanoma survival in Sweden from 1960 to 1998. Invest Ophthalmol Vis Sci. 2003;44(8):3282–3287. doi:10.1167/iovs.03-0081
25. Zimmerman LE, McLean IW, Foster WD. Does enucleation of the eye containing a malignant melanoma prevent or accelerate the dissemination of tumour cells. Br J Ophthalmol. 1978;62(6):420–425. doi:10.1136/bjo.62.6.420
26. Zimmerman LE, McLean IW. An evaluation of enucleation in the management of uveal melanomas. Am J Ophthalmol. 1979;87(6):741–760. doi:10.1016/0002-9394(79)90348-9
27. Affeldt JC, Minckler DS, Azen SP, Yeh L. Prognosis in uveal melanoma with extrascleral extension. Arch Ophthalmol. 1980;98(11):1975–1979. doi:10.1001/archopht.1980.01020040827006
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


