Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 15
Primary Localized Cutaneous Nodular Amyloidosis Presenting as Milia: An Unusual Clinical Manifestation
Authors Wang Y, Zhou H , Wang J, Wang P
Received 12 June 2022
Accepted for publication 10 August 2022
Published 13 August 2022 Volume 2022:15 Pages 1639—1642
DOI https://doi.org/10.2147/CCID.S378253
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Jeffrey Weinberg
Yanqing Wang,1 Hongyu Zhou,1 Jiaqi Wang,1 Ping Wang2
1Department of Dermatology, Hangzhou Third People’s Hospital, Zhejiang Chinese Medical University, Hangzhou, 310009, People’s Republic of China; 2Department of Dermatology, Hangzhou Third People’s Hospital, Affiliated Hangzhou Dermatology Hospital of Zhejiang University School of Medicine, Hangzhou, 310009, People’s Republic of China
Correspondence: Ping Wang, Department of Dermatology, Hangzhou Third People’s Hospital; Affiliated Hangzhou Dermatology Hospital, Zhejiang University School of Medicine, Westlake Ave 38, Hangzhou, 310009, People’s Republic of China, Tel +8613588812862, Email [email protected]
Abstract: Primary localized cutaneous nodular amyloidosis (PLCNA) is rare and clinically noncharacteristic, presenting mostly as plaque-like lesions. We report a case of a progressively larger erythematous plaque following a contusion of the skin on the right zygomatic area, which was strangely covered with recurrent scattered 2 mm whiteish blisters to the extent that it was misdiagnosed as a herpesvirus infection several times over a decade. Pathology and special staining diagnosed nodular amyloidosis with milia.
Keywords: primary localized cutaneous nodular amyloidosis, milia, local trauma, plaque
Introduction
Amyloidosis is the accumulation of proteins of the amyloid type in various tissues.1 Primary localized cutaneous nodular amyloidosis is a rare and distinct type of amyloidosis;2 it usually presents as single or multiple firm, waxy nodules or infiltrated plaque and occurs preferentially on the legs and commonly on the face and trunk but can be present anywhere on the skin.3 Milia are benign keratinous cysts most commonly found on the face, especially on the cheeks and eyelids.4 Primary milia emerge spontaneously, and secondary milia result from skin disease or trauma.5 Here, we report an unusual case of primary localized cutaneous nodular amyloidosis associated with recurrent milia that occurred after local trauma.
Case Report
A 54-year-old Chinese female with limited persistent plaque and recurrent pustules for 11 years had an initial appearance of erythema, followed by whiteish pinhead-sized scattered blisters 1–2 days later. The blisters resolved after approximately 1 to 2 weeks, and topical iodophor was ineffective. The patient had been seen at another hospital and was diagnosed with herpes simplex. Oral valacyclovir and topical penciclovir cream were also ineffective. The patient recalled that she had lacerated her right zygomatic area with a wooden comb a few days before the onset of the plaque. Over the past 11 years, the plaque had gradually enlarged, with scattered blisters on the plaque recurring once every 1–2 months.
Physical examination revealed a 2×2 cm oval-shaped, well-demarcated, orange-yellow plaque with overlying scattered 2 mm whiteish blisters on the right zygomatic area (Figure 1). No similar lesions were found elsewhere. Histopathologically, the overlying epidermis showed atrophic changes, and lymphocytes and plasma cells were found in the superficial dermis and around the blood vessels (Figure 2A). The entire dermis and subcutis were filled with eosinophilic, amorphous, and homogeneous amyloid material that was positive for Congo red and Crystal violet staining (the arrows in Figure 2B and C represent the sites of amyloid deposition). Some keratin cysts were found in the superficial dermis (Figure 2B and C). Arsenicum staining, acid staining, periodic acid-Schiff (PAS) staining and direct immunofluorescence (DIF) results were all negative, and all these findings were consistent with a diagnosis of primary localized cutaneous nodular amyloidosis with milia.
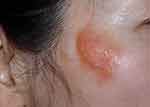 |
Figure 1 Orange‒yellow plaque with overlying scattered 2 mm whiteish blisters on the right zygomatic area. |
The patient has remained in good general health with no evidence of progression to systemic amyloidosis. Her blood count, blood chemistries, liver and kidney function, antinuclear antibody profile, serum protein electrophoresis and urine protein electrophoresis studies were normal. The patient refused our proposed surgical treatment option but indicated that she would be followed up regularly.
Discussion
Primary localized cutaneous amyloidosis is subdivided into papular, macular, and nodular amyloidosis. Nodular amyloidosis is the rarest type of primary localized cutaneous amyloidosis, presenting as waxy infiltrated plaque and nodules; it is characterized by the deposition of amyloid in the dermis, subcutaneous tissue and small blood vessels of the skin.2 Dermoscopically, nodular amyloidosis shows features similar to granulomatous disease, including an orange background and distal vessels; reddish structureless and whitish area and ulcerations were also reported.6 PLCNA represents a localized dyscrasia of plasma cells and is associated with autoimmune diseases, particularly Sjogren syndrome.7 Plasma cell dyscrasia, possibly caused by an autoimmune disease, may induce the local production and deposition of immunoglobulin light chains.8 Therefore, it is recommended to include anti-nuclear, anti-Ro/SSA and anti-La/SSB in the laboratory tests to exclude autoimmune diseases.
Milia are small, white, multiple, benign, superficial keratinous cysts,9 and they can be divided into primary milia and secondary milia.5 Primary milia are thought to develop from the sebaceous collar of vellus hairs, whereas most secondary milia originate from eccrine ducts rather than the overlying epidermis, hair follicles or sebaceous ducts.10 The diagnosis of primary cutaneous nodular amyloidosis with recurrent milia is very rare and has not been reported.
In our case, the clinical presentation was misleading. Due to the rarity of PLCNA and the presence of milia, it could be easily be mistaken for other diseases, such as cutaneous sarcoidosis, lupus tumidus, pseudolymphoma, facial granuloma, benign lymphocytic infiltration, or herpes simplex virus type 1 (HSV-1) infections. This patient was misdiagnosed with herpes simplex for more than 10 years, and the associated medication was ineffective. Therefore, the clinicopathological correlation as well as subsequent follow-up is very important, since it can help with the correct diagnosis and management decisions.
The trigger for nodular amyloidosis is unclear, and suggested causes are speculative, while in secondary milia, the cause can be medication-related, disease-associated, or trauma-related. Milia are commonly caused by traumatizing superficial abrasions.10 The patient had a history of abrasions, which were thought to be the cause of the milia. However, there is no consensus on whether trauma creates milia through epidermal implantation or by stimulating undifferentiated pilosebaceous cells to proliferate. The classic disease-associated type of milia is postbullous,11 and other diseases include herpes zoster and bullous pemphigoid. Milia have not been found to be associated with nodular amyloidosis. Nevertheless, the recurrent appearance of milia in our case suggests a possible association with amyloid irritation.
In most cases, primary cutaneous nodular amyloidosis is benign and limited to the skin; however, it can progress to systemic disease in approximately 7% to 50% of patients.12 Treatment of localized cutaneous nodular amyloidosis is difficult due to the lack of a consistently effective treatment. Various treatment modalities have been reported to improve the appearance of lesions, including surgical excision, cryotherapy, electrodesiccation and curettage, intralesional steroid injection, and more recently, carbon dioxide laser treatment.13 Moreover, bleeding complications as well as the recurrence of skin lesions should be considered.14 In our case, the medical history of primary cutaneous nodular amyloidosis reached 11 years, and no systemic progression was observed. It is unknown whether the presence of milia alters the risk of progression to systemic disease, and long-term follow-up is necessary. Based on relevant case reports, we recommend a follow-up period of 6 months after histopathological diagnosis.15
Conclusion
This case shows a rare clinical presentation of milia in primary localized cutaneous nodular amyloidosis. We present for the first time that milia is associated with PLCNA and that amyloid may be the causative agent of milia, which contributes to the diagnosis of PLCNA. More studies are needed to verify this relationship between primary localized cutaneous nodular amyloidosis and milia.
Consent
Written informed consent was obtained from the patient for the publication of this case report and any accompanying images. Institutional approval was not required for this case study.
Acknowledgments
We thank the patient for her permission to publish this information. This work was supported by the Zhejiang Natural Science Foundation of China (LBZ22H160001).
Disclosure
The authors report no conflicts of interest related to this work.
References
1. Mena L, Carrasco C, Folch H, de la Parra R, Carreño L. Primary cutaneous nodular amyloidosis associated with the injection of autologous fat. Clin Exp Dermatol. 2021;46:552–554. doi:10.1111/ced.14429
2. Vestery JP, Tidman MJ, Mclaren KM. Primary nodular cutaneous amyloidosis – long-term follow-up and treatment. Clin Exp Dermatol. 1994;19:159–162. doi:10.1111/j.1365-2230.1994.tb01148.x
3. Ung CY, Carr NJ, Ardern-Jones MR. Primary cutaneous nodular amyloidosis associated with psoriasis. Clin Exp Dermatol. 2014;39:608–611. doi:10.1111/ced.12347
4. Dickison P, Howard V, Wylie B, Smith SD. Localized axillary milia en plaque: a rare cutaneous case presentation of systemic amyloidosis. Clin Exp Dermatol. 2016;41(7):764–767. doi:10.1111/ced.12914
5. Yahya H. Idiopathic multiple eruptive milia: report of a case in a Nigerian woman. Niger J Clin Pract. 2018;21:395–396. doi:10.4103/njcp.njcp_43_17
6. Rongioletti F, Atzori L, Ferreli C, Pinna A, Aste N, Pau M. A unique dermoscopy pattern of primary cutaneous nodular amyloidosis mimicking a granulomatous disease. J Am Acad Dermatol. 2016;74(1):e9–e10. doi:10.1016/j.jaad.2015.09.026
7. Dupont L, Martins Souza PR, Damiani L, Boff AL. Cutaneous nodular amyloidosis: a disfiguring aspect of the face. Am J Dermatopathol. 2019;41:945–947. doi:10.1097/DAD.0000000000001470
8. Bellinato F, Rosina P, Sina S, Girolomoni G. Primary nodular localized cutaneous amyloidosis of the scalp associated with systemic lupus erythematosus. Arch Rheumatol. 2022;37(1):145–147. doi:10.46497/ArchRheumatol.2022.8817
9. Nambudiri VE, Habib N, Arndt KA, Kane KS. Milia en plaque of the nose: report of a case and successful treatment with topical tretinoin. Pediatrics. 2014;133:e1373–e1376. doi:10.1542/peds.2013-1728
10. Berk DR, Bayliss SJ. Milia: a review and classification. J Am Acad Dermatol. 2008;59(6):1050–1063. doi:10.1016/j.jaad.2008.07.034
11. Hisa T, Goto Y, Taniguchi S, Nakanishi T, Kakudo K, Takigawa M. Post-bullous milia. Australas J Dermatol. 1996;37(3):153–154. doi:10.1111/j.1440-0960.1996.tb01037.x
12. Kalajian AH, Waldman M, Knable AL. Nodular primary localized cutaneous amyloidosis after trauma: a case report and discussion of the rate of progression to systemic amyloidosis. J Am Acad Dermatol. 2007;57:S26–S29. doi:10.1016/j.jaad.2006.12.014
13. Fernandes C, Éthier V, Marouan S, Chababi-Atallah M, Veilleux M. Localized ulcerative nodular amyloidosis presenting as ulcerative panniculitis, an unusual clinical manifestation: a case report. SAGE Open Med Case Rep. 2019;7:1–3.
14. Weidner T, Illing T, Elsner P. Primary localized cutaneous amyloidosis: a systematic treatment review. Am J Clin Dermatol. 2017;18:629–642. doi:10.1007/s40257-017-0278-9
15. Woollons A, Black MM. Nodular localized primary cutaneous amyloidosis: a long-term follow-up study. Br J Dermatol. 2001;145:105–109. doi:10.1046/j.1365-2133.2001.04291.x
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

