Back to Journals » International Journal of General Medicine » Volume 13
Primary Generalized Glucocorticoid Hypersensitivity Treated with Mifepristone: A Case Report
Authors Liu Y , Han M , Yang J, Xu Q, Xu L, Ren Y, Xiang C, Liu Z, Zhang Y
Received 25 July 2020
Accepted for publication 17 September 2020
Published 13 October 2020 Volume 2020:13 Pages 825—831
DOI https://doi.org/10.2147/IJGM.S273969
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Yunfeng Liu,1 Minmin Han,1,2 Jing Yang,1 Qishan Xu,1 Linxi Xu,1 Yi Ren,1 Chenyu Xiang,1 Zi’ang Liu,2 Yi Zhang3
1Department of Endocrinology, First Hospital of Shanxi Medical University, Taiyuan 03000, China; 2First Clinical Medical College, Shanxi Medical University, Taiyuan 03000, China; 3Department of Pharmacology, Shanxi Medical University, Taiyuan 03000 China
Correspondence: Yunfeng Liu
Department of Endocrinology, First Hospital of Shanxi Medical University, No. 85, Jiefang Nan Road, Yingze District, Taiyuan City, Shanxi Province 03000, China
Tel +86-18703416196
Fax +86-0351-4639769
Email [email protected]
Abstract: Here, we report a case of a patient with symptoms of Cushing syndrome, who is diagnosed with primary generalized glucocorticoid hypersensitivity in the end. The patient’s relevant laboratory tests and imaging examinations are described. Mifepristone, a glucocorticoid receptor antagonist, was prescribed and its therapeutic effect on the patient’s electrolyte level, lipid metabolism, and bone metabolism was observed during the treatment. The endocrine assessment indicated normal pituitary-adrenal axis regulation function but reduced cortisol secretion. Quantitative reverse transcription-polymerase chain reaction indicated reduced mRNA level of mineralocorticoid receptor gene. Pituitary magnetic resonance imaging showed normal pituitary anatomy, while adrenal computed tomography scan showed bilateral adrenal atrophy and increased content of visceral and abdominal subcutaneous fat. Moreover, chromosome examination revealed a normal 46, XY chromosome. In this case, mifepristone was administered to treat primary generalized glucocorticoid hypersensitivity. To the best of our knowledge, there are a few reports on mifepristone-treated primary generalized glucocorticoid hypersensitivity. In the one-year follow-up visits, the evaluated results of electrolyte level, lipid metabolism, and bone metabolism indicated that the patient’s symptoms resulting from cortisol hypersensitivity were relieved progressively.
Keywords: primary generalized glucocorticoid hypersensitivity, mifepristone, glucocorticoid receptor, electrolyte, lipid metabolism, bone metabolism
Introduction
To date, primary generalized glucocorticoid hypersensitivity (PGGH) has been reported in a few cases. In all these cases, the patients harbored cushingoid manifestations but with hypo- or normocortisolemia. However, the mechanisms leading to this phenomenon were remained to be elucidated. A notable review recently demonstrated that glucocorticoid receptor (GR) gene mutations as well as N363S and Bcll polymorphisms played a pivotal role in this setting.1 However, only one case has identified a GR gene mutation.2 Other cases postulated that the root causes may be found at transcription levels.3,4 In terms of the approaches to treat PGGH, GR antagonists, specifically mifepristone, were assumed to be the optimum treatment for impeding GR activity and relieving cushingoid symptoms.5–7 Here, we reported a patient with PGGH treated with mifepristone. We delineated the complete diagnosis and treatment process as well as the therapeutic effect on the patient’s electrolyte level, lipid metabolism, and bone metabolism.
Case Presentation
A 27-year-old male was admitted to our hospital with edema of lower extremities and fatigue. Before admission, he had received the hydrochlorothiazide treatment for one week (25mg, qd) at a local hospital. After hydrochlorothiazide administration, he still felt weak, although the swelling was slightly relieved. Consequently, he discontinued the treatment because of its poor therapeutic effect. The patient acknowledged that he had never taken any exogenous hormone.
The patient’s blood pressure and body mass index were 127/88 mmHg and 35kg/m2, respectively. Moreover, his weight had increased by about 25kg during the previous 6 years. The patient’s secondary sex characteristics appeared when he was 15 years old, and although, he had normal external genital organs, but he had never experienced erection and ejaculation. Physical examinations revealed that he presented centripetal obesity, moon face, buffalo hump, and purple striae (Figure 1A). Mild swelling was found in his lower extremities.
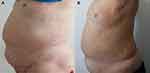 |
Figure 1 Patient’s Cushing phenotype. (A) before mifepristone treatment; and (B) after treatment for one year. |
Laboratory and Imaging Tests
Laboratory tests revealed the following data: adrenocorticotropin (ACTH)<0.220 pmol/L (1.1–13.2 pmol/L); 24-hour urinary 17-hydroxycorticosteroids and 17-ketosteroid was 1.142mg (2–10 mg/24h) and 6.286mg (6–25 mg/24 h), respectively; and 24-hour urinary-free cortisol was 11.95nmol (100–379 nmol/24h). Other data obtained are shown in Table 1. Pituitary magnetic resonance imaging showed normal pituitary anatomy (Figure 2A), while the adrenal computed tomography scan showed bilateral adrenal atrophy and increased content of intra-abdominal fat and abdominal subcutaneous fat (Figure 2B). Dual-energy X-ray absorptiometry revealed that the body mineral density at the levels of lumbar 1-lumbar 4 was 0.685, Z score was −3.7, and the body fat was 48.7%. The patient’s chromosome examination revealed a normal 46, XY chromosome.
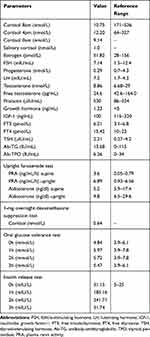 |
Table 1 Laboratory Examination in the Hospital |
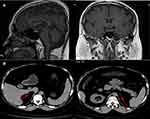 |
Figure 2 (A) Pituitary magnetic resonance imaging showing normal pituitary anatomy; (B) Adrenal computed tomography scan showing atrophic adrenals. |
Family History
The patient’s father had been abandoned as a child. He received inguinal herniorrhaphy at the ages of 10 and 53. He suffered from hypertension at age 45 and took nifedipine sustained release tablets (20 mg/d) and indapamide (1.5 mg/d) to control his blood pressure. His body mass index was 21.5 kg/m2 and he had a few purple striae on the abdomen. His ACTH was also less than 0.22 pmol/L and 24h urinary-free cortisol was 23.62 nmol. The patient’s mother was healthy and had normal serum ACTH and cortisol levels.
Insulin Tolerance Test
The patient and his father were recommended the insulin tolerance test by intravenous injection of insulin (0.15 U/kg). Serum samples were taken at 0, 15, 30, 60, 90, and 120 minutes. During the test, the patient experienced dizziness, sweating, and weakness 3min after the insulin injection and the blood glucose measured by glucose meter was 1.6 mmol/L. Subsequently, he was intravenously administered 50% glucose (60mL). After 15 min, the blood glucose increased to 12.2 mmol/L. Furthermore, his cortisol level was increased by 49% during the test. The plasma cortisol of the patient’s father increased by 105.9% during the test (Table 2).
 |
Table 2 Insulin Tolerance Test |
Renin-Angiotensin-Aldosterone System Function
The function of renin-angiotensin-aldosterone system was also assessed. The blood samples were drawn by venipuncture after 30 minutes of rest in a supine position in the morning, and 2 hours after staying in an upright position with 20mg furosemide intramuscular injection. The aldosterone-to-renin ratio was calculated. The patient’s plasma renin activity was above normal and serum aldosterone was close to the lower limit (Table 1). The patient appeared weak and his potassium decreased from 3.5 to 2.9 mmol/L after the injection of furosemide.
Quantitative Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
RT-PCR was performed to evaluate the mRNA levels of GRα, GRβ, mineralocorticoid receptor (MR), 11β-1-hydroxysteroid dehydrogenase (11β-1-HSD), 11β-2-HSD, hexose-6-phosphate dehydrogenase (H6PD), and 3β-2-HSD in the peripheral blood lymphocytes of the patient and two normal, age-matched men at three different time points. The mRNA levels of MR were found to be lower in our patient. However, the difference of the mRNA levels of other indices between the patient and the normal, age-matched males was not significant (Figure 3).
 |
Figure 3 RT-PCR results for the patient and two normal, age-matched men at three different time points. The results were normalized with the patient’s relative mRNA level as 1. *P<0.05. |
Mifepristone Treatment
The patient was treated with mifepristone 200mg/d for one month, and then 400mg/d for the next eleven months, during which time the patient was instructed to maintain a similar level of physical activity and receive the same calorie intake (25 kcal/kg/day). After one year of treatment with mifepristone (Table 3, Figure 1B), the patient’s body weight decreased from 95 kg to 93 kg. His total body fat decreased from 48.7% to 39.9%. The body mineral density at the levels of lumbar 1-lumbar 4 was increased from 0.685 to 0.702. During the treatment, the patient’s potassium level was always maintained within the normal range. The ACTH and cortisol level remained lower than normal.
 |
Table 3 Comparisons Before and After Treatment |
Discussion
In this case, the diagnosis of Cushing’s syndrome was suspected initially when the patient was admitted to our hospital owing to his typical cushingoid symptoms. However, the endocrine profile abolished the possibility of Cushing syndrome due to his low to normal cortisol and ACTH levels.
In addition, his adrenal computed tomography scan showed bilateral adrenal atrophy. Therefore, the diagnosis of adrenal cortical insufficiency was assumed. After detailed consultation, we were informed that the patient had never suffered hypotension, hyponatremia, and hypoglycemia which are symptoms precipitated in adrenal cortical insufficiency. Moreover, the patient had increased content of body fat and osteoporosis.
We evaluated the patient’s adrenal function. Because ACTH-(1-24) was not available in our hospital at the time, the patient and his father underwent insulin tolerance test to assess their pituitary and adrenal function instead. The patient’s plasma cortisol level elevated by 49% during the test. His father’s cortisol level also increased significantly after the insulin injection, which was followed by hypoglycemia. This seems to indicate that insulin-induced hypoglycemia might restore the secretion of pituitary ACTH and adrenal cortisol. One can postulate that the regulating function of pituitary-adrenal axis was normal in our patient despite his low to normal ACTH and cortisol levels. All of these results negated diagnosis of adrenal cortical insufficiency unlikely.
Detailed interrogation revealed no history of exogenous glucocorticoids administration. The patient was mentally healthy and cooperative during hospitalization. Therefore, the possibility of exogenous Cushing’s syndrome was ruled out.
Due to the combination of subnormal cortisol, normal pituitary-adrenal axis function, and typical Cushing symptoms, the possibility of PGGH was considered. The same holds true for the near lower-limit aldosterone level and significantly low aldosterone to renin ratio accompanied by normal adrenal function, which seems to reflect the aldosterone hypersensitivity. However, further investigation is warranted to prove this speculation.
It is well established that patients with PGGH harbored more GR than normal, age-matched individuals.3,8 Furthermore, these patients tended to have abnormal GR-binding capacity; however, they also exhibit normal nuclear translocation, thermal stability, and heat activation.9 It is also worth noting that GR pertaining to the steroid hormone receptor family of nuclear receptor superfamily of transcription factors, consists of two isoforms, GRα and GRβ.1 Furthermore, it is recognized that 11β-1-HSD and 11β-2-HSD plays an important role in the inter-conversion of cortisol and cortisone.10 Moreover, the role of H6PDH in adjusting the activity of 11β-1-HSD oxidoreductase activity has also been reported.11 Therefore, assessing the levels of GRα, GRβ, 11β-1-HSD, 11β-2-HSD, and H6PD would indirectly indicate the glucocorticoid action pathway.
On the other hand, MR is featured in response to steroid binding,12 especially of mineralocorticoids. 3β-2-HSD gene plays a pivotal role in encoding the 3β-2-HSD, which was the key enzyme for progesterone synthesis. Mineralocorticoids are the downstream products of progesterone. Therefore, MR and 3β-2-HSD can provide us with useful information on the mineralocorticoid action pathway.
Thus, we conducted the quantitative RT-PCR in the peripheral blood lymphocytes of the patients and two normal, age-matched males to evaluate the mRNA levels of GRα, GRβ, MR, 11β-1-HSD, 11β-2-HSD, H6PD, and 3β-2-HSD. The results disclosed that the mRNA level of MR in our patient was found to be lower than the normal, age-matched males. On the contrary, the levels of GRα, GRβ, 11β-1-HSD, 11β-2-HSD, H6PD, and 3β-2-HSD were found to be similar to the normal, age-matched males.
The normal levels of 11β-1-HSD, 11β-2-HSD, and H6PD were substantiated with the quantitative RT-PCR. One can speculate that the activation of the 11β-1-HSD and 11β-2-HSD is not involved in glucocorticoid hyperreaction. Moreover, normal level of GRs presented in RT-PCR indicated that there were not more GRs, which usually occurs in the PGGH, in our case.
Previous studies have introduced a groundbreaking concept, which showed that patients with PGGH may harbored GR mutations.1,2,13,14 We conducted first-generation sequencing assessment, however, no positive outcome was obtained. Therefore, the mechanisms underlying the glucocorticoid hypersensitivity in our patient remained to be further elucidated.
Regarding treatment, ketoconazole and cabergoline previously used to treat a patient with PGGH; however, the therapeutic effect was not satisfactory.5 After treatment with these medications, the patient’s body weight decreased, but his Cushing symptoms were still presented. A recent review recommended that PGGH management should focus on addressing relevant manifestations, such as dyslipidemia, type 2 diabetes, and hypertension.15 Considering that the patient’s ACTH and cortisol levels were lower than normal, ACTH suppressor and adrenal inhibitor were unlikely treatment options. Mifepristone, a GR antagonist, seems to be a suitable and preferred therapy for this patient. Newfield et al reported the relief of PGGH manifestations in a patient with mifepristone treatment.3 Thus, the mifepristone was recommended as therapeutic regimen for our patient. Due to the possibly unsatisfactory effects including hypokalemia, unstable blood pressure, and blood glucose level, during mifepristone treatment,16 200 mg/d was prescribed initially and the patient’s blood pressure, blood glucose, plasma ACTH, plasma cortisol, and plasma potassium were under scrutiny.
During the first month, elevated ACTH and cortisol levels as well as reduced serum potassium level did not occur. In addition, symptoms of nausea did not occur. Then, the dosage of mifepristone was increased to 400 mg/d. During the next 11 months, the patient’s ACTH and cortisol levels were not significantly altered; however, 17-ketosteroid and 17-hydroxycorticosteroid were increased. The patient’s blood pressure and blood glucose did not seem to be affected.
After treatment for one year, the patient’s symptoms of asthenia and edema were relieved. Furthermore, his body fat decreased significantly from 48.7% to 39.9%. However, his body weight decreased merely 2kg, which implied that our treatment substantially improved the fat metabolism in our patient. In addition, the patient’s lumbar mineral density did not decrease, however, it increased slightly after the mifepristone treatment, further suggesting that mifepristone can help to impede osteoporosis.
Conclusion
In conclusion, we have reported a patient with PGGH. Relevant examination results did not provide a reliable clue to the etiology of PGGH, which remains to be investigated further. The mifepristone was administered to the patient with satisfactory effects in terms of improved electrolyte level, lipid metabolism, and bone metabolism, further providing useful information for future treatment of PGGH patients.
Data Sharing Statement
The data that used to support this study are available on reasonable request from the corresponding author, Yunfeng Liu.
Ethics and Consent Statement
This study was approved by the ethics committee in First Hospital of Shanxi Medical University (2018K002).
Consent for Publication
Written informed consent has been provided by the patient and his father to have the case details and any accompanied images published.
Acknowledgments
The authors thank the National Nature Science Foundation of China (NO. 81670710, 81770776), Advanced Programs of Shanxi for the Returned Overseas Chinese Scholars (2016-97), Research Project Supported by Shanxi Scholarship Council of China (2017-053, 2020-172), and Nature science foundation of Shanxi Province (201901D111353) for the support. The authors are grateful to We Health Biomedical technology Co. Ltd in Shanghai for the first-generation sequencing test.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
This study was supported by the grants from National Nature Science Foundation of China (NO. 81670710, 81770776), Advanced Programs of Shanxi for the Returned Overseas Chinese Scholars (2016-97), Research Project Supported by Shanxi Scholarship Council of China (2017-053, 2020-172), and Natural Science Foundation of Shanxi Province (201901D111353), which were mainly for the collection, genetic analysis, and the interpretation of data.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Nicolaides N, Charmandari E. Novel insights into the molecular mechanisms underlying generalized glucocorticoid resistance and hypersensitivity syndromes. Hormones. 2017;16:124–138.
2. Charmandari E, Ichijo T, Jubiz W, et al. Novel point mutation in the amino terminal domain of the human glucocorticoid receptor (hgr) gene enhancing hgr-mediated gene expression. J Clin Endocrinol Metab. 2008;93(12):4963–4968. doi:10.1210/jc.2008-0892
3. Newfield R, Kalaitzoglou G, Licholai T, Chilton D. Normocortisolemic Cushing’s syndrome initially presenting with increased glucocorticoid receptor numbers. J Clin Endocrinol Metab. 2000;85:14–21.
4. Fujii H, Iida S, Gomi M, et al. Augmented induction by dexamethasone of metallothionein IIa messenger ribonucleic acid in fibroblasts from a patient with cortisol hyperreactive syndrome. J Clin Endocrinol Metab. 1993;76:445–449.
5. Krysiak R, Okopien B. Glucocorticoid hypersensitivity syndrome–a case report. West Indian Med J. 2012;61:844.
6. Clark R. Glucocorticoid receptor antagonists. Curr Top Med Chem. 2008;8(9):813–838. doi:10.2174/156802608784535011
7. McMaster A, Ray D. Drug insight: selective agonists and antagonists of the glucocorticoid receptor. Nat Rev Endocrinol. 2008;4:91.
8. Russcher H, Smit P, van Rossum E, van den Akker E, Brinkmann A, de Heide L. Strategies for the characterization of disorders in cortisol sensitivity. J Clin Endocrinol Metab. 2006;91(2):694–701. doi:10.1210/jc.2005-2212
9. Iida S, Nakamura Y, Fujii H, Nishimura J, Tsugawa M, Gomi M. A patient with hypocortisolism and Cushing’s syndrome-like manifestations: cortisolhyperreactive syndrome. J Clin Endocrinol Metab. 1990;70(3):729–737. doi:10.1210/jcem-70-3-729
10. Albertin G, Tortorella C, Malendowicz L, et al. Human adrenal cortex and aldosterone secreting adenomas express both 11beta-hydroxysteroid dehydrogenase type 1 and type 2 genes. Int J Mol Med. 2002;9:495–498.
11. Daniela R, Ryder J, Kelli B, et al. Abnormalities of glucose homeostasis and the hypothalamic-pituitary-adrenal axis in mice lacking hexose-6-phosphate dehydrogenase. Endocrinology. 2007;148(10):5072–5080. doi:10.1210/en.2007-0593
12. Sõber S, Laan M, Annilo T. MicroRNAs miR-124 and miR-135a are potential regulators of the mineralocorticoid receptor gene (NR3C2) expression. Biochem Biophys Res Commun. 2010;391(1):727–732. doi:10.1016/j.bbrc.2009.11.128
13. Ramamoorthy S, Cidlowski J. Corticosteroids: mechanisms of action in health and disease. Rheum Dis Clin North Am. 2016;42(1):15. doi:10.1016/j.rdc.2015.08.002
14. Quax R, Manenschijn L, Koper J, et al. Glucocorticoid sensitivity in health and disease. Nat Rev Endocrinol. 2013;9:670–686.
15. Charmandari E, Kino T, Chrousos GP. Primary generalized familial and sporadic glucocorticoid resistance (chrousos syndrome) and hypersensitivity. Endocr Dev. 2013;24:67–85.
16. Castinetti F, Conte-Devolx B, Brue T. Medical treatment of Cushing’s syndrome: glucocorticoid receptor antagonists and mifepristone. Neuroendocrinology. 2010;92:125–130.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
