Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Primary Cutaneous Follicle Center Lymphoma Presenting as a Solitary Nodule on the Forearm of an Adolescent Girl: A Case Report and Literature Review
Authors Al Harbi SM , Al Natour S, Al Saif NM , Al Saif N, Al Bayat MI
Received 5 November 2022
Accepted for publication 10 January 2023
Published 20 January 2023 Volume 2023:16 Pages 167—172
DOI https://doi.org/10.2147/CCID.S396326
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Anne-Claire Fougerousse
Sadan Mohammed Al Harbi,1 Sahar Al Natour,1 Nasser Mohammed Al Saif,1 Noura Al Saif,1 Methal Isam Al Bayat2
1Department of Dermatology, College of Medicine, Imam Abdulrahman Bin Faisal University, Dammam, Saudi Arabia; 2Department of Pathology, College of Medicine, Imam Abdulrahman Bin Faisal University, Dammam, Saudi Arabia
Correspondence: Sadan Mohammed Al Harbi, Department of Dermatology, College of Medicine, Imam Abdulrahman Bin Faisal University, Post Box No. 1982, Dammam, 31441, Saudi Arabia, Email [email protected]
Abstract: Primary cutaneous B-cell lymphomas (PCBCLs) are very rare to be seen in pediatric and adolescent age group, especially primary cutaneous follicle center lymphoma (PCFCL) which is considered the least occurring main subtype. Here, we describe a 16-year-old girl who developed a slowly growing solitary firm smooth surfaced erythematous nodule over her forearm. Histopathological examination showed a dense dermal nodular, periadnexal and perivascular lymphoid infiltrate extending deep to the subcutis. Immunohistochemical staining showed a B-cell population with positivity for CD20, variable staining for BCL6 and CD10 and uniquely staining for BCL2. Although a primary cutaneous marginal zone lymphoma (PCMZL) was considered but the presences of interfollicular BCL6 and CD10 positivity established the diagnosis of PCFCL. To our knowledge, only 12 cases of pediatric and adolescent PCFCL have been described in the literature.
Keywords: B-cell lymphoma, skin cancer, pediatric, juvenile, lymphoproliferative disorder
Introduction
Primary cutaneous lymphomas are very rare in pediatric and adolescent age group. The most presented subtype is primary cutaneous T-cell lymphoma, particularly mycosis fungoides and CD30+ lymphomas (namely lymphomatoid papulosis and anaplastic large T-cell lymphoma).1,2 Data on the epidemiology of pediatric and adolescent primary cutaneous B-cell lymphomas (PCBCLs) are very limited due to the scarcity of the disease. The incidence of pediatric and adolescent PCBCLs is found to be 0.12 per 1,000,000 person-years, with primary cutaneous marginal zone lymphoma (PCMZL) being the most common subtype (77.1%), followed by primary cutaneous diffuse large B-cell lymphoma (PCDLBCL) (12.5%) and primary cutaneous follicle center lymphoma (PCFCL) (10.4%).3 In this age group, PCFCL is exceptionally rare with only 12 cases having been reported in the literature (Table 1).1,3–9 In this study, we report a case of PCFCL in a 16-year-old girl with unusual clinical and immunohistochemical presentation.
 |
Table 1 Summary of the Clinical Information on Reported Cases of Pediatric and Adolescent PCFCL (Less Than 20 Years of Age) |
Case Report
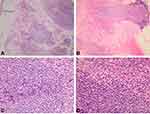
A Saudi 16-year-old girl presented to outpatient dermatology clinic at King Fahad Hospital of the University with a solitary painless erythematous nodule over the left forearm that had been slowly growing over the past 2 years. The patient sought medical advice in multiple hospitals where a course of antibiotic, oral antihistamines as well as topical steroid creams were prescribed to her with no improvement. She had no history of fever, night sweats, weight loss or generalized body pruritis. In addition to that, the patient denied any history of insect bites, trauma or recent travel. On examination, a solitary nodule over the left forearm measuring 1.5 cm × 1 cm, with an erythematous to hyperpigmented smooth surface and firm consistency (Figure 1). Differential diagnosis of dermatofibroma and benign adnexal neoplasms were considered and a skin biopsy was suggested but the patient initially refused. Subsequently, the patient agreed and a skin biopsy was done. Hematoxylin and eosin (H&E) stained sections of skin show a dense dermal nodular, periadnexal and perivascular lymphoid infiltrate. The infiltrate involved the superficial and deep dermis, focally extending to the subcutis, and sparing the epidermis in a follicular growth pattern (Figure 2). It was composed predominantly of small, elongated CD20 positive B cells with irregular nuclei and inconspicuous nucleoli. A small proportion of the neoplastic B cell (less than 10%) showed larger, more rounded nuclei with multiple peripheral nucleoli. CD3 positive reactive T lymphocytes were admixed with the infiltrate. Apoptosis was focally identified; however, mitoses were not prominent. The B-cell population was positive for BCL2 and showed variable BCL6 positivity in more than 30% of the neoplastic cells. Only a few scattered cells were positive for CD10 (Figure 3). Additionally, CD30, CD43 and TDT were negative. CD21 highlighted expanded follicular dendritic meshworks. Notably, BCL6 positive cells were seen extending beyond the meshwork in some areas.
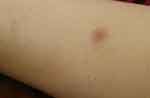 |
Figure 1 A solitary non scaly erythematous nodule over the left forearm with a firm consistency measuring 1.5 cm x 1 cm. |
BCL2 gene rearrangement was not detected by interphase fluorescence in situ hybridization (FISH) performed on paraffin embedded tissue but Immunoglobulin heavy chain gene rearrangement was detected, confirming the monoclonal nature of the B-cell population.
Histological picture and molecular genetic testing confirmed the diagnosis of PCFCL.
Accordingly, a full body examination was done, no hepatosplenomegaly, peripheral lymphadenopathy, or other skin abnormalities were found.
A work-up for metastases included a complete blood count (CBC), peripheral blood flow cytometry, liver function tests (LFT), creatinine, lactate dehydrogenase (LDH), erythrocyte sedimentation rate (ESR), C- reactive protein test (CRP) and computed tomography (CT) scan of the chest, abdomen and pelvis which were all unremarkable.
Discussion
Primary cutaneous lymphomas are a group of T-cell and B-cell lymphomas that present in the skin without evidence of extracutaneous disease at the time of diagnosis. Primary cutaneous B-cell lymphomas (PCBCLs) form only 20–25% of all adult primary cutaneous lymphomas.10 According to the 2018 World Health Organization-European Organization for Research and Treatment of Cancer (WHO-EROTIC) classification of primary cutaneous lymphomas, PCBCLs are classified into primary cutaneous marginal zone lymphoma (PCMZL); primary cutaneous follicle center lymphoma (PCFCL); primary cutaneous diffuse large B-cell lymphoma, leg type (PCDLBCL, LT); Epstein-Bar virus (EBV+) mucocutaneous ulcer; and intravascular large B cell lymphoma.11 PCMZL and PCFCL both have very indolent clinical courses and excellent prognosis with 100% and 95% a 5-year disease-specific survival rate, respectively.11 PCDLBCL, LT has a more of an aggressive course and unfavorable prognosis with a 50–60% 5-year survival rate.11 In the pediatric and adolescent age groups (less than 20 years of age), the incidence of primary cutaneous B-cell lymphomas (PCBCLs) is very rare. Bomze et al found that pediatric PCBCLs incidence is 40 folds lower than in adults (age >20 years), and majority of pediatric cases were PCMZL (77.1%), followed by PCDLBCL (12.5%) and lastly PCFCL (10.4%).3 Pediatric PCFCL seems to be extremely rare with only 12 individuals less than 20 years of age reported in literature (Table 1).1,3–9 The underlying pathogenesis of PCFCL is not well understood and has been linked to chronic antigenic viral stimulation including Epstein Barr virus (EBV), Human Herpes Virus type 8 (HHV8) and Hepatitis B virus (HBV).12 Primary cutaneous follicle center lymphoma (PCFCL) presents clinically as a solitary or grouped of plaques or nodules with a predilection of scalp, forehead, and trunk.13 Review of the literature of reported cases of pediatric PCFCL show a predilection of lesions occurring on the head and neck area with only a single case over the arm, parallelly our patient presentation of solitary nodule over the proximal forearm is a rare occurrence for PCFCL in this age group (Table 1).1,3–9 PCFCL is predominantly composed of a centrocyte cell with cleaved nuclei and centroblast. Its infiltrations can have any of the 3 growth patterns including follicular, diffuse, or follicular and diffuse.14 This infiltration is found in the dermis and can extend deep into the subcutaneous fat. A subepidermal Grenz zone is seen in most cases. In contrast to the reactive follicular infiltrates, neoplastic follicles in PCFCL have no or only few tangible body macrophages.10 These neoplastic cells express B-cell markers including CD20 and CD79a and consistently BCL6 as a marker of follicle center origin, CD10 is variably expressed, it is usually positive in follicular pattern and negative in diffuse growth pattern. MUM1 (multiple myeloma 1) and FOXP1 (Forkhead Box P1) staining is usually negative. The neoplastic cells typically lack BCL2 staining and are always negative for CD5 and CD43.7,10,15–17 Lucioni et al experienced a BCL2 positive expression in at least 25–27% of PCFCL cases.18 BCL2 is considered helpful in the differentiation between PCFCL and systemic follicular lymphoma with secondary cutaneous involvement, which is usually positive in nodal follicular lymphoma and less often found in PCFCL.17,19 The proliferation index (Ki67 staining) is usually lower than those seen in reactive follicles but some cases have shown high proliferation indices.15,16
PCFCL often lack BCL2 gene rearrangements; however, if the rearrangement is present at diagnosis, it is associated with risk of systemic spread.16,20 Immunoglobulin heavy-chain or light-chain gene rearrangements confirm the clonal nature of the infiltrate, particularly if the differential diagnosis includes reactive lymphoid hyperplasia.15,16 PCFCL usually show amplification of REL gene and can have mutations in CREBBP (CREB binding protein encoding gene) and KTM2D (Lysine Methyltransferase 2D encoding gene).16
Our case showed the characteristic morphologic features and lacked BCL2 gene rearrangement. The cells stained for pan B-cell marker (CD20), however, staining for BCL6 was variable. Uniquely, our case showed diffuse staining for BCL2. It is reported that if BCL2 is positive, it is usually only weakly expressed.16 It also showed a low Ki67 proliferation index in the 95 germinal center area, in contrast to the strong Ki67- positivity seen in reactive follicles.21 The exact prognosis of pediatric PCFCL is not well known due to its rarity, but a single study which is a Population-Based Study compared a PCBCLs 5-year cancer-specific survival between pediatric and adult population showed 100% survival rate for the pediatric group and 90.9% for the adult population.3 Follow up of the twelve published cases of pediatric PCFCL showed 9 cases were alive and disease free over a range of 42 weeks, one case disease recurred at 13 months follow-up, the remaining two patients’ disease course was not reported (Table 1), our patient was referred to a specialist hospital for further management.1,3–9
Conclusion
We report this case of primary cutaneous Follicle center lymphoma (PCFCL) for the rarity in pediatric and adolescent age group, the unusual site of occurrence over the forearm, the unusual immunohistochemical testing, as an addition to the reported cases of pediatric PCFCL in the literature, also to shed light on the importance of skin biopsies in diagnosing PCFCL which can be easily misdiagnosed or missed for other benign cutaneous neoplasms.
Abbreviations
PCBCLs, primary cutaneous B-cell lymphomas; PCFCL, primary cutaneous follicle center lymphoma; PCMZL, primary cutaneous marginal zone lymphoma; PCDCBCL, primary cutaneous diffuse large B-cell lymphoma; H&E, hematoxylin and eosin; FISH, fluorescence in situ hybridization; CBC, complete blood count; LFT, liver function tests; LDH, lactate dehydrogenase; ESR, erythrocyte sedimentation rate; CRP, C- reactive protein test; CT, computed tomography; WHO-EROTIC, World Health Organization-European Organization for Research and Treatment of Cancer; PCDLBCL, LT, primary cutaneous diffuse large B-cell lymphoma, leg type; EBV, Epstein Barr virus; HHV8, Human Herpes Virus type 8; HBV, Hepatitis B virus; MUM1, multiple myeloma 1; FOXP1, Forkhead Box P1; CREBBP, CREB binding protein encoding gene; KTM2D, Lysine Methyltransferase 2D encoding gene.
Ethics Approval and Consent for Publication
This case report has been performed in accordance with the principles stated in the Declaration of Helsinki. Written informed consent provided by the Taylor & Francis group for publication of this work including photography and medical data was obtained and signed by the patient’s mother. Institutional ethical approval was not required to publish this case report.
Acknowledgments
The authors would like to thank all members who contributed in writing this case report.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Fink-Puches R, Chott A, Ardigó M, et al. The spectrum of cutaneous lymphomas in patients less than 20 years of age. Pediatr Dermatol. 2004;21(5):525–533. doi:10.1111/j.0736-8046.2004.21500.x
2. Boccara O, Blanche S, de Prost Y, Brousse N, Bodemer C, Fraitag S. Cutaneous hematologic disorders in children. Pediatr Blood Cancer. 2012;58(2):226–232. doi:10.1002/pbc.23103
3. Bomze D, Sprecher E, Goldberg I, Samuelov L, Geller S. Primary cutaneous B-cell lymphomas in children and adolescents: a SEER population-based study. Clin Lymphoma Myeloma Leuk. 2021;21(12):e1000–e1005. doi:10.1016/j.clml.2021.07.021
4. Ghislanzoni M, Gambini D, Perrone T, Alessi E, Berti E. Primary cutaneous follicular center cell lymphoma of the nose with maxillary sinus involvement in a pediatric patient. J Am Acad Dermatol. 2005;52(5 Suppl 1):S73–S75. doi:10.1016/j.jaad.2004.05.025
5. Condarco T, Sagatys E, Prakash AV, Rezania D, Cualing H. Primary cutaneous B-cell lymphoma in a child. Fetal Pediatr Pathol. 2008;27(4–5):206–214. doi:10.1080/15513810802319442
6. Amitay-Laish I, Feinmesser M, Ben-Amitai D, et al. Juvenile onset of primary low-grade cutaneous B-cell lymphoma. Br J Dermatol. 2009;161(1):140–147. doi:10.1111/j.1365-2133.2009.09121.x
7. Sayej WN, Isakoff MS, DiGiuseppe JA, et al. Primary cutaneous follicle center lymphoma in a 16-year-old boy with Crohn's disease exposed to infliximab and methotrexate. J Pediatr Gastroenterol Nutr. 2020;70(2):e49–e50. doi:10.1097/MPG.0000000000002578
8. Edmonds N, Hernández-Pérez M, Holsinger M, Gru AA. Primary cutaneous follicle center lymphoma in a 16-year-old girl. J Cutan Pathol. 2021;48(5):663–668. doi:10.1111/cup.13939
9. D’Alessandro PR, Lo AC, Spencer MH, et al. Primary cutaneous follicle center lymphoma of the medial canthus of the eye in an 11-year old. Pediatr Blood Cancer. 2022;69(9):e29630. doi:10.1002/pbc.29630
10. Burg G, Kempf W, Cozzio A, et al. WHO/EORTC classification of cutaneous lymphomas 2005: histological and molecular aspects. J Cutan Pathol. 2005;32(10):647–674. doi:10.1111/j.0303-6987.2005.00495.x
11. Willemze R, Cerroni L, Kempf W, et al. The 2018 update of the WHO-EORTC classification for primary cutaneous lymphomas [published correction appears in Blood. 2019 Sep 26;134(13):1112]. Blood. 2019;133(16):1703–1714. doi:10.1182/blood-2018-11-881268
12. Lima M. Cutaneous primary B-cell lymphomas: from diagnosis to treatment. An Bras Dermatol. 2015;90(5):687–706. doi:10.1590/abd1806-4841.20153638
13. Selva R, Violetti SA, Delfino C, et al. A literature revision in primary cutaneous B-cell lymphoma. Indian J Dermatol. 2017;62(2):146–157. doi:10.4103/ijd.IJD_74_17
14. Kempf W, Kazakov DV, Belousova IE, Mitteldorf C, Kerl K. Paediatric cutaneous lymphomas: a review and comparison with adult counterparts. J Eur Acad Dermatol Venereol. 2015;29(9):1696–1709. doi:10.1111/jdv.13044
15. Skala SL, Hristov B, Hristov AC. Primary cutaneous follicle center lymphoma. Arch Pathol Lab Med. 2018;142(11):1313–1321. doi:10.5858/arpa.2018-0215-RA
16. Louissaint A, Jansen PM, Sander CA, et al. Primary cutaneous follicle centre lymphoma. In: WHO Classification of Tumours Editorial Board. Haematolymphoid Tumours [Internet; Beta Version Ahead of Print]. WHO classification of tumours series,
17. Jaffe ES, Arber DA, Campo E, Harris NL, Quintanilla-Fend L. Hematopathology E-Book. Elsevier Health Sciences; 2016.
18. Lucioni M, Fraticelli S, Neri G, et al. Primary cutaneous B-cell lymphoma: an update on pathologic and molecular features. Hemato. 2022;3(2):318–340. doi:10.3390/hemato3020023
19. Cerroni L, Volkenandt M, Rieger E, Soyer HP, Kerl H. bcl-2 protein expression and correlation with the interchromosomal 14;18 translocation in cutaneous lymphomas and pseudolymphomas. J Invest Dermatol. 1994;102(2):231–235. doi:10.1111/1523-1747.ep12371768
20. Leinweber B, Colli C, Chott A, Kerl H, Cerroni L. Differential diagnosis of cutaneous infiltrates of B lymphocytes with follicular growth pattern. Am J Dermatopathol. 2004;26(1):4–13. doi:10.1097/00000372-200402000-00002
21. Barasch NJK, Liu YC, Ho J, et al. The molecular landscape and other distinctive features of primary cutaneous follicle center lymphoma. Hum Pathol. 2020;106:93–105. doi:10.1016/j.humpath.2020.09.014
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.