Back to Journals » Cancer Management and Research » Volume 10
Primary clear cell carcinoma of the thymus and literature comparison of features
Received 29 November 2017
Accepted for publication 29 January 2018
Published 16 March 2018 Volume 2018:10 Pages 513—518
DOI https://doi.org/10.2147/CMAR.S158452
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Kenan Onel
Video abstract presented by Xiaomin Dai.
Views: 270
Xiaomin Dai, Li Zhao, Fang Peng
Department of Pathology, Zhejiang Hospital, Xihu District, Hangzhou, Zhejiang, China
Abstract: Clear cell carcinoma arising from the thymus is considered exceedingly rare. It shows aggressive clinical behavior and demonstrates frequent local recurrences as well as widespread metastasis. The detailed clinical data of one patient with thymic clear cell carcinoma were compiled, and a review of relevant reported studies was performed. We summarized the clinical characteristics, pathological diagnosis of the patient and other reported cases. The analysis showed that older male patients were more likely to suffer, and the manifestations included chest pain and dyspnea. Some patients are asymptomatic, with the tumor being discovered during physical examination. Histologically, thymic clear cell carcinoma is composed of lobulated structures arranged in hyperchromatic fibrous stroma; the tumor cells are uniform with obvious nucleoli and clear cytoplasm. To establish the correct diagnosis, consideration and exclusion of metastasis and other original tumors in the differential diagnosis by immunohistochemistry, clinical and radiologic correlation is important.
Keywords: thymic carcinoma, clear cell carcinoma, differential diagnosis
Introduction
Thymic carcinoma is a rare, highly mediastinal malignancy derived from the thymic epithelium. It shows the classical histological features with prominent cell atypia, increased proliferation and lack of immature T lymphocytes.1 Thymic carcinomas are a group that includes tumors which are heterogeneous for histological appearance. Based on the clinical characteristics and histologic features, it has been classified into low-grade and high-grade malignancy groups. However, this category has been excluded from the current World Health Organization (WHO) classification.2 Thymic carcinoma can also be divided into further 10 subtypes including thymic clear cell carcinoma. Thymic clear cell carcinoma is particularly rare and represents approximately 3% of all thymic carcinoma.3 Unfortunately, there are not much data on pathogenesis, prevalence, clinical characteristics, immunohistochemical features and treatment. The patient series of primary thymic clear cell carcinoma in the literature comprises a few such cases. Here, we present one rare case of primary thymic clear cell carcinoma of a patient in combination with left breast infiltrative ductal carcinoma, including the pathologic description of this thymic carcinoma. We then compared the features of our patient with those of 18 others with similar tumors described in the literature.
Materials and methods
The presented case was obtained from the files of the Department of Pathology at Zhejiang Hospital. This case was derived from a thymectomy specimen, and hematoxylin and eosin (H&E)-stained sections were available for review. Immunohistochemical staining was performed using antibodies against pan-cytokeratin (CKpan) (1:100; Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd, Beijing, China), 34βE12 (1:100; Zsbio), p63 (1:100, Zsbio), thyroid transcription factor 1 (TTF-1) (1:25, Zsbio), GATA binding protein 3 (GATA-3) (1:100, Zsbio), human melanoma black 45 (HMB45) (1:100, Zsbio), CD5 (1:100; Zsbio), c-kit (1:50; Zsbio), and terminal deoxynucleotidyl transferase (TdT) (1:50; Zsbio). The relevant clinical data and follow-up of this case were obtained using a retrospective survey.
The literature selection for this review included a PubMed database search from 1982 to December 2017 of reported cases in English. The search was performed by using various combinations of searching keywords involving “(thymic OR thymus) and (clear cell carcinoma OR clear cell feature).” Reports with detailed clinical data including age, gender, symptoms, tumor size, treatment, follow-up, and immunohistochemistry were summarized. In addition, cross-referencing of related papers was analyzed in the articles from the research cited. The data in the relevant literatures were summarized, and the clinical and pathological features of new cases and previously reported cases were discussed.
Consent statement
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the author of this paper.
Results
Case report
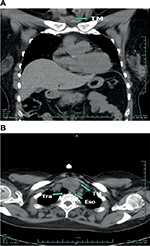
A 50-year-old female was admitted to our hospital after the diagnosis of infiltrative ductal carcinoma of the breast. She had received the diagnosis 2 months ago and had begun chemotherapy according to the EC schedule (epirubicin 140 mg/m2 and cyclophosphamide 800 mg/m2). The physical examination after admission revealed a remarkable abnormal finding, a palpable mass was examined at the anterior clavicle of the neck. Chest computed tomography (CT) findings demonstrated a mass in the anterosuperior mediastinum (Figure 1). Blood tests including alpha-fetoprotein, acetylcholine receptor antibody (AchR-Ab) and titin antibody (Titin-Ab) as markers for myasthenia gravis, beta-human chorionic gonadotropin (β-HCG), CA19.9 and human immunodeficiency virus (HIV) were all within normal range. Abdominal CT, brain magnetic resonance imaging (MRI), colonoscopy, otolaryngological and gynecological examinations revealed no evidence of other abnormalities.
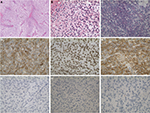
Partial thymectomy via median sternotomy and radical mastectomy were performed, and the surgically resected tumor was a 4.0 × 3.0 × 3.0 cm3 solid white mass obtained from the anterosuperior mediastinum. Histopathological examination with H&E staining showed the tumor with a lobulated architecture composed of undifferentiated clear cells, with the abundance and density of fibrous stroma (Figure 2A). The tumor appeared to have radially penetrated the peripheral adipose tissue. Figure 2B showed homogeneous neoplastic cells with abundant, clear cytoplasm, round or ovoid nuclei and a low nuclear/cytoplasmic ratio. Nuclear atypia was not marked, and nuclear chromatin was finely dispersed. Mucicarmine stain was negative in this case when tested, while the material was positive for Periodic acid Schiff (PAS) (Figure 2C). The neoplastic cells were positive for CKpan, p63 and 34βE12 (Figure 2D–F). In contrast, neoplastic cells were negative for TTF-1, GATA-3 and HMB45 (Figure 2G–I), and histological examination from left breast tumor demonstrated the typical breast infiltrating ductal carcinoma. Immunohistochemistry with estrogen receptor (ER) and progesterone receptor (PR) demonstrated negative cells in the breast tumor. The human epidermal growth factor (HER2/neu) IHC score was 3+, and the Ki-67 labeling index was 30% in the resected breast tumor.
We made a diagnosis of thymic clear cell carcinoma based on the histological and immunophenotypical findings. According to the Masaoka staging system, the tumor at the diagnosis presented in stage IIb. The patient was also evaluated as having stage IIIa (T3N1M0) breast cancer. Later, our patient was treated systemically with four cycles of chemotherapy which consisted of epirubicin, cyclophosphamide, docetaxel and trastuzumab (herceptin) after surgery. She underwent another operation for total thymectomy and the removal of surrounding adipose and received adjuvant radiotherapy administered to mediastinal lesion in the other hospital after our treatment. The patient performed well within 12 months after surgery, and the follow-up tests showed no symptoms of local recurrence or metastasis.
Systematic literature review
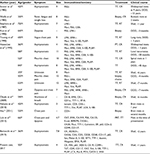
Our comprehensive review of reported literature of primary thymic clear cell carcinoma revealed only 18 cases. The detailed clinical and pathological information collected from all 19 cases (including our case) are summarized in Table 1. Eighteen patients had been diagnosed thymic clear cell carcinoma histologically either by biopsy or by surgical resection, and only one case was confirmed by cytology. Among the 19 patients, the reported age ranged between 33 and 84 years (average 52.6 years) with a slight male predominance (63.2%). The most common clinical manifestation was a mediastinum mass discovered incidentally. Eight patients were asymptomatic on admission, and five patients had chest pain. The reported tumor size ranged between 3 and 12 cm (average 7.8 cm). In 13 cases, including ours, complete resection was performed to obtain the diagnosis of thymic clear cell carcinoma. The treatment for thymic clear cell carcinoma was described in 18 cases, only six patients received chemoradiation therapy in which the tumor was resected. Of the 18 cases reported, eight had spread metastases, with survival ranging from 2 weeks to 13 years, while the other five patients remained alive without significant recurrence during their follow-up. In our case, the patient was in good health with no detectable recurrence at the time of writing (October 2017).
Discussion
Thymic epithelial tumors (TETs) are the second most common tumors of the mediastinum (21%) and the most predilection sites of the anterosuperior mediastinum.15 However, thymic carcinoma is extremely rare and represents less than 1% of the thymic malignancies. Thymic carcinoma was defined as a malignant tumor that was completely different from thymoma in cell morphology and immunohistochemistry by Levine and Rosai in 1978.16 Thymic carcinoma shows much more aggressive clinical behavior than thymoma, generally tends to spread radially into the adjacent thymus and adipose tissue and widespread metastases are common. Thymic carcinoma can be divided into further subtypes, i.e., squamous cell carcinoma, basaloid carcinoma, mucoepidermoid carcinoma, lymphoepithelioma-like carcinoma, clear cell carcinoma, sarcomatoid carcinoma, adenocarcinoma, NUT carcinoma, undifferentiated carcinoma and other rare thymic carcinoma according to the 4th edition of WHO classification of tumors of the lung, pleura, thymus and heart.2 Several staging systems have been evolved over the years, the WHO staging system using the TNM classification and the Masaoka staging system are the most commonly used.3,15,17
Thymic clear cell carcinoma, as histologic of cells with an optically clear cytoplasm, was first reported by Snover et al in 1982.4 Microscopically, clear cell of the thymus is often not active in cytology, which is in contrast to its clinical invasion. Thymic clear cell carcinoma usually shows the lobulated structure, and the tumor cells are nested, lobed, and flaky, which are surrounded by hyperchromatic fibrous stroma. The blood sinus vascular structure of the metastatic lesion of renal cell carcinoma is lacking.9 The tumors have a broad range of cytological features, ranging from uniform clear cells with minimal atypia to the pleomorphic neoplasm cells with obvious nucleoli. Cytoplasm is abundant, mostly transparent or granular, and can be mildly eosinophilic. In some cases, scattered lymphocytes, focal necrosis and transitions from areas of conventional squamous cell carcinoma can be observed. Although sometimes the tumor has well-defined periphery, it is characterized by the biological features of infiltrative, and surrounding fatty tissue or residual thymus around the mediastinal could be invaded.
The top differential diagnosis is metastatic renal cell carcinoma or a metastasis from other clear cell tumors.18 Although renal cell carcinoma bears some similarities in cytologic and architectural, features such as glands with extensive luminal hemorrhage and sinusoidal vasculature are lacking in thymic clear cell carcinoma. Immunoreactivity for high-molecular weight keratins would support thymic clear cell carcinoma, and conversely, strong staining for PAX8 and vimentin supports metastatic renal cell carcinoma. In our case, the tumor cells were positive for 34βE12 and p63 and negative for PAX8 and vimentin. Additionally, a normal abdominal CT scan would exclude a renal primary. Primary lung and thyroid carcinoma both may show clear cell changes, frequently metastasize or extend into the mediastinum, but they exhibit histologic and immunohistochemical differences from thymic clear cell carcinoma.9 Again, the radiographic exclusion of a lung mass at the time of diagnosis of thymic neoplasm is important. In our case, given the absence of a pulmonary mass on initial radiographic evaluation, the tumor cells were negative for TTF-1; a diagnosis of thymic clear cell carcinoma was rendered, excluding primary from metastatic lung.
Differential diagnosis should be discussed with other primary tumors, including mediastinal seminoma, parathyroid carcinoma, balloon cell melanoma and thymoma with clear cell component. Primary mediastinal seminomas, which occur almost exclusively in men, are morphologically similar to testicular seminomas, with small tumor lobules in a stroma containing a marked lymphocytic infiltrate.19 Cytologically, seminoma cells are more pleomorphic, with vesicular nuclei and obvious nucleoli, in contrast to the bland nuclear feature of thymic clear cell carcinoma.9 Seminomas in other extragonadal sites as well as primary testicular tumors are generally positive for placental alkaline phosphatase (PLAP).20 In contrast, thymic clear cell carcinoma is focally positive for keratins and is generally unreactive for PLAP. Parathyroid carcinoma, although cytologically similar to thymic clear cell carcinoma, has broad trabecular, rather than lobular, architecture (or both) of endocrine differentiation and is more diffusely parathyroid hormone (PTH)-positive in the clear cells. Pure clear-cell variants of melanoma composed predominantly or entirely of cells with large amounts of clear, vacuolated cytoplasm, due to intracytoplasmic glycogen may be confused with thymic clear cell carcinoma. In such cases, immunohistochemical staining is a valuable diagnostic adjunct, as melanoma is uniformly positive for S-100, HMB-45, and Melan-A. The changes of clear cells were often found in WHO Type B3 thymoma and are almost focal lesions. In most tumors, these are gradual migrations of clear cell lesions, with the prominent traditional Type B3 thymoma region observed. Necrotic, hyperproliferative, fibroplasia or P53 overexpression were lacking and PAS were negative.
In recent years, a large number of studies have been published in the literature, attempting to identify specific immunohistochemical markers that can help diagnose and identify primary thymic carcinoma.18 The use of immunomarkers is rather limited as there is not a single immunohistochemical stain that can unequivocally separate thymic clear cell carcinoma from other tumors. In general, CK (pan) is strongly positive in the tumor cells of thymic clear cell carcinoma. Variable staining has been described for CD5, c-kit and EMA, whereas Pax8, S-100, PLAP and TTF-1 are usually negative.21 These markers are not specific, and especially, the clear cell variant of thymic carcinoma can show variable expression for CD522,23 and is often negative for c-kit.13,24 In addition, tumor cells usually show strong cytoplasmic diastase-labile periodic acid-Schiff positivity, but the Mucicarmine stain is negative. All the immunophenotypic features described above are illustrated in our case, which supports the diagnosis of clear cell carcinoma of the thymus.
Clear cell carcinomas of the thymus are highly malignant, aggressive mediastinal neoplasms with frequent local recurrences and distant metastases. The specific criteria to confirm this tumor have not yet been established. However, based on its rarity, challenging histologic diagnosis, more cases should be collected to better define clinical behavior, histopathology, pathogenesis and other relevant information.
Disclosure
The authors report no conflicts of interest in this work.
References
Suster S, Rosai J. Thymic carcinoma. A clinicopathologic study of 60 cases. Cancer. 1991;67:1025–1032. | ||
Hartmann CA, Roth C, Minck C, Niedobitek G. Thymic carcinoma. Report of five cases and review of the literature. J Cancer Res Clin Oncol. 1990;116:69–82. | ||
Kim DJ, Yang WI, Choi SS, Kim KD, Chung KY. Prognostic and clinical relevance of the World Health Organization schema for the classification of thymic epithelial tumors: a clinicopathologic study of 108 patients and literature review. Chest. 2005;127:755–761. | ||
Snover DC, Levine GD, Rosai J. Thymic carcinoma. Five distinctive histological variants. Am J Surg Pathol. 1982;6:451–470. | ||
Wolfe JT, Wick MR, Banks PM, Scheithauer BW. Clear cell carcinoma of the thymus. Mayo Clin Proc. 1983;58:365–370. | ||
Stephens M, Khalil J, Gibbs AR. Primary clear cell carcinoma of the thymus gland. Histopathology. 1987;11:763–765. | ||
Kuo TT, Chang JP, Lin FJ, Wu WC, Chang CH. Thymic carcinomas: histopathological varieties and immunohistochemical study. Am J Surg Pathol. 1990;14:24–34. | ||
Truong LD, Mody DR, Cagle PT, Jackson-York GL, Schwartz MR, Wheeler TM. Thymic carcinoma. A clinicopathologic study of 13 cases. Am J Surg Pathol. 1990;14:151–166. | ||
Hasserjian RP, Klimstra DS, Rosai J. Carcinoma of the thymus with clear-cell features. Report of eight cases and review of the literature. Am J Surg Pathol. 1995;19:835–841. | ||
Okuda M, Huang CL, Haba R, Yokomise H. Clear cell carcinoma originating from ectopic thymus. Gen Thorac Cardiovasc Surg. 2009;57:269–271. | ||
Nakano T, Endo S, Tsubochi H, Nokubi M, Watanabe Y, Koyama S. Thymic clear cell carcinoma. Gen Thorac Cardiovasc Surg. 2010;58:98–100. | ||
Hsu Y-H. Clear cell carcinoma of the thymus. Tzu Chi Med J. 2011;23: 151–152. Available from: https://www.sciencedirect.com/science/article/pii/S1016319011000231. Accessed March 03, 2018. | ||
Lale SA, Tiscornia-Wasserman PG, Aziz M. Diagnosis of thymic clear cell carcinoma by cytology. Case Rep Pathol. 2013;2013:617810. | ||
Bertocchi P, Meriggi F, Zambelli C, Zorzi F, Zaniboni A. Clear cell thymic carcinoma: a case report. Tumori. 2015;101:e73–74. | ||
Venuta F, Anile M, Diso D, et al. Thymoma and thymic carcinoma. Eur J Cardiothorac Surg. 2010;37:13–25. | ||
Levine GD, Rosai J. Thymic hyperplasia and neoplasia: a review of current concepts. Hum Pathol. 1978;9:495–515. | ||
Tomaszek S, Wigle DA, Keshavjee S, Fischer S. Thymomas: review of current clinical practice. Ann Thorac Surg. 2009;87:1973–1980. | ||
Moran CA, Suster S. Thymic carcinoma: current concepts and histologic features. Hematol Oncol Clin North Am. 2008;22:393–407. | ||
Bergh NP, Gatzinsky P, Larsson S, Lundin P, Ridell B. Tumors of the thymus and thymic region: III. Clinicopathological studies on teratomas and tumors of germ cell type. Annals Thoracic Surg. 1978;25:107–111. | ||
Bentley AJ, Parkinson MC, Harding BN, Bains RM, Lantos PL. A comparative morphological and immunohistochemical study of testicular seminomas and intracranial germinomas. Histopathology. 1990;17:443–449. | ||
Weissferdt A, Moran CA. Immunohistochemistry in the diagnosis of thymic epithelial neoplasms. Appl Immunohistochem Molec Morphol. 2015;22:479–487. | ||
Dorfman DM, Shahsafaei A, Chan JK. Thymic carcinomas, but not thymomas and carcinomas of other sites, show CD5 immunoreactivity. Am J Surg Pathol. 1997;21:936–940. | ||
Kuo TT, Chan JK. Thymic carcinoma arising in thymoma is associated with alterations in immunohistochemical profile. Am J Surg Pathol. 1998;22:1474–1481. | ||
Weissferdt A, Moran CA. Thymic carcinoma, part 1: a clinicopathologic and immunohistochemical study of 65 cases. Am J Clin Pathol. 2012;138:103–114. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.