Back to Journals » Cancer Management and Research » Volume 12
Primary Causes of Death in Patients with Non-Hodgkin’s Lymphoma: A Retrospective Cohort Study
Authors Mei M, Wang Y, Song W, Zhang M
Received 24 December 2019
Accepted for publication 8 April 2020
Published 6 May 2020 Volume 2020:12 Pages 3155—3162
DOI https://doi.org/10.2147/CMAR.S243672
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Eileen O'Reilly
Mei Mei,1,2 Yingjun Wang,1,2 Wenting Song,1,2 Mingzhi Zhang1
1Department of Oncology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan Province, People’s Republic of China; 2The Academy of Medical Sciences, Zhengzhou University, Zhengzhou, Henan Province, People’s Republic of China
Correspondence: Mingzhi Zhang
Department of Oncology, The First Affiliated Hospital of Zhengzhou University, &Ngr;o. 1 Jianshe East Road, Zhengzhou, Henan, People’s Republic of China
Tel +8613838565629
Fax +86 371 6629 5561
Email [email protected]
Purpose: Non-Hodgkin’s lymphoma (NHL) comprises many serious hematologic malignancies from lymphocytes. The incidence of NHL is 5.1 per 100,000, with a mortality rate of 2.5 per 100,000 worldwide. However, the causes of death among patients with NHL vary. This retrospective cohort study aimed to elucidate the primary causes and specific risk factors for NHL.
Patients and Methods: The study included patients who were diagnosed from January 2006 to January 2018. Grouped by sex, Ann Arbor stage, date of diagnosis, age, B symptom, NHL type, international prognostic index, and Eastern Cooperative Oncology Group (ECOG) performance score, the Log-rank test was performed, and survival curves were drawn using the Kaplan–Meier method. The competing-risks regression model was used to analyze the specific causes of death.
Results: T-cell lymphoma, B symptoms and worse performance were associated with a lower survival. Mortality from NHL accounted for most and other common causes that contributed to death included circulatory and respiratory diseases. Patients diagnosed with T-cell lymphoma were more likely to die of NHL rather than other causes. Moreover, patients with B symptoms on admission were more likely to die of diseases of the circulatory system. Lastly, patients diagnosed at an earlier age suffered more from diseases of the digestive system and immune mechanism or other uncommon causes.
Conclusion: Classifications of subtypes, age and occurrence of B symptoms were factors providing references for a specific cause of death owing to NHL, which might enable physicians to decrease cause-specific mortality rates.
Keywords: NHL, competing-risks regression, survival analysis, overall survival, ECOG score, cause-specific mortality
Introduction
Non-Hodgkin’s lymphoma (NHL) comprises many hematologic malignancies,1 originating from B lymphocytes, T lymphocytes or natural killer lymphocytes, with approximately 85–90% arising from B lymphocytes.2 It has been reported that 386,000 cases of NHL occurred worldwide.3 The incidence of NHL is 5.1 per 100,000, with a mortality rate of 2.5 per 100,000 worldwide.3,4 The American Cancer Society projected that 77,240 new cases of NHL would be diagnosed in 2020 in the USA, with 19,940 deaths occurring due to NHL during this period.5 The mortality rate of NHL reduced from 1970 to 2017.5 There were 68,500 new cases of NHL in 2016 in China, accounting for 14.9% newly diagnosed NHL worldwide, and 37,600 patients were reported to have died.6
The causes of death among patients with NHL vary. It is previously reported an increased mortality in NHL, other malignant tumors, cardiac disease and pneumonia in childhood NHL survivors.7 Risk factors of developing NHL include organ transplantation, HIV infection and autoimmune diseases.8 Treatments such as radiotherapy and stem cell transplantation have been used to treat NHL but were risky since they increased the possibilities of late sequelae.9,10 This retrospective cohort study aimed to analyze the various causes of death and influencing factors for NHL to determine the most common causes, in order to provide references for clinical practice.
Patients and Methods
Patients
This follow-up study collected data from 155 NHL patients who had exact data on the date and causes of death recorded. The eligibility criteria for patients in this study included: (1) patients were diagnosed or received treatment for NHL at the department of oncology, the First Affiliated Hospital of Zhengzhou University, from January 2006 to 2018; (2) histopathology and immunohistochemical staining were performed by the Pathology Department of The First Affiliated Hospital of Zhengzhou University to ensure the diagnosis. The exclusion criteria included: (1) patients lost to follow-up or still alive; (2) the lack of date or cause of death. The death data were collected and recorded via telephonic follow-up or by reviewing medical records by a specially assigned staff member.
The following clinical data were retrieved from the hospital’s database: sex, age, date of diagnosis, international prognostic index (IPI), Eastern Cooperative Oncology Group (ECOG) performance score, lymphoma subtype, disease stage, the occurrence of B symptoms. All patients were staged according to the Ann Arbor staging system. B symptoms were defined as unexplained fever with a temperature >38°C for three consecutive days, drenching night sweats, and/or unexplained weight loss exceeding 10% of the baseline value. IPI score based on age, tumor stage, LDH, performance status, and number of extranodal disease sites from International Non-Hodgkin’s Lymphoma Prognostic Factors Project.11 The ECOG score was used to evaluate performance status.
Outcome Ascertainment
We described the distributions of major causes of death in patients diagnosed with NHL. The study analyzed the primary cause but not the direct cause. All causes of death were categorized into NHL-specific and non-NHL specific causes, with the latter one being further classified into (1) infectious and parasitic diseases, (2) diseases of the circulatory system, (3) diseases of the respiratory system, and (4) other causes. NHL-specific causes included progress, relapse and complication of NHL according to the medical history. Classification was based on the International Classification of Diseases (ICD-10).
Statistical Analysis
Distributions of patients were grouped by basic clinical information listed above. Overall survival (OS) was defined as the period from clinical diagnosis to death. The Log rank test was performed on groups divided by sex, Ann Arbor stage, date of diagnosis, age at diagnosis, B symptom, NHL type, IPI score, and ECOG score. Survival curves were drawn using the Kaplan–Meier method. All statistical analyses except the competing-risks regression were performed using SPSS Statistics for Windows, version 21 (IBM Corp., Armonk, NY, USA).
Potential confounding bias may exist in this reptrospective study, so events were analyzed via a competing-risks regression model using STATA 15 (StataCorp LLC, College Station, TX, USA). When we analysed NHL-specific mortality, causes except NHL were set as the competing events. Risks were showed by cause-specific sub-hazard ratios (SHR) and cumulative incidence function (CIF).12,13 We also calculated the SHRs of NHL-specific and non-NHL causes mortality respectively, for NHL type, sex, B symptoms, ECOG score, IPI score, Ann Arbor stage, age at diagnosis, and date of diagnosis. Statistical significance was defined as P < 0.05.
Results
Distribution of Patients
This study included 155 participants who died during the follow-up period. All patients were Asian. The average age was 54.8±17.15, range from 12 to 85 years old. The mean duration from diagnosis until death was 14.000±1.243 months. Except for 8 patients who abandoned treatment, others all received chemotherapy in our department. All the patients were categorized according to sex, Ann Arbor Stage, date of diagnosis, age at diagnosis, B symptom, NHL type, IPI score and ECOG (Table 1).
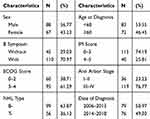 |
Table 1 Distributions of Characteristics of Patients Diagnosed with Non-Hodgkin’s Lymphoma |
Clinical Features
Patients with B-cell lymphoma had a longer OS time than those with T-cell lymphoma (P=0.019, Log-rank test) (Figure 1A). Patients with B symptoms on admission had a lower survival fraction (P=0.014, Log-rank test) (Figure 1B). Patients with an ECOG score of 4 had a lower survival rate (P=0.010, Log-rank test) (Figure 1C). The median survival durations, according to IPI scores, were 15±1.786 (IPI = 0–3) and 6±1.053 (IPI = 4–5) (P=0.032, Log-rank test) (Figure 1D). Other variables showed no significant difference between groups. In the Cox proportional hazards regression model with NHL type, B symptoms, ECOG score and IPI score included as covariates, a significant statistics difference was found between groups (P<0.001). Among them, IPI (P=0.028), NHL types (P=0.008) and B symptom (P=0.018) significantly related to death.
 |
Figure 1 Kaplan–Meier curves for comparison of patients diagnosed with NHL according to (A) NHL type, (B) B symptom, (C) ECOG and (D) IPI. |
Causes of Death
Mortality from NHL was the most common independent cause of death, accounting for 70.3%. The other common causes were diseases of the circulatory and respiratory systems. The results presented in Table 2 only include causes that were found to be significant in this study.
 |
Table 2 Distribution of Causes of Death of Follow-Up in Patients Diagnosed with Non-Hodgkin’s Lymphoma |
Competing Risk Regression
The 155 patients were divided into two groups: death attributed to NHL and death attributed to other causes (Table 3). Secondly, patients were further classified into four groups: (1) infectious and parasitic diseases, (2) diseases of the circulatory system, (3) diseases of the respiratory system and (4) other causes (Table 4). For patients diagnosed with T-cell lymphoma, the cumulative incidence of the death rate attributed to NHL was much higher (Figure 2). On the contrary, patients diagnosed with B-cell lymphoma had greater risks for other causes rather than NHL. A significant difference was shown between the groups that were diagnosed later than 2014 compared to their counterparts; patients in this group had a higher probability of death from other causes.
 |
Table 3 Sub-Hazard Ratios of Cause-Specific Death by Competing-Risks Regression |
 |
Table 4 Sub-Hazard Ratios of Cause-Specific Death by Competing-Risks Regression of Causes Other Than Non-Hodgkin’s Lymphoma |
 |
Figure 2 Cumulative incidence plot comparing NHL type in patients dead attribute to NHL. |
Moreover, patients with B symptoms on admission were more likely to die of diseases of the circulatory system. Lastly, patients diagnosed at an earlier age suffered more from diseases of the digestive system and immune mechanism or other uncommon causes.
Discussion
The age-standardized incidence rate of NHL increased from 2006 to 2016, while the mortality rate increased from 2006 to 2013 and remained stable.6 The age-standardized mortality rate was higher in older or male patients, and in Tibet, Hebei and Xinjiang province in China.6 In our study, T-cell lymphoma, B symptoms, and worse performance status (ECOG score of 4) in NHL patients were associated with a lower survival.
Patients with B-cell lymphoma had a longer survival time, in accordance with a study in primary intestinal NHL.14 The 5-year OS rate of patients diagnosed with B-cell lymphoma was significantly higher than that of patients diagnosed with T-cell lymphoma (71% versus 28%, p<0.001).14 The efficacy of Rituximab and anti-CD20 monoclonal antibody in the treatment of B-cell lymphoma might be a possible reason for these differences.15–17
Patients with B symptoms had worse survival, probobaly due to abnormal endogenous cytokines.18 Interestingly, B symptoms were more common in men than in women and were seen primarily in patients with advanced stage NHL.19
An ECOG of 3 and 4, which is considered a poor performance status, predicted worse outcomes for event-free survival (EFS) and OS in pediatric B-NHL in India.20 Another study showed that elevated lactate dehydrogenase levels, poor performance status, advanced stage, IPI score ≥3, conservative treatment, and high-grade histological subtype were associated with poor survival in patients with primary gastric lymphoma.21
This retrospective cohort study demonstrated that the most common cause of death among patients with NHL was NHL, followed by diseases of the circulatory system and respiratory system. This finding is similar to that of previous studies that attempted to determine the cause of death in cancer patients. Zaorsky et al conducted an analysis of 28 cancers and found that patients with lung, pancreatic, and brain cancer were most likely to die of primary cancer.22 Väkevä et al reported that among 31 patients with cutaneous T-cell lymphomas who died during a follow-up period of 10 years, the most common causes of death were cutaneous lymphoma, coronary artery disease, and lung cancer.23 In our previous study on extranodal natural killer/T cell lymphoma in the USA based on the SEER program, the most common cause of death was NHL.24 The mortality rate of cardiovascular disease in those with NHL was 5.35 times that of the general population.25 A SEER-based study showed that 5.8% of NHL patients died of cardiovascular disease.26
Several factors, including sex, NHL type, ECOG score, IPI score, B symptoms, age at diagnosis, stage and date of diagnosis, were analyzed for cause-specific mortality. The competing-risks regression model was constructed to evaluate the relationship between variables and cause-specific failures,27 the sub-distribution hazard rate of the specific causes and cumulative incidence of those causes were shown. The results showed that patients diagnosed with T-cell lymphoma are more likely to die of NHL, probably due to the subtype and treatment regimen.28 Thus, a risk factor for an NHL-induced death was the diagnosis of T-cell lymphoma.
In cases, in which the patients were diagnosed before 2014, the cause of death was more likely attributed to diseases of the circulatory system, respiratory system, infection and parasitic diseases, and rare causes. This is probably due to the supplement of electronic medical record data and a better follow-up system in recent years.
Limitations exist in this study. We only included patients with specific death dates and recorded causes, which may have led to selection bias. Due to the retrospective nature of the study, some information may have been missing. Moreover, this is a single-center study, which may limit the generalizability of the study due to regional or racial differences among patients. Furthermore, the number of deaths attributed to non-NHL diseases was relatively small, probably resulted in statistics deviations in uncommon causes. Lastly, autopsies information was not included because of the lack of cases.
Conclusion
In conclusion, T-cell lymphoma, poor ECOG performance, and the presence of B symptoms were found to be the primary factors leading to a poor prognosis. This cohort study revealed that the most common cause of death among patients with NHL is NHL, followed by diseases of the circulatory and respiratory systems. Patients diagnosed with T-cell lymphoma are more likely to die of NHL rather than any other cause. In addition, NHL patients with a poor ECOG performance were more likely to die of infectious and parasitic diseases. Moreover, patients exhibiting B symptoms on admission were more likely to die of diseases of the circulatory system. More efforts should be made in implementing a comprehensive prognostic evaluation and treatment plan in preventing high-risk factors in NHL patients.
Abbreviations
NHL, Non-Hodgkin’s lymphoma; IPI, international prognostic index; ECOG, Eastern Cooperative Oncology Group; ICD, International Classification of Diseases; OS, overall survival; CIF, cumulative incidence function.
Acknowledgments
We would like to show gratitude to all members in the oncology department of the First Affiliated Hospital of Zhengzhou University.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
This study was supported by the National natural Science Foundation of China (81570203, 81970184, U1904139) and Department of Science & Technology of Henan province (182102310114).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375–2390. doi:10.1182/blood-2016-01-643569
2. Chiu BCH, Hou N. Epidemiology and etiology of non-hodgkin lymphoma. Cancer Treat Res. 2015;165:1–25. doi:10.1007/978-3-319-13150-4_1
3. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–386. doi:10.1002/ijc.29210
4. Kamel MG, Elqushayri AE, Thach TQ, Huy NT. Cardiovascular mortality trends in non-Hodgkin’s lymphoma: a population-based cohort study. Expert Rev Anticancer Ther. 2018;18(1):91–100. doi:10.1080/14737140.2018.1409626
5. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. doi:10.3322/caac.21590
6. Liu W, Liu J, Song Y, et al. Burden of lymphoma in China, 2006–2016: an analysis of the Global Burden of Disease Study 2016. J Hematol Oncol. 2019;12(1):115. doi:10.1186/s13045-019-0785-7
7. Bluhm EC, Ronckers C, Hayashi RJ, et al. Cause-specific mortality and second cancer incidence after non-Hodgkin lymphoma: a report from the Childhood Cancer Survivor Study. Blood. 2008;111(8):4014–4021. doi:10.1182/blood-2007-08-106021
8. Shankland KR, Armitage JO, Hancock BW. Non-Hodgkin lymphoma. Lancet. 2012;380(9844):848–857. doi:10.1016/S0140-6736(12)60605-9
9. Aviles A, Delgado S, Fernandez R, Talavera A, Neri N, Huerta-Guzman J. Combined therapy in advanced stages (III and IV) of follicular lymphoma increases the possibility of cure: results of a large controlled clinical trial. Eur J Haematol. 2002;68(3):144–149. doi:10.1034/j.1600-0609.2002.01542.x
10. Philip T, Guglielmi C, Hagenbeek A, et al. Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin’s lymphoma. N Engl J Med. 1995;333(23):1540–1545. doi:10.1056/NEJM199512073332305
11. Project IN-HsLPF. A predictive model for aggressive non-Hodgkin’s lymphoma. N Engl J Med. 1993;329(14):987–994. doi:10.1056/NEJM199309303291402
12. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509. doi:10.1080/01621459.1999.10474144
13. Scrucca L, Santucci A, Aversa F. Regression modeling of competing risk using R: an in depth guide for clinicians. Bone Marrow Transplant. 2010;45(9):1388–1395. doi:10.1038/bmt.2009.359
14. Kim SJ, Choi CW, Mun YC, et al. Multicenter retrospective analysis of 581 patients with primary intestinal non-hodgkin lymphoma from the Consortium for Improving Survival of Lymphoma (CISL). BMC Cancer. 2011;11:321. doi:10.1186/1471-2407-11-321
15. Găman AM. Follicular non-Hodgkin’s lymphoma: correlation between histology, pathophysiology, cytogenetic, prognostic factors, treatment, survival. Rom J Morphol Embryol. 2013;54(1):71–76.
16. Găman A, Bold A, Găman G. The unexpected evolution of a case of diffuse large B-cell non-Hodgkin lymphoma. Rom J Morphol Embryol. 2011;52(2):719–722.
17. Subramanian J, Cavenagh J, Desai B, Jacobs I. Rituximab in the treatment of follicular lymphoma: the future of biosimilars in the evolving therapeutic landscape. Cancer Manag Res. 2017;9:131–140. doi:10.2147/CMAR.S120589
18. Kurzrock R, Redman J, Cabanillas F, Jones D, Rothberg J, Talpaz M. Serum interleukin 6 levels are elevated in lymphoma patients and correlate with survival in advanced Hodgkin’s disease and with B symptoms. Cancer Res. 1993;53(9):2118–2122.
19. Dehghani M, Haddadi S, Vojdani R. Signs, Symptoms and Complications of Non-Hodgkin’s Lymphoma According to Grade and Stage in South Iran. Asian Pac J Cancer Prev. 2015;16(8):3551–3557. doi:10.7314/APJCP.2015.16.8.3551
20. Patel A, Sharma MC, Mallick S, Patel M, Bakhshi S. Poor performance status, urban residence and female sex predict inferior survival in pediatric advanced stage mature B-NHL in an Indian tertiary care center. Pediatr Hematol Oncol. 2018;35(1):23–32. doi:10.1080/08880018.2018.1424279
21. Wang YG, Zhao LY, Liu CQ, et al. Clinical characteristics and prognostic factors of primary gastric lymphoma: a retrospective study with 165 cases. Medicine (Baltimore). 2016;95(31):e4250. doi:10.1097/MD.0000000000004250
22. Zaorsky NG, Churilla TM, Egleston BL, et al. Causes of death among cancer patients. Ann Oncol. 2017;28(2):400–407. doi:10.1093/annonc/mdw604
23. Vakeva L, Lipsanen T, Sintonen H, Ranki A. Morbidity and causes of death in patients with cutaneous T-cell lymphoma in Finland. Acta Derm Venereol. 2017;97(6):735–738. doi:10.2340/00015555-2629
24. Mei M, Wang Y, Zhang M. Causes of mortality in cases with extra nodal natural killer/T-cell lymphoma, nasal type: a cohort study. PLoS One. 2019;14(4):e0214860. doi:10.1371/journal.pone.0214860
25. Boyne DJ, Mickle AT, Brenner DR, et al. Long-term risk of cardiovascular mortality in lymphoma survivors: a systematic review and meta-analysis. Cancer Med. 2018;7(9):4801–4813. doi:10.1002/cam4.1572
26. Abuamsha H, Kadri AN, Hernandez AV. Cardiovascular mortality among patients with non-Hodgkin lymphoma: differences according to lymphoma subtype. Hematol Oncol. 2019;37(3):261–269. doi:10.1002/hon.2607
27. Dignam JJ, Zhang Q, Kocherginsky MN. The use and interpretation of competing risks regression models. Clin Cancer Res. 2012;18(8):2301–2308. doi:10.1158/1078-0432.CCR-11-2097
28. Armitage JO. The aggressive peripheral T-cell lymphomas: 2017. Am J Hematol. 2017;92(7):706–715. doi:10.1002/ajh.24791
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
