Back to Journals » OncoTargets and Therapy » Volume 10
Preventive effect of kampo medicine (hangeshashin-to, TJ-14) plus minocycline against afatinib-induced diarrhea and skin rash in patients with non-small cell lung cancer
Authors Ichiki M, Wataya H, Yamada K, Tsuruta N , Takeoka H, Okayama Y, Sasaki J, Hoshino T
Received 6 July 2017
Accepted for publication 4 October 2017
Published 24 October 2017 Volume 2017:10 Pages 5107—5113
DOI https://doi.org/10.2147/OTT.S145613
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Ingrid Espinoza
Masao Ichiki,1 Hiroshi Wataya,2 Kazuhiko Yamada,3 Nobuko Tsuruta,4 Hiroaki Takeoka,1 Yusuke Okayama,1 Jun Sasaki,1 Tomoaki Hoshino3
1Department of Respiratory Medicine, Clinical Research Institute, National Hospital Organization, Kyushu Medical Center, 2Division of Internal Medicine, Saiseikai Fukuoka General Hospital, Fukuoka City, 3Division of Respirology, Neurology, and Rheumatology, Department of Internal Medicine, Kurume University, Kurume City, 4Department of Respiratory Medicine, Hamanomachi Hospital, Fukuoka City, Fukuoka, Japan
Purpose: Diarrhea and oral mucositis induced by afatinib can cause devastating quality of life issues for patients undergoing afatinib treatment. Several studies have shown that hangeshashin-to (TJ-14) might be useful for chemotherapy-induced diarrhea and oral mucositis. In this study, we investigated the prophylactic effects of TJ-14 for afatinib-induced diarrhea and oral mucositis and minocycline for afatinib-induced skin rash.
Patients and methods: First- and second-generation epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitors have become the standard first-line treatment in patients with EGFR-mutated non-small cell lung cancer. The incidence of diarrhea was higher with afatinib than with gefitinib, and we conducted a single-arm Phase II study with afatinib. Patients who had previously undergone treatment with afatinib were ineligible. Both TJ-14 (7.5 g/day) and minocycline (100 mg/day) were administered simultaneously from the start of afatinib administration. The primary end point was the incidence of ≥ grade 3 (G3) diarrhea (increase of ≥7 stools/day over baseline) during the first 4 weeks of treatment. The secondary end points were the incidence of ≥ G3 oral mucositis (severe pain interfering with oral intake) and ≥ G3 skin toxicity (severe or medically significant but not immediately life-threatening).
Results: A total of 29 patients (nine men and 20 women; median age, 66 years; performance status, 0/1/2: 18/10/1) were enrolled from four centers. Four patients had undergone prior treatment with chemotherapy, including gefitinib or erlotinib. In all, 20 (68.9%) patients and one (3.4%) patient had diarrhea of any grade and ≥ G3, respectively. One (3.4%) patient had ≥ G3 oral mucositis; no patients had ≥ G3 skin rash. A total of 18 (62%) of the 29 patients achieved a partial response.
Conclusion: The present study indicated a trend in which TJ-14 reduced the risk of afatinib-induced diarrhea and minocycline reduced the risk of afatinib-induced skin rash.
Keywords: epidermal growth factor receptor, hangeshashin-to, afatinib, adverse events
Plain language summary
Adverse effects induced by afatinib can cause devastating quality of life issues for patients undergoing afatinib treatment. We conducted a study to evaluate the prophylactic efficacy of TJ-14 (hangeshashin-to, a traditional Japanese kampo medicine) on afatinib-induced diarrhea and oral mucositis because the previous reports proved that TJ-14 was effective against chemotherapy-induced diarrhea and oral mucositis. We also evaluated the prophylactic efficacy of minocycline (+TJ-14) on afatinib-induced skin toxicity. A total of 29 patients with epidermal growth factor receptor mutation-positive non-small cell lung cancer were treated with afatinib and prophylactic treatments. Only one patient had grade 3 (G3) diarrhea, and other three patients had grade 2 diarrhea; no one developed G3 skin rash. On the contrary, 11 patients had ≥ grade 2 oral mucositis. The results indicated a trend in which TJ-14 reduced the risk of afatinib-induced diarrhea and minocycline (+TJ-14) reduced the risk of afatinib-induced skin rash. We concluded that TJ-14 and minocycline are promising prophylactic treatments for afatinib-induced diarrhea and skin rash.
Introduction
Non-small cell lung cancer (NSCLC) accounts for approximately 85% of lung cancer cases and remains the leading cause of cancer death worldwide.1 Epidermal growth factor receptor (EGFR) mutations are important drivers of NSCLC tumors. The frequency of EGFR mutations in NSCLC in Asian populations is approximately 50% to 60%.2,3 First-generation EGFR-tyrosine kinase inhibitors (TKIs) such as gefitinib and erlotinib have high antitumor activity and are associated with long progression-free survival in NSCLC patients with tumors that harbor an activating EGFR mutation, including the common mutations exon 21 L858R (L858R) and exon 19 deletion (Del 19).4,5
Afatinib, a second-generation EGFR-TKI, is an oral irreversible ErbB family blocker that is associated with longer progression-free survival compared with platinum-based chemotherapy for first-line treatment. A significant improvement in overall survival (OS) with afatinib was observed in patients with Del 19 mutations in the LUX-Lung 3 trial (first-line afatinib versus first-line cisplatin and pemetrexed) and in the LUX-Lung 6 trial (first-line afatinib versus first-line cisplatin and gemcitabine).6–8 Therefore, in patients with these common EGFR mutations, EGFR-TKIs have become the standard of care for first-line treatment. Afatinib has shown an adverse event profile similar to that of other EGFR-TKIs. Three of the most common adverse events with afatinib are rash, diarrhea, and oral mucositis, which can cause reduction in the afatinib dose and even early suspension. The incidence rates of treatment-related diarrhea and oral mucositis were higher with afatinib than with gefitinib or erlotinib. On the other hand, the frequency and severity of all adverse events, including rash, were similar with afatinib and gefitinib or erlotinib.9,10 Here we focus on diarrhea and oral mucositis. Pharmacologic management of EGFR-TKI-induced diarrhea is based on the grade of diarrhea and is usually limited to loperamide.
The median progression-free survival of the first- and second-generation EGFR-TKIs is approximately 9 to 13 months.11 Second-generation afatinib has been shown to significantly improve progression-free survival compared to first-generation gefitinib.9 T790M gatekeeper mutation is the most common resistance mechanism of first- and second-generation EGFR-TKIs, and third-generation EGFR-TKI osimertinib is effective against T790M-positive NSCLC patients and has already been used in many countries.12,13 Furthermore, the mechanisms of resistance such as C797S mutation against third-generation EGFR-TKI have been revealed.12,14 If the mechanism of resistance can be confirmed, it is important to administer the next effective agents at an early stage, leading to long-term survival. Therefore, countermeasures against adverse events of afatinib are extremely important.
Hangeshashin-to (TJ-14), a traditional Japanese kampo medicine, is composed of seven medicinal herbs: Pinelliae tuber, Scutellariae radix, Glycyrrhizae radix, Zizyphi fructus, Ginseng radix, Coptidis rhizome, and Zingiberis siccatum rhizome. TJ-14 is prescribed in Japan to treat inflammatory diarrhea, gastritis, and stomatitis. Mori et al15 reported that TJ-14 was effective in preventing and controlling irinotecan (CPT-11)-induced diarrhea. Kono et al16 found that TJ-14 was effective as a gargle therapy for the treatment of chemotherapy-induced oral mucositis. TJ-14 suppresses the elevation in colonic prostaglandin E2 (PGE2) that is closely associated with diarrhea and enhances colonic water absorption.17–19 TJ-14 inhibits the cyclooxygenase-2 (COX-2) expression and PGE2 elevation associated with oral mucositis.20,21 On the other hand, Arrieta et al22 reported that tetracycline was tolerated and reduced the incidence and severity of rash associated with afatinib. Melosky et al23 reported that prophylactic treatment with minocycline significantly lengthened the time to the most severe grade of erlotinib-induced skin rash.
The aim of the present study was to evaluate the prophylactic effects of TJ-14 on afatinib-induced diarrhea and oral mucositis and minocycline on afatinib-induced skin toxicity. Minocycline is commonly used to treat skin toxicity due to EGFR-TKIs in Japan.
Patients and methods
Study design
This prospective, multicenter trial was performed in patients receiving afatinib for NSCLC with EGFR mutations. The primary end point was the incidence of ≥ grade 3 (G3) diarrhea (increase of ≥7 stools/day over baseline) during the first 4 weeks of treatment. As a measurement of the severity of diarrhea, the worst grade of the day was used instead of the mean circadian change. The secondary end points were the incidence rates of ≥ G3 oral mucositis (severe pain interfering with oral intake) and skin toxicity (severe or medically significant but not immediately life-threatening).
Eligibility
Patients with histologically or cytologically confirmed NSCLC with EGFR mutations were eligible. The inclusion criteria were clinical stage IIIB or IV or postoperative recurrence, age ≥20 years, Eastern Cooperative Oncology Group-performance status 0–2, appropriate organ functions (lung: SpO2 ≥92%; heart: normal 12-lead electrocardiogram; bone marrow: hemoglobin ≥9.0 g/dL, white blood cells ≥3,000/mm3, neutrophils ≥1,500/mm3, platelets ≥100,000/mm3; liver: aspartate aminotransferase [AST] and alanine aminotransferase [ALT] less than or equal to two times the upper normal limit, total bilirubin ≤1.5 mg/dL), life expectancy of at least 3 months, and no prior use of afatinib, but patients who had received prior chemotherapy, including first-generation EGFR-TKIs such as gefitinib or erlotinib, were eligible. The exclusion criteria were hypersensitivity to any medicine, including kampo and minocycline; impaired intestinal absorption; and interstitial pneumonia. Prophylactic topical or systemic steroid therapy was not allowed in this trial. Patients with oral mucositis or dermatopathy at registration were excluded from the study.
This study followed the ethical principles of the Declaration of Helsinki, and the study protocol was approved by the institutional review boards of each participating institution. All patients provided written informed consent before study-related procedures were performed.
Assessment of study treatment and toxicity
All patients received afatinib 40 mg orally once daily, until disease progression or unacceptable toxicity occurred. If a patient had any ≥ G3 drug-related adverse events or any selected prolonged grade 2 adverse events despite best supportive care, the study drug was paused for no more than 14 days. After interruption of treatment and recovery to ≤ grade 1 (or to the grade present at baseline), the afatinib dose was reduced by 10 mg decrements to a minimum dose of 20 mg. Treatment was withdrawn in patients who did not recover to ≤ grade 1 or baseline grade. Salvage regimens were not restricted for patients after discontinuation of the protocol study.
Both TJ-14 and minocycline hydrochloride were administered from the start of afatinib administration. The patients who received TJ-14 were instructed to rinse and gargle with a 100 mL oral rinse solution containing 2.5 g of TJ-14 in water three times daily before meals and to hold the solution in the mouth for 5–10 s and swallow it. All patients also received one 50 mg tablet of minocycline every 12 h after meals. The patients used a moisturizing agent (Hirudoid®) as an emollient cream on the face, neck, hands, feet, chest, and the back twice daily and applied a >30 SPF sunscreen to areas exposing to the sun before going out.
All patients underwent comprehensive baseline assessments, including clinical laboratory tests and imaging studies. The patients also received follow-up assessments at weeks 1, 2, and 4 after the initiation of afatinib administration and monitoring at regular intervals. All adverse events were followed until they either resolved or the investigator determined that the event was no longer clinically significant. Evaluations of toxicity were based on the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0 (NCI-CTCAE v4.0) and nursing investigation including time, frequency, and property of diarrhea.
Statistical analysis
All patients who received at least 4 weeks of the study treatment were included in the safety and efficacy analysis, as well as patients with early suspension due to grade 2 or higher toxicity. In the LUX-Lung 3 trial, the incidence of afatinib-related ≥ G3 diarrhea was 14.4%. On the basis of previous data,6 we assumed that an incidence of afatinib-related ≥ G3 diarrhea of 1% would indicate the potential usefulness of this treatment, whereas an incidence of ≥ G3 diarrhea of 14.4% would be the upper limit of interest. Based on this assumption, the number of patients needed for analysis of the toxicity data under 90% power for a one-sided type I error rate of 0.05 was calculated to be 27. After assuming an unevaluability rate of <10%, we planned an accrual of 29 patients. All statistical analyses were performed with the JMP statistical package, version 10 (SAS Institute Inc., Cary, NC, USA).
This study was registered with the University Hospital Medical Information Network (UMIN) in Japan, number UMIN000018392. The research protocol was approved by the ethics committee of Kyushu Medical Center (registration number 15A086) and that of the Kurume University.
Results
Patient characteristics
Accrual was started in July 2015 and stopped in March 2017. A total of 29 patients with EGFR-mutant NSCLC were treated with afatinib and prophylactic therapy at four institutions. All patients received afatinib and prophylactic treatment for at least 4 weeks (median, 24 weeks; range, 4–91 weeks). Patient characteristics are summarized in Table 1. Four patients had minor EGFR mutations (G719C, G719A, L861Q, and G719X). In all, 25 of the 29 patients received afatinib as first-line chemotherapy, while the remaining four patients had a history of prior treatment (second-, third-, fourth- and fifth-line treatment in one patient each). The patient who had received fourth-line afatinib therapy had received gefitinib, cisplatin plus pemetrexed, and docetaxel; the patient who had received fifth-line afatinib therapy had received gefitinib, erlotinib, pemetrexed, and docetaxel.
Adverse events
The toxicity profile is summarized in Table 2. The adverse events of all grades during the first 4 weeks after the initiation of afatinib therapy were diarrhea in 20 (68.9%) patients, mucositis in 20 (68.9%) patients, and skin rash in 13 (44.8%) patients. The first onset of major adverse events such as diarrhea, skin rash, and oral mucositis occurred within 4 weeks after the initiation of afatinib therapy (Figure 1). In comparison with previous reports, prophylactic treatment with TJ-14 reduced the incidence of afatinib-induced major toxicities except for oral mucositis (Table 3). However, only one patient developed G3 diarrhea during the first 4 weeks, and the incidence of afatinib-related ≥ G3 diarrhea, the primary endpoint in this trial, was 3.4% (95% CI, 0.04%–17.7%). Three patients with grade 2 diarrhea (increase of 4–6 stools/day over baseline) improved with loperamide treatment within a few days, and one patient with G3 diarrhea improved after a short interruption followed by reduction of the afatinib dose. However, two patients discontinued afatinib for >4 weeks after the initiation of treatment. One of these patients had G3 nausea/vomiting (defined as inadequate oral caloric or fluid intake, tube feeding, total parenteral nutrition, or hospitalization indicated or more than or equal to six vomiting episodes in 24 h) at week 5. The other patient had grade 2 interstitial lung disease (defined as symptomatic, medical intervention indicated, and limiting instrumental activities of daily living) at week 7. Only three patients had ≥ G3 adverse events during the first 4 weeks or later (nausea/vomiting at week 5, mucositis at week 3, and diarrhea at week 2). In all, 18 (62%) patients required reduction of the afatinib dose during follow-up due to adverse events; the most common adverse events were oral mucositis (seven patients) and diarrhea (five patients). All patients with adverse events ≥ grade 2 recovered to grade 1 or less after reduction or discontinuation of afatinib. No patients withdrew from treatment or had any adverse events from TJ-14 or minocycline during the first 4 weeks, but one patient discontinued TJ-14 due to grade 1 constipation at week 9.
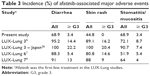  | Table 3 Incidence (%) of afatinib-associated major adverse events |
Antitumor efficacy
Two patients were considered nonevaluable for response (one for refusal to be evaluated at week 5 and the other for nontarget lesions). Of the 29 patients, 18 (62%) achieved a partial response (PR; 95% CI: 42.2%–79.3%), 6 (20.6%) had stable disease, and in 3 (10.3%), the disease progressed during treatment. Interestingly, all four patients with minor mutations achieved PR.
The last patient was enrolled in this study in March 2017, and we performed a final investigation of adverse events, frequency of dose reduction, and response in June 2017. Therefore, the results for progression-free survival and OS are not mature.
Discussion
Although afatinib, an irreversible ErbB family blocker, is generally better tolerated than cytotoxic agents, it often causes diarrhea, rash, and oral mucositis, which impair a patient’s quality of life. In particular, diarrhea can result in fluid and electrolyte losses, leading to dehydration, electrolyte imbalances, and renal insufficiency, in addition to nutritional deficiencies.24,25 These adverse events are most likely to occur in the first 4 weeks after treatment initiation. Kato et al26 showed that the time to first onset of diarrhea or rash was 1–14 days after starting afatinib for most patients. It is very important to suppress these adverse events in the early stages. Therefore, we evaluated adverse events within the first 4 weeks in this trial with TJ-14 as the prophylactic.
The incidence of G3 diarrhea of patients who received afatinib in the LUX-Lung 3 trial and LUX-Lung 6 trial was 14.4% and 5.4%, respectively.6,7 We initially expected the incidence of afatinib-induced diarrhea, the primary end point of this trial, to be lower than the rates obtained in the LUX-Lung 3 and LUX-Lung 6 trials. In the present study, only one (3.4%) patient had G3 diarrhea, and 20 (68.9%) patients had diarrhea of any grade. The primary end point was not met in this trial because the actual upper limit value (17.7%) of 95% CI exceeded the expected upper limit value (14.4%). However, the patient with G3 diarrhea was 80 years old and received two prior treatments (gefitinib and carboplatin plus pemetrexed plus bevacizumab). Of the 25 patients with no prior treatment, only two patients had grade 2 diarrhea and none had ≥ G3 diarrhea. According to the subgroup analysis of the Japanese population in the LUX-Lung 3 trial, all 54 (100%) patients had diarrhea of any grade and 12 (22.2%) patients had ≥ G3 diarrhea.26 As a result, a trend was observed in which TJ-14 reduced the risk of afatinib-induced diarrhea. Uribe et al showed that epidermal growth factor has a potent effect in inhibiting Ca2+-dependent Cl− secretion in human colonic epithelial cells. One theory is that the diarrhea induced by EGFR-TKIs is a result of excess Cl− secretion and colonic water secretion, leading to a secretory form of diarrhea.27 It has been suggested that TJ-14 might act by repression of the increase in the amount of proinflammatory prostaglandins such as PGE2, as well as promotion of the water absorbing capacity of the colon in animal models.17,18 Anaplastic lymphoma kinase (ALK) fusion oncogene-associated NSCLC as well as EGFR-mutated NSCLC is a clear subset of lung cancer suitable for targeted therapy.28,29 Pharmacologic inhibition of ALK fusion oncogene-associated NSCLC using ALK-TKIs leads to the downregulation of Ras/Mek/Erk and PI3K/Akt and apoptosis; these mechanisms of inhibition are similar to those of EGFR-TKIs.28,29 ALK-TKIs, such as crizotinib, alectinib, and ceritinib, have been reported to have adverse events such as diarrhea, and it has been pointed out that the frequency of diarrhea with ceritinib use is particularly high.30 TJ-14 may be effective against diarrhea caused by these ALK-TKIs in view of the mechanism of action.
On the other hand, 20 (68.9%) patients in this trial had all oral mucositis of any grade, and one (3.4%) patient had G3 oral mucositis. We concluded that prophylactic treatment with TJ-14 did not reduce afatinib-induced oral mucositis, because the incidence of oral mucositis was not different from that in previous reports.6,7,9,26 Kono et al reported that TJ-14 had a therapeutic effect in patients with chemotherapy-induced oral mucositis compared with baseline.16 Yamashita et al31 reported that TJ-14 reduced severe grade oral mucositis induced by chemoradiation in patients with head and neck cancers compared with placebo. In these trials, the patients were instructed to rinse and gargle with an oral rinse solution of TJ-14 and to hold the solution in the mouth for 10–30 s, followed by expectoration. It remains to be seen if chemotherapy-induced oral mucositis and afatinib-induced oral mucositis are caused by different mechanisms and if the latter is possibly inflammation mediated. The mechanisms of action of TJ-14 inhibit mucositis via anti-inflammatory effects through enhancement of corticosterone and suppression of COX-2 and PGE2.32,33 TJ-14 might have a direct effect against oral mucositis via suppression of PGE2 or COX-2. Therefore, it was suggested that it is important to perform the rinse and gargle therapy, whether or not TJ-14 is taken orally. In our trial, only 14 (48%) patients were instructed to rinse and gargle, and the other patients took the TJ-14 without rinsing and gargling. As a result, almost half of the patients had moderate-to-severe oral mucositis.
EGFR-TKIs impair keratinocyte growth, migration, and chemokine expression, which lead to recruitment of inflammatory cells and cutaneous injury.34 Several clinical studies on the prophylactic treatment of skin rash with antibiotics such as doxycycline, tetracycline, and minocycline have been published. In these reports, there was a mixture of effects and noneffects, leading to inconsistent results.22,23,35,36 Although Melosky et al23 reported that prophylactic treatment with minocycline significantly lengthened the time to the most severe grade of erlotinib-induced skin rash, the incidence of rash of any grade did not differ significantly between patients receiving prophylactic treatment with minocycline (100 mg orally twice daily) and those receiving treatment from rash occurrence. In our trial, prophylactic treatment with half dose minocycline (50 mg orally twice daily) reduced afatinib-induced skin rash. These differences may be due to the types of EGFR-TKI used, the presence or absence of the combined use of moisturizing and UV scattering agents, and/or the synergistic effect with TJ-14. Our results were very encouraging compared with those of previous reports.6,7,9,26 In the LUX-Lung 3 and LUX-Lung 6 global trials, the incidence rates of paronychia of any grade were 32.6%–56.8%.6,7 In our trial, the incidence of paronychia of any grade was 17.2% without ≥ G3 paronychia. Many patients receiving afatinib suffer from paronychia, and therefore, the low incidence of paronychia is an advantage of prophylactic treatment with low-dose minocycline (+TJ-14).
Conclusion
TJ-14 and minocycline are promising prophylactic treatments for afatinib-induced diarrhea and skin rash. This was a small Phase II study, and a higher level of evidence from large sample size trials is required to confirm the efficacy of TJ-14, including rinse and gargle therapy, and minocycline for afatinib-induced diarrhea, oral mucositis, and skin rash.
Acknowledgment
The authors would like to thank all registered patients, their families, participating physicians, and Enago for the English language review.
Disclosure
The authors report no conflicts of interest in this work.
References
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. | ||
Chou TY, Chiu CH, Li LH, et al. Mutation in the tyrosine kinase domain of epidermal growth factor receptor is a predictive and prognostic factor for gefitinib treatment in patients with non-small cell lung cancer. Clin Cancer Res. 2005;11(10):3750–3757. | ||
Mitsudomi T, Kosaka T, Endoh H, et al. Mutations of the epidermal growth factor receptor gene predict prolonged survival after gefitinib treatment in patients with non-small-cell lung cancer with postoperative recurrence. J Clin Oncol. 2005;23(11):2513–2527. | ||
Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12(8):735–742. | ||
Maemondo M, Inoue A, Kobayashi K, et al; North-East Japan Study Group. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362(25):2380–2388. | ||
Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31(27):3327–3334. | ||
Wu YL, Zhou C, Hu CP, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol. 2014;15(2):213–222. | ||
Yang JC, Wu YL, Schuler M, et al. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol. 2015;16(2):141–151. | ||
Park K, Tan EH, O’Byrne K, et al. Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer (LUX-Lung 7): a phase 2B, open-label, randomised controlled trial. Lancet Oncol. 2016;17(5):577–589. | ||
Soria JC, Felip E, Cobo M, et al; LUX-Lung 8 Investigators. Afatinib versus erlotinib as second-line treatment of patients with advanced squamous cell carcinoma of the lung (LUX-Lung 8): an open-label randomised controlled phase 3 trial. Lancet Oncol. 2015;16(8):897–907. | ||
Wang S, Cang S, Liu D. Third-generation inhibitors targeting EGFR T790M mutation in advanced non-small cell lung cancer. J Hematol Oncol. 2016;9(1):34. | ||
Wang S, Tsui ST, Liu C, Song Y, Liu D. EGFR C797S mutation mediates resistance to third-generation inhibitors in T790M-positive non-small cell lung cancer. J Hematol Oncol. 2016;9(1):59. | ||
Khozin S, Weinstock C, Blumenthal GM, et al. Osimertinib for the treatment of metastatic EGFR T790M mutation-positive non-small cell lung cancer. Clin Cancer Res. 2017;23(9):2131–2135. | ||
Wang S, Song Y, Yan F, Liu D. Mechanisms of resistance to third-generation EGFR tyrosine kinase inhibitors. Front Med. 2016;10(4):383–388. | ||
Mori K, Kondo T, Kamiyama Y, Kano Y, Tominaga K. Preventive effect of Kampo medicine (Hangeshashin-to) against irinotecan-induced diarrhea in advanced non-small-cell lung cancer. Cancer Chemother Pharmacol. 2003;51(5):403–406. | ||
Kono T, Satomi M, Chisato N, et al. Topical application of Hangeshashinto (TJ-14) in the treatment of chemotherapy-induced oral mucositis. World J Oncol. 2010;1(6):232–235. | ||
Kase Y, Hayakawa T, Ishige A, Aburada M, Komatsu Y. The effects of Hange-shashin-to on the content of prostaglandin E2 and water absorption in the large intestine of rats. Biol Pharm Bull. 1997;20(9):954–957. | ||
Kase Y, Hayakawa T, Aburada M, Komatsu Y, Kamataki T. Preventive effects of Hange-shashin-to on irinotecan hydrochloride-caused diarrhea and its relevance to the colonic prostaglandin E2 and water absorption in the rat. Jpn J Pharmacol. 1997;75(4):407–413. | ||
Kase Y, Saitoh K, Ishige A, Komatsu Y. Mechanisms by which Hange-shashin-to reduces prostaglandin E2 levels. Biol Pharm Bull. 1998;21(12):1277–1281. | ||
Kono T, Kaneko A, Matsumoto C, et al. Multitargeted effects of hangeshashinto for treatment of chemotherapy-induced oral mucositis on inducible prostaglandin E2 production in human oral keratinocytes. Integr Cancer Ther. 2014;13(5):435–445. | ||
Logan RM, Gibson RJ, Sonis ST, Keefe DM. Nuclear factor-kappaB (NF-kappaB) and cyclooxygenase-2 (COX-2) expression in the oral mucosa following cancer chemotherapy. Oral Oncol. 2007;43(4):395–401. | ||
Arrieta O, Vega-González MT, López-Macías D, et al. Randomized, open-label trial evaluating the preventive effect of tetracycline on afatinib induced-skin toxicities in non-small cell lung cancer patients. Lung Cancer. 2015;88(3):282–288. | ||
Melosky B, Anderson H, Burkes RL, et al. Pan Canadian rash trial: a randomized phase III trial evaluating the impact of a prophylactic skin treatment regimen on epidermal growth factor receptor-tyrosine kinase inhibitor-induced skin toxicities in patients with metastatic lung cancer. J Clin Oncol. 2016;34(8):810–815. | ||
Hirsh V. Managing treatment-related adverse events associated with EGFR tyrosine kinase inhibitors in advanced non-small-cell lung cancer. Curr Oncol. 2011;18(3):126–138. | ||
Hirsh V, Blais N, Burkes R, Verma S, Croitoru K. Management of diarrhea induced by epidermal growth factor receptor tyrosine kinase inhibitors. Curr Oncol. 2014;21(6):329–336. | ||
Kato T, Yoshioka H, Okamoto I, et al. Afatinib versus cisplatin plus pemetrexed in Japanese patients with advanced non-small cell lung cancer harboring activating EGFR mutations: subgroup analysis of LUX-Lung 3. Cancer Sci. 2015;106(9):1202–1211. | ||
Uribe JM, Gelbmann CM, Traynor-Kaplan AE, Barrett KE. Epidermal growth factor inhibits Ca (2+)-dependent Cl- transport in T84 human colonic epithelial cells. Am J Physiol. 1996;271(3):914–922. | ||
Shaw AT, Solomon B. Targeting anaplastic lymphoma kinase in lung cancer. Clin Cancer Res. 2011;17(8):2081–2086. | ||
Dholaria B, Hammond W, Sherders A, Lou Y. Emerging therapeutic agents for lung cancer. J Hematol Oncol. 2016;9(1):138. | ||
Kim DW, Mehra R, Tan DSW, et al. Activity and safety of ceritinib in patients with ALK-rearranged non-small-cell lung cancer (ASCEND-1): updated results from the multicenter, open-label, phase 1 trial. Lancet Oncol. 2016;17(4):452–463. | ||
Yamashita T, Araki K, Tomifuji M, Kamide D, Tanaka Y, Shiotani A. A traditional Japanese medicine Hangeshashinto (TJ-14) alleviates chemoradiation induced mucositis and improves rates of treatment completion. Support Care Cancer. 2015;23(1):29–35. | ||
Samosorn S, Tanwirat B, Muhamad N, et al. Antibacterial activity of berberine-NorA pump inhibitor hybrids with a methylene ether linking group. Bioorg Med Chem. 2009;17(11):3866–3872. | ||
Grycová L, Dostál J, Marek R. Quaternary protoberberine alkaloids. Phytochemistry. 2007;68(2):150–175. | ||
Melosky B, Leighl NB, Rothenstein J, Sangha R, Stewart D, Papp K. Management of egfr tki-induced dermatologic adverse events. Curr Oncol. 2015;22(2):123–132. | ||
Lacouture ME, Keefe DM, Sonis S, et al. A phase II study (ARCHER 1042) to evaluate prophylactic treatment of dacomitinib-induced dermatologic and gastrointestinal adverse events in advanced non-small-cell lung cancer. Ann Oncol. 2016;27(9):1712–1718. | ||
Jatoi A, Dakhil SR, Sloan JA, et al; North Central Cancer Treatment Group. Prophylactic tetracycline does not diminish the severity of epidermal growth factor receptor (EGFR) inhibitor-induced rash: results from the North Central Cancer Treatment Group (Supplementary N03CB). Support Care Cancer. 2011;19(10):1601–1607. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



