Back to Journals » Journal of Inflammation Research » Volume 14
Preventive Effect of Flavonoid Extract from the Peel of Gonggan (Citrus reticulata Blanco Var. Gonggan) on CCl4-Induced Acute Liver Injury in Mice
Authors Wu Y, He Y , Wang R, Zhao X
Received 1 August 2021
Accepted for publication 29 September 2021
Published 5 October 2021 Volume 2021:14 Pages 5111—5121
DOI https://doi.org/10.2147/JIR.S332134
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ning Quan
Ya Wu,1,2,* Yongpeng He,3,4,* Rui Wang,1,2 Xin Zhao1
1Chongqing Collaborative Innovation Center for Functional Food, Chongqing Engineering Research Center of Functional Food, Chongqing Engineering Laboratory for Research and Development of Functional Food, Chongqing University of Education, Chongqing, 400067, People’s Republic of China; 2College of Biological and Chemical Engineering, Chongqing University of Education, Chongqing, 400067, People’s Republic of China; 3Chongqing Key Laboratory of Translational Research for Cancer Metastasis and Individualized Treatment, Chongqing University Cancer Hospital & Chongqing Cancer Institute & Chongqing Cancer Hospital, Chongqing, 400030, People’s Republic of China; 4Department of Biochemistry and Molecular Biology, College of Basic Medical Sciences, Southwest Medical University, Luzhou, 646000, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xin Zhao Tel/Fax +86-23-62653650
Email [email protected]
Objective: Citrus peel, a waste product of citrus consumption and processing, is rich in flavonoids. This study aimed to study the protective effect of flavonoid extract from the peel of gonggan (Citrus reticulata Blanco var. gonggan) on acute chemical liver injury.
Materials and Methods: We established a chemical liver injury model induced by carbon tetrachloride (CCl4) in mice. The flavonoid composition in gonggan (Citrus reticulata Blanco var. gonggan) peel was detected by HPLC. The histopathological sections of liver, related biochemical indicators in serum and liver, and related genes were examined to evaluate the protective effect of gonggan peel flavonoid extract (GPFE).
Results: The results showed that GPFE contained narirutin, hesperidin, nobiletin, tangeretin, and 5-demethylnobiletin. After 14 days of intragastric administration of GPFE, the result showed GPFE could reduce the increase in liver index, serum alanine aminotransferase (ALT), and aspartate transaminase (AST) levels caused by CCl4. At the same time, pathological sections of liver confirmed that GPFE alleviated the damage to liver tissue. Moreover, biochemical indicator results showed that GPFE increased the activities of superoxide dismutase (SOD) and catalase (CAT) in liver tissue and reduced the content of malondialdehyde (MDA). Also, it reduced the levels of inflammation factors: tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ), interleukin (IL)-1β, and IL-6. In addition, q-PCR results showed that GPFE upregulated mRNA expression levels of nuclear factor erythroid 2-related factor 2 (Nrf2), copper/zinc superoxide dismutase (SOD1), manganese superoxide dismutase (SOD2), glutathione peroxidase (GSH-Px), γ-glutamylcysteine synthetase (γ-GCS), CAT, and downregulated IL-6 and TNF-α mRNA expression levels. The mechanism of GPFE may be related to the inhibition of oxidative stress and inflammation.
Conclusion: The experiment indicates GPFE has a good protective effect on acute chemical liver injury in mice induced by CCl4 via antioxidant and anti-inflammatory pathways.
Keywords: anti-inflammation, anti-oxidative, citrus peel, liver injury
Introductions
Chemical liver injury is caused by alcohol, chemical toxic substances, drugs, and other hepatotoxic substances. It is a precursor of liver disease, and further development could cause different degrees of hepatocyte necrosis, fatty degeneration, hepatocirrhosis, liver cancer, and other serious liver diseases. It is one of the most common types of liver injury in clinical practice.1,2 Acute liver injury has the characteristics of high morbidity and mortality, which is increasing in recent years.3 Current studies indicated that the pathogenesis of liver injury mainly involves oxidative stress, mitochondrial damage, inflammation, immune regulation, and cell apoptosis, etc.4–6 The occurrence of liver injury usually involves the participation of multiple mechanisms. Therefore, it is of great significance to study the prevention and treatment of acute liver injury.
Flavonoids are common components of natural products and have various physiological activities.7 Natural flavonoids have become important effective components of anti-chemical liver injury. Studies have shown that total flavonoid extracts and monomeric compounds have good anti-chemical liver injury activity.8–10 The mechanisms are related to the inhibition of oxidative stress, inflammation, hepatocyte apoptosis, and other methods of exerting hepatoprotective effects. Citrus flavonoids are one of the main sources of dietary flavonoids and have many biological activities. Studies about the effects of citrus on liver injury are concentrated on the monomer of citrus flavonoids and citrus extract. The extracts of Citrus aurantium L., Citrus depressa, Citrus aurantium, natsumikan (Citrus natsudaidai) and shekwasha (Citrus depressa) have certain protective effect on liver injury, and the liver injury model is established by acetaminophen, alcohol, or D-galactose.11–15 Nobiletin, as a monomer of citrus flavonoids, had protective effect on lipopolysaccharide/D‑galactosamine‑induced liver injury.16 Naringin and hesperidin could inhibit liver injury induced by CCl4.17 The CCl4-induced liver injury is a typical liver injury model, and its pathophysiology is like the pathogenesis of human liver.18 The research on the effect of citrus extract on liver damage caused by CCl4 is few. Citrus peel, a waste product of citrus consumption and processing, is a more convenient source compared with the monomer. Therefore, the study of functional components from citrus peel on liver injury induced by CCl4 could can not only enrich related research but also make full use of the by-products of citrus processing and improve the added value of citrus products.19
In this study, the mouse model of acute liver injury induced by CCl4 was used. The pathological sections, serum, and liver biochemical indicators were analyzed, and the q-PCR results of related genes were explored to evaluate the protective effect and possible mechanism of gonggan peel flavonoid extract (GPFE) on acute liver injury in mice. This study would provide the experimental basis for the development of citrus peel and the research of protecting chemical liver injury.
Materials and Methods
Preparation of GPFE
The peel of gonggan (Citrus reticulata Blanco var. gonggan from Yulin City, Guangxi Zhuang Autonomous Region, China) was freeze-dried and ground into a powder. A solution of citrus peel powder (100 mg) and 80% ethanol solution with a liquid-to-material ratio of 20:1 was heated at 80°C for 4 h.20,21 To purify the crude extract, AB-8 macroreticular resin (Beijing Solarbio Science & Technology Co., Ltd., Beijing, China) was used with a 90% ethanol eluent. When the eluent became colorless, the separation was stopped and the eluates were combined, and evaporated under reduced pressure. The residue was freeze-dried and then ground into powder to be used as the source of GPFE. The total flavonoids of GPFE were obtained by detecting the absorbance at the wavelength of 500nm (Evolution 300 ultraviolet spectrophotometer, Thermo Fisher Scientific, Inc., Waltham, MA, USA) with rutin as standard substance.
Determination of GPFE Composition
Two milligrams each of narirutin, hesperidin, nobiletin, tangeretin, and 5-demethylnobiletin (Shanghai Yuanye Biological Technology Co., Ltd., China) were dissolved in methanol (2 mL, HPLC grade) separately to afford the standard solutions.
A liquid chromatography system (UltiMate3000 HPLC System, Thermo Fisher Scientific, Waltham, MA, USA) with a Welch C18 column (4.6 × 250 mm long, 5 μm) was employed. The mobile phase A was acetonitrile (HPLC grade) and mobile phase B was 0.5% glacial acetic acid aqueous solution. The mobile phase gradient was: 0 min, 12% A; 0~20 min, 25% A; 20~35 min, 45% A; 35~40 min, 100% A. The flow rate was 1.0 mL/min and column temperature was 35°C. After 10 μL test solution was injected, the chromatographic peak area was obtained at detection wavelength of 285 nm.22 The compounds in GPFE were analyzed according the chromatographic peak area of standard substance.
Animal Models and Treatment
Fifty specific pathogen-free (SPF) Kunming mice aged six weeks (20 ± 2g, male) were housed at room temperature 22± 2°C, 55± 5% humidity with 12 h light–dark cycle conditions. After one week of adaptive feeding, the mice were randomly divided into normal group, model group, silymarin group, low concentration GPFE extract group (GPFE-L group), and high concentration GPFE extract group (GPFE-H group). All mice were free to eat and drink. At the same time, the mice in silymarin group were given intragastric administration of 200 mg/kg silymarin; the mice in GPFE-L group and GPFE-H group were given intragastric administration of 150 mg/kg and 300 mg/kg GPFE separately. After 2 weeks, all mice, except the normal group, were peritoneally injected with 0.8% CCl4/olive oil mixture and the induction dose was 10 mL/kg.23,24 After fasting for 16 hours, the blood was taken from the orbit and quickly put at 4°C for 0.5 h, followed by centrifugated at 4000 rpm/min for 10 min.23,24 After the mice were sacrificed by severed neck, the liver was removed immediately and washed in normal saline. The serum and liver were stored at −80°C for further testing. Additionally, the formula: Liver index (%) = organ weight (g)/body weight of mice (g) ×100% was used to calculate the liver index.23
Histological Analysis of the Liver Tissues
The liver tissues (~0.5 cm2) were fixed in 10% formalin solution for 48 h and then embedded in paraffin. The tissues were sectioned and stained with hematoxylin and eosin to observe the pathological changes of liver tissue in each group by an optical microscope (BX43, Olympus, Tokyo, Japan).
Determination of SOD, CAT, and MDA Levels in Liver Tissues
Liver tissue (100 mg) was ground in 900 μL saline solution in ice bath, and then the homogenate was centrifugated at 10,000 rpm for 15 min at 4°C to obtain the test supernatant. The SOD, CAT, and MDA levels in liver tissue were detected by using the kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, Jiangsu, China).
The method of determining SOD is as follows. According to the kit instruction, the distilled water (20 μL) in blank experiment and supernatant of tissue homogenate (20 μL) in test experiment were added with the enzyme working solution (20 μL) or enzyme diluent (20 μL) as required. All samples were added with applied solution (200 μL). After mixing, the solution was incubated at 37°C for 20 minutes, and the absorbance was measured at 450 nm with Varioskan LUX Multimode Microplate Reader Fluoroskan (Thermo Fisher Scientific, Waltham, MA, USA).
The method of determining MDA is as follows. The reagents provided in the kit were used to prepare reagent 1, reagent 2, reagent 3, and standard solution. Ethanol (0.1 mL), standard solution (0.1 mL) and supernatant of tissue homogenate (0.1 mL) were added separately to different centrifuge tubes, followed by the addition of reagent 1(0.1 mL), reagent 2 (3 mL), and reagent 3 or 50% glacial acetic acid (1 mL) according to the instruction. Then, the mixing solution was heated at 95°C for 40 minutes, and the absorbance of the supernatant was measured at 532 nm after centrifugation. The MDA content was calculated according to the formula.
The method of determining CAT is as follows. The solution of reagent 1 (1 mL) and reagent 2 (0.1 mL) provided in the kit was mixed, then supernatant of tissue homogenate (0.05 mL) was added in test experiment, but not in blank experiment. The solution was reacted at 37°C for 1 minute. After adding reagent 3 (1 mL) and 4 (0.1 mL) in both test and blank experiment, only the blank group was added with supernatant of tissue homogenate (0.05 mL). The absorbance of the solution was measured at 405 nm.
Determination of ALT, AST, TNF-α, IFN-γ, IL-1β and IL-6 in Serum
After the serum thawed, the determination of ALT, AST, TNF-α, IFN-γ, IL-1β, and IL-6 levels in serum were carried out by using the corresponding enzyme kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, Jiangsu, China).
The method of determining ALT and AST is as follows. In the test experiment, 5 μL serum was added to matrix fluid (20 μL). In the blank experiment, matrix fluid (20 μL) without serum was present. Subsequently, the solution was heated at 37°C for 30 minutes, followed by the addition of 2,4-dinitrophenylhydrazine (20 μL). Then, serum (5 μL) was added only in the blank experiment. After the mixture was heated at 37°C for 20 minutes, 0.4 mol/L NaOH solution (200 μL) was added in two experiments. The absorbance of the solution at 510 nm was determined.
The method of determining inflammatory cytokines is as follows. Serum samples (10 μL) and diluents (40 μL) are added to the ELISA plate, followed by the conjugate reagent (100 μL). After incubating at 37°C for 60 minutes, the solution was discarded and ELISA plate was washed 5 times with the washing solution. Then, the ELISA plate was added with the chromogenic reagent and left in the dark at 37°C for 15 minutes. After termination reaction with stop solution, the absorbance of sample was measured at 450 nm to calculate the concentration of cytokine according to the standard curve.
Reverse Transcriptase Quantitative Polymerase Chain Reaction Assay
After liver tissue (100 mg) was ground in 1 mL of Trizol (Invitrogen, Carlsbad, CA, USA), 200 μL chloroform was added to the homogenate and allowed to stand at 0°C for 5 minutes. After centrifuging the homogenate at 14,000 rpm/min for 15 min, the supernatant was separated, and equal volumes of isopropanol was added. The mixed solution was allowed to stand on ice for 15 minutes, then centrifuged at 14,000 rpm/min for 20 min. After discarding the supernatant, 500 μL ethanol/DEPC water (volume ratio 3:1) was used to wash the precipitate. The solution was centrifuged and the precipitate was dissolved in 20 μL DEPC water. The RNA concentration of the sample was measured using an ultra-microspectrophotometer (Nano-100, All for Life Science, Hangzhou, Zhejiang, China). The reverse transcription kit (Tiangen Biotech Co., Ltd., Beijing, China) and 1 μL diluted total RNA (1 μg/μL) were used to synthesize a cDNA template. The q-PCR reaction system consists of SYBR Green PCR Master Mix (10 μL), the cDNA template (1 μL), and the upstream and downstream primers (Tables 1, 1 μL). The reaction was performed as follows (StepOnePlus Real-Time PCR System; Thermo Fisher Scientific): 95°C for 90 s, 40 cycles of 95°C for 30 s, 60°C for 30 s, 72°C for 30 s, then 95°C for 30 s, and 55°C for 35 s. The 2−ΔΔCt method with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as housekeeping genes was selected to calculate relative gene expression.
 |
Table 1 Sequences of Primers Used in This Study |
Statistical Analysis
The experimental data were analyzed with SPSS 17.0 (SPSS Inc., Chicago, IL, USA) and GraphPad Prism 7 statistical software (Graph Pad Software Inc., La Jolla, CA, USA). The results are expressed as mean ± standard deviation. Comparisons among groups were made by one-way analysis of variance followed by Tukey’s test. It indicated that the difference was statistically significant when P ≤ 0.05.
Results
Composition Analysis of GPFE Extract
The total flavonoids in GPFE were 20.3% with rutin as reference substance. The kind and content of flavonoid in GPFE were analyzed by HPLC (Figure 1). The HPLC results were represented as follows: narirutin (10 mg/g), hesperidin (84.9 mg/g), nobiletin (27.3mg/g), tangeretin (28.3 mg/g), 5-demethylnobiletin (8.4 mg/g).
Histopathological Examination of Liver Tissue
In Figure 2, hepatocytes of the normal group were intact and clear, and arranged radially in the center of the central vein. The hepatocyte in the model group had irregular arrangement accompanied by cellular steatosis and necrosis. Compared with the model group, hepatocyte’s arrangement in treatment group was basically orderly and the cell structure is relatively clear with few fat vacuoles. The histopathological results showed that the silymarin, GPFE could alleviate liver injury induced by CCl4 at different degrees, and the effect of high-dose-GPFE was better than that of low-dose-GPFE.
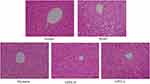 |
Figure 2 Histopathological observation of liver tissues sections in mice of the different groups after staining with hematoxylin and eosin (H&E). |
Inhibition of Liver Injury in Mice
The organ index is one of the important indicators of biological research, which reflects the degree of pathological swelling of tissue cells.25 The liver index of mice in the model group was significantly higher than that in normal group (Table 2, P < 0.05). After treatment, liver index of mice in the silymarin and GPFE-H group compared with the model group was decreased by 8.7% and 6.9%, respectively (P < 0.05), but there was no significant difference between the treatment groups. AST and ALT are the most sensitive indexes to detect liver function, and their activity level reflects the degree of liver damage. An increase of serum ALT and AST levels in model mice was observed compared with normal mice (Table 2, P < 0.05). The result showed that mouse serum ALT and AST levels were decreased by silymarin and GPFE (P < 0.05). The reduction of ALT level in silymarin, GPFE-H and GPFE-L group was 65%, 50% and 31%, respectively. And AST level in silymarin, GPFE-H and GPFE-L group was decreased by 65%, 47% and 33%, respectively. The high-dose GPFE had better performance than low-dose in decreasing ALT and AST activities.
 |
Table 2 The Liver Injury Degree in Mice |
SOD, CAT, and MDA Levels in Liver Tissues
In the model group, the result showed that CCl4 decreased SOD and CAT activities and elevated the MDA level in the liver tissue (Table 3, P < 0.05). It indicated that CCl4 caused the body’s oxidation and anti-oxidation imbalance, and the ability to clear free radicals is significantly decreased. In the treatment group, the SOD and CAT activities were increased compared with the model group (P < 0.05). The increase ratio of SOD was 113%, 123% and 91%, respectively, in the silymarin group, GPFE-H group and GPFE-L group. And increase ratio of CAT was 33%, 38%, 18%, respectively. However, MDA level was decreased by 42%, 44%, and 25%, respectively, in the silymarin group, GPFE-H group and GPFE-L group (P < 0.05). The effect of high-dose GPFE was like that of silymarin.
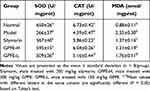 |
Table 3 Liver Tissue Levels of ALT, AST, SOD, CAT and MDA in Mice from Each Group |
Serum TNF-α, IL-6, IL-1β, and IFN-γ Levels in Mice
Table 4 reveals that the serum inflammatory cytokines TNF-α, IFN-γ, IL-1β, and IL-6 of the normal group were lowest (P < 0.05). Conversely, the inflammatory cytokine contents in liver injury mice induced by CCl4 were highest, indicating that the mice were in an inflammatory state. After gavage with GPFE and silymarin, a decrease of TNF-α, IFN-γ, IL-1β and IL-6 levels was detected compared with the model group (P < 0.05). TNF-α level in the silymarin group, GPFE-H group and GPFE-L group was decreased by 30%, 16% and 11%, respectively. The reduction of IFN-γ level was 31%, 31% and 24%, respectively. IL-1β level in the silymarin group, GPFE-H group and GPFE-L group was decreased by 24%, 34% and 19%, respectively. Similarly, IL-6 level was reduced by 35%, 35%, and 32%, respectively. The result indicated GPFE and silymarin efficiently alleviated the inflammatory response.
 |
Table 4 Serum Levels of TNF-α, IL-1β, IL-6, and IFN-γ in Mice of Each Groups |
Nrf2, SOD1, SOD2, GSH-Px, CAT, γ-GCS, IL-6 and TNF-α mRNA Expression Levels in Liver Tissue
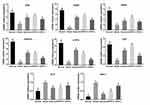
To explore the possible mechanism of GPFE, the mRNA expression of antioxidant-related genes was examined, including Nrf2, SOD1, SOD2, γ-GCS, GSH-Px, and CAT. Also, the inflammatory genes IL-6 and TNF-α were detected. CCl4 suppressed the mRNA expression levels of Nrf2, SOD1, SOD2, γ-GCS, GSH-Px, and CAT, and enhanced the mRNA expressions of IL-6 and TNF-α in liver injury mice compared with the normal mice (P < 0.05, Figure 3). After intervention with GPFE and silymarin, the mRNA expression of Nrf2, SOD1, SOD2, γ-GCS, GSH-Px, and CAT were upregulated, simultaneously IL-6 and TNF-α mRNA expressions were downregulated. GPFE had better effect than silymarin in improving the mRNA expression of SOD and CAT, which was basically consistent with the previous experiment.
Discussion
Citrus flavonoids play a critical role in plant flavonoids. At present, most of the flavonoids found in citrus exist in the form of glycosides, mainly flavanones, flavones and flavonols.19,20 The content and kind of flavonoids are different in different varieties of citrus fruits. Flavanones are the highest content of flavonoids in the citrus. The common aglycones are hesperetin and naringenin, which are mainly in the form of glycosides.19,20 The glycosides could be divided into rutinoside and neohesperidoside. Rutinoside includes hesperidin, narirutin, and didymin.19,20 Neohesperidoside includes neohesperidin and naringin.26 The common flavones include nobiletin, tangeretin, luteolin, diosmetin and so on. The common flavonols are quercetin, rutin, kaempferol and so on. HPLC analysis of the components of GPFE included narirutin, hesperidin, nobiletin, tangeretin and 5-demethylnobiletin, which was consistent with common citrus flavonoids. In the investigation of dietary flavonoid intake and disease, it was found that people who consume about 500 mg of flavonoids a day have the low risk of cancer or cardiovascular disease.27 Above the 500 mg, there are no additional benefits. Based on this study, 500 mg was selected as the reference value for the intake of flavonoids. The total flavonoids in GPFE were 20.3%. For dose conversion, the weight of adults is 70 kg and that of mice is 20g. The dose of GPFE in mice was about 300 mg/kg, which was calculated by dose conversion and the flavonoids content in GPFE. Therefore, we finally chose the high dose is 300 mg/kg and the low dose is 150 mg/kg.
CCl4 could be quickly absorbed by the liver after intake and is metabolized by hepatic cytochrome P450 to generate trichloromethyl radical (CCl3·), trichloromethyl peroxide (OOCCl3·), and a series of reactive oxygen species (ROS), which cause serious damage to hepatocytes.28,29 At the same time, CCl4 induce liver inflammation by activating Kupffer cells to release pro-inflammatory factors. Under the combined action of lipid peroxidation and pro-inflammatory factors, the hepatocyte becomes necrotic.28,29 When the hepatocyte is damaged, ALT and AST, which are mainly distributed in the liver, are released resulting in the serum levels of those increasing. So, serum ALT and AST levels are often used as liver function test indicators to determine whether the liver is damaged.30 The experimental results showed that GPFE significantly reduced the serum ALT and AST levels in liver injury mice. Moreover, the results of histologic sections and liver index showed that it alleviated the degree of hepatocyte lesions.
Oxidative stress caused by free radicals is one of the important mechanisms of liver damage induced by CCl4.28,29 MDA, as a product of lipid peroxidation, destroys the membrane structure, causing swelling and necrosis of hepatocytes.31 SOD and CAT are important antioxidant enzymes in the body. Superoxide radicals are converted into hydrogen peroxide by SOD, and CAT further degrades hydrogen peroxide into water.31 In this study, the level of MDA in mice of the model group was increased, and SOD and CAT activities were inhibited. The pretreatment of GPFE could significantly inhibit MDA content and enhance SOD and CAT activities, which indicated that GPFE alleviated the oxidative stress in mice with acute liver injury.
In CCl4-induced liver injury, inflammation is another important pathological mechanism. CCl4-mediated oxidative stress could activate inflammatory cells, causing them to release excessive pro-inflammatory factors, such as TNF-α and IL-6.32,33 TNF-α is an important inflammatory cytokine that could activate neutrophils to release proteases and oxygen-free radicals, causing hepatocyte apoptosis or necrosis.34 In the occurrence and development of inflammation, IL-6 promotes the activation of lymphocytes, induces endothelial cells to produce chemokines, and promotes the infiltration of inflammatory cells.35 IL-1β can not only cause severe inflammation by itself but also induce the expression of inflammatory factors, to participate in acute inflammation.36 IFN-γ activates macrophages and promotes the secretion of IL-1, TNF-α, and IL-6 to regulate inflammatory response.37,38 Experimental results showed that GPFE effectively reduced TNF-α, IL-6, IL-1β, and IFN-γ levels in liver injury mice to inhibit inflammation.
The anti-inflammatory activity of citrus flavonoids is closely related to its structure. Flavonoids with 3- or 4-hydroxy substituents on the B ring could be used as selective lipoxygenase inhibitors, while flavonoids with 5 or more methoxy substituents have higher phosphodiesterase inhibitory activity.39 Hesperidin achieves anti-inflammatory effect by inhibiting the synthesis and biological activity of different pro-inflammatory mediators such as arachidonic acid derivatives, prostaglandin E2 and F2.40 Hesperidin can be used as a lead drug for highly specific anti-inflammatory drugs against phospholipase A2 isoenzymes for clinical inflammation treatment.41 Nobiletin inhibited lipopolysaccharide (LPS)-induced TNF-α and IL-1β production in microglia by NF-κB signal pathway.42 Three polymethoxyflavones (nobiletin, tangeretin and 5-demethylnobiletin) offered the collective effect on inhibiting the secretion of NO, TNF-α, IL-1β and IL-6 induced by LPS, which possesses potent anti-neuroinflammatory capacity.43 Subsequent studies found that the mechanism of three polymethoxyflavones may be related to the regulation of JAK2/STAT3 pathway.44
Numerous studies have shown that the Nrf2/ARE signal pathway has an important role in resisting oxidative stress and inflammation. When the body experiences oxidative stress under the influence of various factors, the Nrf2 signaling pathway is activated, and then regulates the downstream gene expression, including γ-GCS, SOD, CAT, GSH-Px.45 In addition, related literature reported that the degree of liver injury is obviously severe, and related inflammatory factors (IL-1α, TNF-α, IFN-γ) are significantly increased in liver fibrosis mice induced by CCl4 with Nrf2 gene deletion.46 It suggested that Nrf2 signaling pathway was closely related to the inflammatory response. The experimental results showed that GPFE enhanced Nrf2 mRNA expression level, then the mRNA expression of SOD1, γ-GCS, SOD2, CAT, and GSH-Px were upregulated by Nrf2 pathway. Meanwhile, IL-6 and TNF-α mRNA expressions were downregulated, indicating that the GPFE could relieve liver injury induced by CCl4 via inhibiting oxidative stress and inflammation. According to previous reports, hesperidin activates the Keap1-Nrf2-ARE signaling pathway to improve the antioxidant capacity.47,48 At the same time, the extracts of nobiletin and tangeretin could also protect liver injury caused by alcohol through the Nrf2 pathway.13 The contents of these flavonoids are high in GPFE, which is why GPFE may protect the liver through the Nrf2 pathway.
Recently, research has found that dietary flavonoids have a good ability to prevent and treat liver injury and its complications. Narirutin fraction from citrus peels attenuates liver injury induced by alcohol via protecting the antioxidant system and inhibiting the production of pro-inflammatory cytokines.49 Hesperidin could regulate Nrf2, PPAR-γ, and TGF-β1/Smad3 signals to suppress oxidative stress and inflammation, thereby protecting the occurrence of chemically induced liver cancer.50 The phytochemicals nobiletin, tangeretin, and 5-demethylnobiletin are the representative polymethoxyflavones found in citrus peels. Through activating the Nrf2 antioxidant pathway and subsequently inhibiting NF-κB-mediated cytokine production, nobiletin exerts a protective effect in LPS/GALN-induced acute liver injury.16 Tangeretin attenuates oxidative damage in HepG2 cells induced by tert-butyl hydroperoxide through the MAPK-Nrf2-ARE signaling pathway to upregulate the antioxidant enzymes NQO1 and HO-1.51 5-Demethylnobiletin could effectively inhibit CCl4-induced ROS production, cell proliferation, and inflammation to relieve acute liver injury.52 The flavonoids contained in GPFE, as active ingredients against liver injury, may work synergistically with each other to offer strong positive effects on acute liver injury.
Conclusion
In this study, we found that GPFE could effectively reduce liver index, liver function-related index enzymes, and inflammatory cytokines in mice with liver injury caused by CCl4. At the same time, it can effectively improve the antioxidant capacityand improve the pathological state of liver injury. The GPFE have a good protective effect on the acute chemical liver injury caused by CCl4. The mechanism of GPFE may be associated with the inhibition of oxidative stress and inflammation through the Nrf2 signaling pathway. This experiment provides research considerations for the development of citrus peel and the basis for developing health food to protect against chemical liver injury. However, because citrus peel extract is used in this study, the exact physiological effects of flavonoids monomer and the in-depth mechanism of GPFE need to be further studied.
Ethical Statement
This study was approved by the Ethics Committee of Chongqing Collaborative Innovation Center for Functional Food (20210132B, Chongqing, China) and followed the national standard of the People’s Republic of China (GB/T 35892-2018) laboratory animal-guidelines for ethical review of animal welfare.
Acknowledgments
This research was supported by the Chongqing University Innovation Research Group Project (CXQTP20033), Research Program of Chongqing University of Education (KY201908B), Science and Technology Project of Chongqing Education Commission (KJ1501415) and Chongqing Kewei Joint Medical Research Project (2021MSXM337).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Fyfe B, Zaldana F, Liu C. The pathology of acute liver failure. Clin Liver Dis. 2018;22(2):257–268. doi:10.1016/j.cld.2018.01.003
2. Gu X, Manautou JE. Molecular mechanisms underlying chemical liver injury. Expert Rev Mol Med. 2012;14:e4. doi:10.1017/S1462399411002110
3. Bernal W, Auzinger G, Dhawan A, et al. Acute liver failure. Lancet. 2010;376(9736):190–201. doi:10.1016/S0140-6736(10)60274-7
4. Tipoe GL, Leung TM, Liong EC, et al. Epigallocatechin-3-gallate (EGCG) reduces liver inflammation, oxidative stress and fibrosis in carbon tetrachloride (CCl4)-induced liver injury in mice. Toxicology. 2010;273(1–3):45–52. doi:10.1016/j.tox.2010.04.014
5. Liu Y, Wen PH, Zhang XX, et al. Breviscapine ameliorates CCl4-induced liver injury in mice through inhibiting inflammatory apoptotic response and ROS generation. Int J Mol Med. 2018;42(2):755–768. doi:10.3892/ijmm.2018.3651
6. Xu T, Zheng L, Xu L, et al. Protective effects of dioscin against alcohol-induced liver injury. Arch Toxicol. 2014;88(3):739–753. doi:10.1007/s00204-013-1148-8
7. Huang WY, Cai YZ, Zhang Y. Natural phenolic compounds from medicinal herbs and dietary plants: potential use for cancer prevention. Nutr Cancer. 2010;62(1):1–20. doi:10.1080/01635580903191585
8. Sobeh M, Mahmoud MF, Petruk G, et al. Syzygium aqueum: a polyphenol- rich leaf extract exhibits antioxidant, hepatoprotective, pain-killing and anti-inflammatory activities in animal models. Front Pharmacol. 2018;9:566. doi:10.3389/fphar.2018.00566
9. Xie W, Wang M, Chen C, et al. Hepatoprotective effect of isoquercitrin against Acetaminophen-induced liver injury. Life Sci. 2016;152:180–189. doi:10.1016/j.lfs.2016.04.002
10. Sobeh M, Youssef FS, Esmat A, et al. High resolution UPLC-MS/MS profiling of polyphenolics in the methanol extract of Syzygium samarangense leaves and its hepatoprotective activity in rats with CCl4-induced hepatic damage. Food Chem Toxicol. 2018;113:145–153. doi:10.1016/j.fct.2018.01.031
11. Shu Y, He D, Li W, et al. Hepatoprotective effect of Citrus aurantium L. against APAP-induced liver injury by regulating liver lipid metabolism and apoptosis. Int J Biol Sci. 2020;16(5):752–765. doi:10.7150/ijbs.40612
12. Lee EY, Kim SH, Chang SN, et al. Efficacy of polymethoxylated flavonoids from Citrus depressa extract on alcohol-induced liver injury in mice. Biotechnol Bioproc E. 2019;24:907–914. doi:10.1007/s12257-019-0310-4
13. Choi BK, Kim TW, Lee DR, et al. A polymethoxy flavonoids-rich Citrus aurantium extract ameliorates ethanol-induced liver injury through modulation of AMPK and Nrf2-related signals in a binge drinking mouse model. Phytother Res. 2015;29(10):1577–1584. doi:10.1002/ptr.5415
14. Yamaura K, Nakayama N, Shimada M, et al. Protective effects of natsumikan (Citrus natsudaidai) extract on Acetaminophen-induced lethal hepatotoxicity in mice. Pharmacognosy Res. 2012;4(4):234–236. doi:10.4103/0974-8490.102274
15. Akachi T, Shiina Y, Ohishi Y, et al. Hepatoprotective effects of flavonoids from shekwasha (Citrus depressa) against D-galactosamine-induced liver injury in rats. J Nutr Sci Vitaminol (Tokyo). 2010;56(1):60–67. doi:10.3177/jnsv.56.60
16. He Z, Li X, Chen H, et al. Nobiletin attenuates lipopolysaccharide/D‑galactosamine‑induced liver injury in mice by activating the Nrf2 antioxidant pathway and subsequently inhibiting NF‑κB‑mediated cytokine production. Mol Med Rep. 2016;14(6):5595–5600. doi:10.3892/mmr.2016.5943
17. Dong D, Xu L, Yin L, et al. Naringin prevents carbon tetrachloride-induced acute liver injury in mice. J Funct Foods. 2015;12:179–191. doi:10.1016/j.jff.2014.11.020
18. Zarezade V, Moludi J, Mostafazadeh M, et al. Antioxidant and hepatoprotective effects of Artemisia dracunculus against CCl4-induced hepatotoxicity in rats. Avicenna J Phytomed. 2018;8(1):51–62.
19. Tripoli E, Guardia ML, Giammanco S, et al. Citrus flavonoids: molecular structure, biological activity and nutritional properties: a review. Food Chem. 2007;104(2):466–479. doi:10.1016/j.foodchem.2006.11.054
20. Sharma K, Mahato N, Lee Y. Extraction, characterization and biological activity of citrus flavonoids. Rev Chem Eng. 2019;35:265–284. doi:10.1515/revce-2017-0027
21. Prasad KN, Kong KW, Ramanan RN, et al. Determination and optimization of flavonoid and extract yield from brown Mango using response surface methodology. Sep Sci Technol. 2012;47(1):73–80. doi:10.1080/01496395.2011.606257
22. Wu Y, Sun H, Yi R, et al. Malus hupehensis leaves extract attenuates obesity, inflammation, and dyslipidemia by modulating lipid metabolism and oxidative stress in high-fat diet-induced obese mice. J Food Biochem. 2020;44:e13484. doi:10.1111/jfbc.13484
23. Wang R, Yang Z, Zhang J, et al. Liver injury induced by carbon tetrachloride in mice is prevented by the antioxidant capacity of Anji White Tea polyphenols. Antioxidants. 2019;8(3):64. doi:10.3390/antiox8030064
24. Niu X, Liu F, Li W, et al. Hepatoprotective effect of fraxin against carbon tetrachloride-induced hepatotoxicity in vitro and in vivo through regulating hepatic antioxidant, inflammation response and the MAPK-NF-κB signaling pathway. Biomed Pharmacother. 2017;95:1091–1102. doi:10.1016/j.biopha.2017.09.029
25. Liu B, Li J, Yi R, et al. Preventive effect of alkaloids from Lotus plumule on acute liver injury in mice. Foods. 2019;8(1):36. doi:10.3390/foods8010036
26. Gao Z, Gao W, Zeng SL, et al. Chemical structures, bioactivities and molecular mechanisms of citrus polymethoxyflavones. J Funct Foods. 2018;40:498–509. doi:10.1016/j.jff.2017.11.036
27. Bondonno NP, Dalgaard F, Kyrø C, et al. Flavonoid intake is associated with lower mortality in the Danish diet cancer and health cohort. Nat Commun. 2019;10(1):3651. doi:10.1038/s41467-019-11622-x
28. Clawson GA. Mechanisms of carbon tetrachloride hepatotoxicity. Pathol Immunopathol Res. 1989;8(2):104–112. doi:10.1159/000157141
29. Jia R, Cao LP, Du JL, et al. Effects of carbon tetrachloride on oxidative stress, inflammatory response and hepatocyte apoptosis in common carp (Cyprinus carpio). Aquat Toxicol. 2014;152:11–19. doi:10.1016/j.aquatox.2014.02.014
30. Weber LW, Boll M, Stampfl A. Hepatotoxicity and mechanism of action of haloalkanes: carbon tetrachloride as a toxicological model. Crit Rev Toxicol. 2003;33(2):105–136. doi:10.1080/713611034
31. Birben E, Sahiner UM, Sackesen CE, et al. Oxidative stress and antioxidant defense. World Allergy Organ J. 2012;5(1):9–19. doi:10.1097/WOX.0b013e3182439613
32. Kiso K, Ueno S, Fukuda M, et al. The role of Kupffer cells in carbon tetrachloride intoxication in mice. Biol Pharm Bull. 2012;35(6):980–983. doi:10.1248/bpb.35.980
33. Ren X, Li X, Jia L, et al. A small-molecule inhibitor of NF-κB-inducing kinase (NIK) protects liver from toxin-induced inflammation, oxidative stress, and injury. FASEB J. 2017;31(2):711–718. doi:10.1096/fj.201600840R
34. Sato A, Nakashima H, Nakashima M, et al. Involvement of the TNF and FasL produced by CD11b Kupffer cells/macrophages in CCl4-induced acute hepatic injury. PLoS One. 2014;9(3):e92515. doi:10.1371/journal.pone.0092515
35. Mohammadi S, Karimzadeh Bardei L, Hojati V, et al. Anti-inflammatory effects of curcumin on insulin resistance index, levels of interleukin-6, C-reactive Protein, and liver histology in polycystic ovary syndrome-induced rats. Cell J. 2017;19(3):425–433. doi:10.22074/cellj.2017.4415
36. Negash AA, Ramos HJ, Crochet N, et al. IL-1β production through the NLRP3 inflammasome by hepatic macrophages links hepatitis C virus infection with liver inflammation and disease. PLoS Pathog. 2013;9(4):e1003330. doi:10.1371/journal.ppat.1003330
37. Wesemann DR, Benveniste EN. STAT-1 alpha and IFN-gamma as modulators of TNF-alpha signaling in macrophages: regulation and functional implications of the TNF receptor 1: STAT-1alpha complex. J Immunol. 2003;171(10):5313–5319. doi:10.4049/jimmunol.171.10.5313
38. He Y, Sun Q. IFN-γ induces upregulation of TNF-α, downregulation of MMP-2 and MMP-9 expressions in abortion rat. Eur Rev Med Pharmacol Sci. 2018;22(15):4762–4767. doi:10.26355/eurrev_201808_1560
39. Chen XM, Tait AR, Kitts DD. Flavonoid composition of Orange peel and its association with antioxidant and anti-inflammatory activities. Food Chem. 2017;218:15–21. doi:10.1016/j.foodchem.2016.09.016
40. Manthey JA, Grohmann K, Guthrie N. Biological properties of citrus flavonoids pertaining to cancer and inflammation. Curr Med Chem. 2001;8(2):135–153. doi:10.2174/0929867013373723
41. Abhithaj J, Arun KG, Sharanya CS, et al. Isozymes inhibited by active site blocking: versatility of calcium indifferent hesperidin binding to phospholipase A2 and its significance. J Recept Signal Transduct Res. 2019;39(1):60–66. doi:10.1080/10799893.2019.1606239
42. Cui Y, Wu J, Jung SC, et al. Anti-neuroinflammatory activity of nobiletin on suppression of microglial activation. Biol Pharm Bull. 2010;33(11):1814–1821. doi:10.1248/bpb.33.1814
43. Ho SC, Kuo CT. Hesperidin, nobiletin, and tangeretin are collectively responsible for the anti-neuroinflammatory capacity of tangerine peel (Citri reticulatae pericarpium). Food Chem Toxicol. 2014;71:176–182. doi:10.1016/j.fct.2014.06.014
44. Wang Y, Zang W, Ji S, et al. Three polymethoxyflavones purified from Ougan (Citrus reticulata Cv. Suavissima) inhibited LPS-induced NO elevation in the neuroglia BV-2 cell line via the JAK2/STAT3 pathway. Nutrients. 2019;11(4):791. doi:10.3390/nu11040791
45. Zhang H, Davies KJA, Forman HJ. Oxidative stress response and Nrf2 signaling in aging. Free Radic Biol Med. 2015;88(PtB):314–336. doi:10.1016/j.freeradbiomed.2015.05.036
46. More VR, Cheng Q, Donepudi AC, et al. Alcohol cirrhosis alters nuclear receptor and drug transporter expression in human liver. Drug Metab Dispos. 2013;41(5):1148–1155. doi:10.1124/dmd.112.049676
47. Liu WY, Liou SS, Hong TY, et al. Protective effects of hesperidin (Citrus Flavonone) on high glucose induced oxidative stress and apoptosis in a cellular model for diabetic retinopathy. Nutrients. 2017;9(12):1312. doi:10.3390/nu9121312
48. Hong Y, An Z. Hesperidin attenuates learning and memory deficits in APP/PS1 mice through activation of Akt/Nrf2 signaling and inhibition of RAGE/NF-κB signaling. Arch Pharm Res. 2018;41(6):655–663. doi:10.1007/s12272-015-0662-z
49. Park HY, Ha SK, Eom H, et al. Narirutin fraction from citrus peels attenuates alcoholic liver disease in mice. Food Chem Toxicol. 2013;55:637–644. doi:10.1016/j.fct.2013.01.060
50. Mahmoud AM, Mohammed HM, Khadrawy SM, et al. Hesperidin protects against chemically induced hepatocarcinogenesis via modulation of Nrf2/ARE/HO-1, PPARγ and TGF-β1/Smad3 signaling, and amelioration of oxidative stress and inflammation. Chem Biol Interact. 2017;277:146–158. doi:10.1016/j.cbi.2017.09.015
51. Liang F, Fang Y, Cao W, et al. Attenuation of tert-butyl hydroperoxide (t-BHP)-induced oxidative damage in HepG2 cells by tangeretin: relevance of the Nrf2-ARE and MAPK signaling pathways. J Agric Food Chem. 2018;66(25):6317–6325. doi:10.1021/acs.jafc.8b01875
52. Chang SN, Kim SH, Dey DK, et al. 5-O-Demethylnobiletin alleviates CCl4-induced acute liver injury by equilibrating ROS-mediated apoptosis and autophagy induction. Int J Mol Sci. 2021;22(3):1083. doi:10.3390/ijms22031083
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.