Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 15
Prevention of Acute Exacerbation in Subjects with Moderate-to-very Severe COPD by Modulating Lower Respiratory Microbiome: Protocol of a Prospective, Multicenter, Randomized Controlled Trial
Authors Hua J, Hu W , Zuo Y, Zhang J
Received 26 July 2020
Accepted for publication 26 October 2020
Published 17 November 2020 Volume 2020:15 Pages 2985—2990
DOI https://doi.org/10.2147/COPD.S274005
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Richard Russell
Jian-lan Hua, Wei-ping Hu, Yi-hui Zuo, Jing Zhang
Department of Pulmonary and Critical Care Medicine, Zhongshan Hospital, Shanghai Medical College, Fudan University, Shanghai, People’s Republic of China
Correspondence: Jing Zhang
Department of Pulmonary and Critical Care Medicine, Zhongshan Hospital, Shanghai Medical College, Fudan University, 180 Feng Lin Road, Shanghai 200032, People’s Republic of China
Email [email protected]
Background: COPD is a global respiratory disease that has produced a worldwide health care burden. Acute exacerbation of COPD (AECOPD) is the leading cause of death in patients with COPD and accounts for the majority of expenditure of COPD management. The colonization of potential pathogenic bacteria in the lower respiratory tract is an important cause of the acute exacerbation especially in patients with moderate and severe COPD. Some clinical studies have shown the potential of oral probiotics, aerosol-inhaled amikacin and combined vaccination to prevent AECOPD.
Methods and Analysis: We hypothesize that patients with stable COPD will benefit from aerosol-inhaled amikacin, oral probiotics or combined vaccination in terms of preventing acute exacerbation of COPD, slowing the progression of the disease and improving their quality of life. The trial aimsto investigate the efficacy and safety of the above interventions to decolonize bacteria in the lower respiratory tract and prevent acute exacerbation of COPD. In the study, 144 patients with stable phase of moderate-to-very severe COPD will be recruited and randomized into aerosol-inhaled amikacin group, oral probiotics group, combined vaccination group and the control group at a 1:1:1:1 ratio. The primary outcome is time to the first COPD exacerbation. Other endpoints include colonization of potential pathogenic bacteria in induced sputum, microbiome in induced sputum, pulmonary function and symptoms of patients, inflammation level and adverse events, serious adverse events, and death.
Keywords: chronic obstructive pulmonary disease, probiotics, amikacin, vaccination
Introduction
COPD is a global respiratory disease that has produced a worldwide health-care burden. It is a chronic respiratory disease characterized by incomplete reversible airflow limitation, small airway obstruction, and alveolar structural damage.1 About three million patients die from COPD every year around the world. Chronic noncommunicable diseases including COPD are projected to impose a worldwide burden of 47 trillion health dollars by 2030.2 The prevalence of COPD in Chinese people over the age of 40 is as high as 12% and has risen by 50% in the past nine years.3
Acute exacerbation of COPD (AECOPD) is the leading cause of death in patients with COPD, and accounts for the majority of expenditure of COPD management. Each COPD patient suffers from 0.5 to 3.5 episodes of acute exacerbations on average per year, and AECOPD is a major cause of death in COPD patients. The studies in China have shown that the average hospital costs of every AECOPD hospitalization for each patient was as high as 11,598 RMB.4 The colonization of potential pathogenic bacteria in the lower respiratory tract is an important cause of the acute exacerbation especially in patients with moderate and severe COPD.5–8
Some clinical studies show that aerosol-inhaled antibiotics are promising in reducing acute exacerbation,9 but these exploratory studies have some defects so that they cannot back up the clinical application of aerosol-inhaled antibiotics. Amikacin, a kind of aminoglycoside, is sensitive to the common pathogens of acute exacerbation of COPD in China.10 Therefore it is necessary to carry out a clinical trial to verify its efficacy and safety in pathogen decolonization and prevention of acute exacerbation. In addition, owing to bacterial resistance and insufficient antibiotic concentration in the lower respiratory tract, developing new strategies is necessary. Oral probiotics are another potential way to regulate the bacterial load and inflammatory response in the lower respiratory tract, which might have clinical benefits for school children suffering from asthma as well as prevent nosocomial pneumonia suggested by some research.11,12 Likewise, airway bacterial burden and inflammation are two main mechanisms of acute exacerbation in COPD. Hence, considering that it is convenient and the safety of oral probiotics, we performed a trial for clinical evaluation. What is more, influenza and Streptococcus pneumoniae vaccines are separately recommended for patients with COPD in the Global Initiative for Chronic Obstructive Pulmonary Disease (GOLD),13 but there have been no studies on the effects of combined vaccination on bacterial load in the lower respiratory tract or on the prevention of acute exacerbation.
Effects of these above methods on the decolonization of potential pathogenic bacteria in the lower respiratory tract and the inflammatory reaction are not clear. Therefore, we planto perform a multicenter, prospective, randomized, controlled trial to investigate the efficacy and safety of aerosol-inhaled amikacin, oral probiotics, and combined vaccination to modulating bacterial load and compositions in the lower respiratory tract and prevent acute exacerbation of COPD. The trial is also designed to analyze the relationship between microbial changes in the lower respiratory tract, the level of inflammatory mediators, the frequency and severity of acute exacerbations to reveal the underlying mechanisms.
Methods and Analysis
Trial Design
This is a multicenter, parallel, prospective, randomized, controlled trial. We hypothesize that patients with stable COPD will benefit from aerosol-inhaled amikacin, oral probiotics or combined vaccination in terms of preventing acute exacerbation of COPD, slowing the progression of the disease and improving quality of life. The trial aimsto investigate the efficacy and safety of aerosol-inhaled amikacin, oral probiotics, or combined vaccination to decolonize bacteria in lower respiratory tract and prevent acute exacerbation of COPD. The primary outcome is time to the first COPD exacerbation. Other endpoints include colonization of potential pathogenic bacteria in induced sputum, microbiome in induced sputum, forced expiratory volume in 1 second (FEV1), COPD assessment test (CAT) score, modified Medical Research Council (mMRC) scale, serum C-reactive protein (CRP) levels, IL-6 in induced sputum, IL-8 in induced sputum, IL-1β in induced sputum and adverse events, serious adverse events, and death.
The trial has been approved in the Ethics Committee of Zhongshan Hospital of Fudan University (B2017-197R) and registered at Clinical Trials (NCT03449459).
Inclusion Criteria
The subjects will be enrolled and randomized into the study group if all of the following criteria are met: (1) written informed consent must be obtained before any assessment is performed; (2) male or female adults aged 18–80 years; (3) diagnosed with COPD according to GOLD 2019 (The ratio of postbronchodilator (salbutamol 400 μg) FEV1 to force vital capacity (FVC) <0.70); (4) moderate-to-very severe airflow limitation (postbronchodilator FEV1 <80% of the predicted value); (5) a documented history of at least two COPD exacerbation in the previous 12 months that required treatment with systemic glucocorticoids and/or antibiotics, or at least one exacerbation in the previous 12 months that required hospitalization; (6) in the stable stage of COPD.
Exclusion Criteria
Exclusion criteria include: (1) patients who have clinically significant and chronic hepatic, renal and gastrointestinal abnormalities or malignant tumor (except for lung cancer) which could interfere with the assessment of the efficacy and safety of the study treatment; (2) patients who are in critical conditions; (3) patients who have had a COPD exacerbation that required treatment with antibiotics and/or systemic corticosteroids or an acute exacerbation of any other diseases in the four weeks prior to screening; (4) patients with concomitant pulmonary disease including, but not limited to, bronchiectasis, interstitial lung disease, asthma; (5) patients who are highly likely to be lost during the three-month treatment and the one-year follow up; (6) pregnant or nursing (lactating) women; (7) patients who have been vaccinated against influenza in the current year, or against S. pneumoniae within five years, or have vaccination contraindications; (8) patients who are allergic to amikacin or other aminoglycosides; (9) patients who have participated in any interventional clinical trials in the three months prior to screening; (10) patients with mental diseases or cognitive disorders which could interfere with treatment and follow-up; (11) patients with long-term use of oral corticosteroids; (12) patients with α-1 antitrypsin deficiency.
Study Outline
The flow chart of the study design is shown in Figure 1.
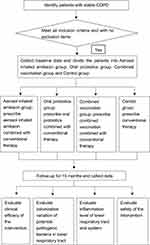 |
Figure 1 Flow chart of the study. Patients’ recruitment, intervention, visits and data processing are described in the figure. |
The study will recruit 144 patients who are compliant with the inclusion criteria and without the exclusion criteria. After enrollment, the participants will be randomly allocated into oral probiotics group, aerosol-inhaled amikacin group, combined vaccination group and a control group at a 1:1:1:1 ratio. All subjects will be followed up for 15 months. Six visits will be performed at baseline, three, six, nine, twelve, and fifteen months after enrollment.
Randomization
Enrollment will be performed at The First Affiliated Hospital of Guangzhou Medical University, Ruijin Hospital of Shanghai Jiao Tong University, Zhongshan Hospital of Fudan University. After enrollment, patients will be assigned into four groups in a 1:1:1:1 ratio to oral probiotics group, aerosol-inhaled amikacin group, combined vaccination group and a control group by random number table method. The random number table will be generated by SPSS in central unit.
Interventions
All the participants will be managed by appropriate medication including, but not limited to, bronchodilators, inhaled glucocorticoids, and long-term oxygen therapy, according to the subjects’ personal characteristics and guidance of The Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2019.13
For control group, subjects will be prescribed long-acting muscarinic antagonists (LAMA) or long-acting beta 2 agonists/long-acting muscarinic antagonists (LABA/LAMA) or inhaled corticosteroid/long-acting beta 2 agonists (ICS/LABA) or LAMA/LABA/ICS according to GOLD report13 and tobacco cessation support will be provided. Subjects of the other groups will be given corresponding additional treatment combined with the abovementioned conventional therapy.
For the oral probiotics group, in addition,subjects will be given Culturelle™ Digestive Health 30 CT (VCAP) (10 billion claim) which consists of 100% Lactobacillus rhamnosus GG (one tablet, once a day) for three months. For the aerosol-inhaled amikacin group, in addition subjects will be given 0.4 g amikacin sulfate injection configured with 5 mL saline in the form of aerosol inhalation intermittently for three months (b.i.d., 5–7 days per month). In order to observe and cope with adverse events timely, subjects will be admitted to the ward during the course of nebulized amikacin. For the combined vaccination group, influenza vaccine recommended by the World Health Organization (WHO) in that year and imported 23-Valent Pneumococcal Polysaccharide Vaccine approved by China Food and Drug Administration (CFDA) will be vaccinated by professional nurses. The interval between two vaccinations is three-to-five days to avoid the overlap of adverse events.
Endpoints
The primary endpoint is time to the first moderate-to-severe COPD exacerbation. COPD exacerbation refers to deterioration of patients’ daily symptoms requiring treatment with antibiotics or systemic glucocorticoid therapy.
Secondary endpoints include colonization of potential pathogenic bacteria in induced sputum, microbiome in induced sputum, FEV1, CAT score, mMRC scale, serum CRP levels, IL-6 in induced sputum, IL-8 in induced sputum, IL-1β in induced sputum and number of patients with adverse events, serious adverse events, and death.
Haemophilus influenzae, S. pneumoniae, Moraxella catarrhalis and Pseudomonas aeruginosa, which are the top four bacteria involved in acute exacerbation of COPD, are defined as potential pathogenic bacteria. Colonized bacteria in the lower respiratory tract is defined as the colony number over 100 cfu/mL for the indicated bacteria in the induced sputum sampled in the stable stage. Bacterial genomic DNA will be isolated from sputum and the 16S bacterial ribosomal RNA genes will be PCR-amplified with the appropriate controls against reagent contamination. Amplified DNA fragments will be sequenced using the specific sequencing platform. Sequencing reads will be processed and analyzed by the specific algorithm and software. The composition and diversity of microbiome are represented by major taxonomic groups at both phylum and genus levels. If necessary, quantitative PCR for 16S rRNA gene will be performed to validate the results of sequencing. The microbiome’s composition and its shift will then be analyzed based on these data.
Pulmonary function assessments will be performed using centralized spirometry according to international standards. FEV1 will be measured after 15 min of inhaling salbutamol 400 μg.
The COPD Assessment Test (CAT) is an eight-item unidimensional measure of health status impairment in COPD, containing six grades from 0 to 5. The modified Medical Research Council scale (mMRC) is a simple and powerful tool to evaluate the breathlessness, containing five grades from 0 to 4. CAT and mMRC will be both utilized to evaluate the symptoms of patients.
Local and systemic inflammation will be evaluated by the sputum levels of IL-6, IL-8 and IL-1β, and serum CRP, respectively.
The overall rate of adverse events will report from initiation of intervention to the last follow-up visit. Laboratory examinations, including routine blood test, hepatorenal function and electrocardiogram, will be performed at baseline and completion of intervention (three months) in order to evaluate the safety of interventions.
Statistical Analysis
Statistical analyses will be performed using SPSS 22.0. Correlation analysis or regression analysis will be used for the endpoint variables (including time to the first COPD exacerbation, colonization of potential pathogenic bacteria in induced sputum, microbiome in induced sputum, FEV1, CAT Score, mMRC scale, serum CRP levels, IL-6 in induced sputum, IL-8 in induced sputum, IL-1β in induced sputum and number of patients with adverse events, serious adverse events, and death). A P-value of 0.05 will be set for significance. In addition, to rule out the influence of confounding factors resulting from interclass discrepancy of conventional therapy and identify optimal subpopulation, subgroup analysis will be performed based on whether the subject uses ICS.
Discussion
Exacerbations play an important role in the progression of COPD. In the study in which bronchoscopic sampling was performed from patients in the stable phase of COPD, the colonization rate of potential pathogenic bacteria was up to 63%, considerably higher than the healthy population.14 Evidence suggests that potential pathogenic bacteria in the lower respiratory tract actively contribute to slight chronic airway inflammation resulting in an increased exacerbation frequency, an accelerated decline in lung function and impaired patients’ quality of life in clinically stable COPD patients. These potential pathogenic bacteria include H. influenzae, Streptococcus pneumoniae, M. catarrhalis, Staphylococcus aureus, P. aeruginosa and some members of a large Enterobacteriaceae family.6,15 Available studies have verified complex reasons for the increase in the colonization rate of these bacteria due to COPD, including reduced mucociliary clearance, mucus hypersecretion, bronchiectasis, defective phagocytosis and hyporesponsiveness of alveolar macrophages to bacterial antigens.6,16
Preventing acute exacerbation is an important part of management of the stable phase of COPD. Previous studies have shown that long-term use of antibiotics in patients with COPD in the stable phase contribute to prevent exacerbation.9 Albert et al and He et al reported that long-term use of erythromycin or azithromycin was associated with a decrease in the frequency of exacerbations and significantly prolonged median time to an exacerbation. It also reduced inflammatory markers significantly including total cells in the sputum and neutrophil elastase in sputum supernatant.17,18 However, there are few studies on the efficacy of intermittent amikacin in preventing the exacerbation of COPD.
In addition, the potential of probiotics and vaccines to prevent exacerbation of COPD has received increasing attention. It is estimated that the protective effects of probiotics against pathogenic bacteria relies on direct antimicrobial activity, reinforcement of the epithelium barrier function and immunomodulation.12 The results of randomized controlled studies has demonstrated that administration of probiotics was associated with a decreased rate of the prevalence of lower respiratory tract infections and colonization of pathogenic bacteria.11,12 A retrospective cohort study by Montserrat-Capdevila et al found that influenza vaccine was effective in preventing hospitalization resulting from AECOPD.19 Similarly, research by Figueira-Gonçalves et al has shown that 13-valent pneumococcal conjugate polysaccharide vaccine on COPD patients reduced the risk of hospitalization for exacerbation by three times.20 However, there are still relatively few well-designed studies on the efficacy of oral probiotics and combined vaccination to prevent exacerbation of COPD. Through this study, we aim to clarify their efficacy and safety of oral probiotics, aerosol-inhaled amikacin and combined vaccination in modulating bacterial load and composition in the lower respiratory tract and prevent exacerbations, thereby providing potential methods to prevent AECOPD in patients at high risk of exacerbation.
Ethics and Dissemination
The trial has been approved by the Ethics Committee of Zhongshan Hospital of Fudan University (B2017-197R) and registered in Clinical Trials (NCT03449459). All authors have confirmed that the trial will be conducted in accordance with the Declaration of Helsinki.
Acknowledgments
This study was supported by the National Key R&D Program of China (grant Nos. 2017YFC1309300 and 2017YFC1309303).
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. MacNee W. Pathogenesis of chronic obstructive pulmonary disease. Clin Chest Med. 2007;28(3):479–513. doi:10.1016/j.ccm.2007.06.008
2. Rosenbaum L, Lamas D. Facing a “Slow-Motion Disaster” — the UN Meeting on Noncommunicable Diseases. N Engl J Med. 2011;365(25):2345–2348. doi:10.1056/NEJMp1112235
3. Wang C, Xu J, Yang L, et al. Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China Pulmonary Health [CPH] study): a national cross-sectional study. Lancet. 2018;391(10131):1706–1717. doi:10.1016/S0140-6736(18)30841-9
4. Cai BQ, Cai SX, Chen RC, et al. Expert consensus on acute exacerbation of chronic obstructive pulmonary disease in the People’s Republic of China. Int J Chron Obstruct Pulmon Dis. 2014;9:381–395.
5. Sethi S, Murphy TF. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med. 2008;359(22):2355–2365. doi:10.1056/NEJMra0800353
6. Matkovic Z, Miravitlles M. Chronic bronchial infection in COPD. Is there an infective phenotype? Respir Med. 2013;107(1):10–22. doi:10.1016/j.rmed.2012.10.024
7. Wilkinson TMA, Patel IS, Wilks M, Donaldson GC, Wedzicha JA. Airway Bacterial Load and FEV 1 Decline in Patients with Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med. 2003;167(8):1090–1095. doi:10.1164/rccm.200210-1179OC
8. Sethi S. Infectious etiology of acute exacerbations of chronic bronchitis. Chest. 2000;117(Suppl 5):380s–385s. doi:10.1378/chest.117.5_suppl_2.380S
9. Miravitlles M, Anzueto A. Chronic Respiratory Infection in Patients with Chronic Obstructive Pulmonary Disease: what Is the Role of Antibiotics? Int J Mol Sci. 2010;45(7):7. doi:10.3390/ijms18071344
10. Ma X, Cui J, Wang J, et al. Multicentre investigation of pathogenic bacteria and antibiotic resistance genes in Chinese patients with acute exacerbation of chronic obstructive pulmonary disease. J Int Med Res. 2015;43(5):699–710. doi:10.1177/0300060515587577
11. Lin Y-L, Jan R-L, Chen -H-H, Wang J-Y. Randomized placebo-controlled trial of lactobacillus on asthmatic children with allergic rhinitis. Pediatr Pulmonol. 2010;45(11):1111–1120. doi:10.1002/ppul.21296
12. Alexandre Y, Le Blay G, Boisrame-Gastrin S, et al. Probiotics: a new way to fight bacterial pulmonary infections? Med Mal Infect. 2014;44(1):9–17. doi:10.1016/j.medmal.2013.05.001
13. Lopez-Campos JL, Soler-Cataluna JJ, Miravitlles M. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2019 Report: future Challenges. Arch Bronconeumol. 2019;201.
14. Weinreich UM, Korsgaard J. Bacterial colonisation of lower airways in health and chronic lung disease. Clin Respir J. 2008;2(2):116–122. doi:10.1111/j.1752-699X.2008.00048.x
15. Sethi S, Sethi R, Eschberger K, et al. Airway bacterial concentrations and exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;176(4):356–361. doi:10.1164/rccm.200703-417OC
16. Taylor AE, Finney-Hayward TK, Quint JK, et al. Defective macrophage phagocytosis of bacteria in COPD. Eur Respir J. 2010;35(5):1039–1047. doi:10.1183/09031936.00036709
17. He ZY, Ou LM, Zhang JQ, et al. Effect of 6 months of erythromycin treatment on inflammatory cells in induced sputum and exacerbations in chronic obstructive pulmonary disease. Respir Int Rev Thoracic Dis. 2010;80(6):445–452.
18. Albert RK, Connett J, Bailey WC, et al. Azithromycin for prevention of exacerbations of COPD. N Engl J Med. 2011;365(8):689–698. doi:10.1056/NEJMoa1104623
19. Montserrat-Capdevila J, Godoy P, Marsal J-R, Cruz I, Solanes M. Efectividad de la vacunación antigripal para evitar el ingreso hospitalario por agudización de la enfermedad pulmonar obstructiva crónica. Enfermedades Infecciosas y Microbiología Clínica. 2014;32(2):70–75. doi:10.1016/j.eimc.2013.02.009
20. Figueira-Gonçalves JM, Bethencourt-Martin N, Perez-Mendez LI, et al. Impact of 13-valent pneumococcal conjugate polysaccharide vaccination in exacerbations rate of COPD patients with moderate to severe obstruction. Revista espanola de quimioterapia. 2017;30(4):269–275.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
