Back to Journals » Therapeutics and Clinical Risk Management » Volume 16
Prevention and Treatment of Postoperative Nausea and Vomiting (PONV): A Review of Current Recommendations and Emerging Therapies
Authors Jin Z, Gan TJ, Bergese SD
Received 5 October 2020
Accepted for publication 5 December 2020
Published 31 December 2020 Volume 2020:16 Pages 1305—1317
DOI https://doi.org/10.2147/TCRM.S256234
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Garry Walsh
Zhaosheng Jin,1 Tong J Gan,1 Sergio D Bergese1,2
1Department of Anesthesiology, Stony Brook University Health Science Center, Stony Brook, NY 11794-8480, USA; 2Department of Neurological Surgery, Stony Brook University Health Science Center, Stony Brook, NY 11794-8480, USA
Correspondence: Sergio D Bergese
Stony Brook University Health Science Center, Stony Brook, NY 11794-8480, USA
Tel +1 631-444-2975
Email [email protected]
Abstract: Postoperative nausea and vomiting is one of the most frequent adverse events after surgery and anesthesia. It is distressing for the patient and can lead to other postoperative complications. Management of PONV involves a framework of risk assessment, multimodal risk reduction, and prophylactic measures, as well as prompt rescue treatment. There has been a significant paradigm shift in the approach towards PONV prevention. There have also been several emerging therapeutic options for PONV prophylaxis and treatment. In this review, we will discuss the up-to-date PONV management guidelines and highlight novel therapeutic options which have emerged in the last few years.
Keywords: antiemetics, enhanced recovery after surgery, postoperative care, postoperative nausea and vomiting
Introduction
Postoperative nausea and vomiting (PONV) remains one of the most common adverse events after surgery. It is distressing for patients, increases the risk of other adverse events such as readmission, and has a financial impact for the healthcare institution.1 Management of PONV involves a framework of risk assessment, multimodal risk reduction, and prophylactic measures, as well as prompt rescue treatment. In this review, we aim to summarize up-to-date recommendations on PONV management, as well as the evidence on newer treatment options.
Epidemiology and Healthcare Cost of PONV in the USA
The risk of PONV in the general surgical population is approximately 30%.2 In high-risk patient groups, or high-risk surgical procedures, the risk of PONV can be as high as 80%.3 PONV is a distressing experience for the patient and can have a significant impact on patient satisfaction.4,5 PONV may prolong post-anesthesia care unit (PACU) stay and increase the risk of postoperative complications. Parra-Sanchez et al6 conducted a prospective observational study and analyzed the healthcare resource utilization associated with PONV in the ambulatory surgical population. They found, on average, the occurrence of PONV increases the PACU stay by an hour and cost by 74 US dollars. PONV has also been shown to be the most common reason for unplanned readmission in bariatric patients.7
In recent years, healthcare remuneration in the United States of America (USA) has transitioned from volume-based systems to value-based systems. This means healthcare institutions are financially incentivized to provide care according to evidence based best practice, and assume financial responsibility for the occurrence of potentially avoidable complications.8 In the context of PONV management, institutions are now paid under the Merit-based Incentive Payment System for administering appropriate PONV prophylaxis based on risk factors.9 However, the introduction of Bundled Payments for Care Improvement means the institution will receive a fixed remuneration for a surgical procedure, regardless of any delay in discharge or readmission that occurs due to PONV.8
Summary of Current Recommendations
Gan et al2 have published several evidence-based guidelines on the management of PONV, based on a comprehensive literature search and the consensus of an international panel of experts. The proposed framework for PONV management involves the assessment of risk factors, risk reduction interventions, PONV prophylaxis, and rescue treatment. Patient risk factors (including female gender, non-smoker, history of PONV, or motion sickness) could be quantified using risk scores such as the Apfel score and the Koivuranta score,3,10 while surgical procedures such as laparotomy and cholecystectomy confer additional PONV risk.11 Other perioperative risk factors of PONV includes the length of surgery, the use of volatile anesthesia, including nitrous oxide, as well as perioperative opioid administration.3,10,12 Perioperative risk reduction interventions include multimodal, opioid sparing anesthesia, avoidance of volatile anesthetic, as well as nitrous oxide exposure. Gan et al2 have extensively reviewed various options for PONV prophylaxis and rescue treatment, which includes pharmacological and non-pharmacological interventions. The authors acknowledged that currently the biggest challenge in PONV management is often low compliance to the guidelines.
Implementation of General Multimodal Prophylaxis
There has been a paradigm shift towards the use of general multimodal prophylaxis for PONV, that is the administration of multiple antiemetics, as a standard of care (Figure 1).1,13,14 This represents a significant change from the previous approach of administering none or one PONV prophylaxis in patients who are considered low risk. Reasons for this paradigm shift include: 1) PONV risk scores only provide an estimated risk stratification;3 2) patients identified as low risk may still develop PONV;3 3) PONV scores do not take into account factors such as the emetogenic risk of the surgical procedure;11 and 4) anti-emetics effectiveness varies between patients.13,14 Instituting multimodal prophylaxis as a standard of care also reduces the practice variability that is commonly seen in perioperative care. As there are now robust clinical data supporting the safety and efficacy of multimodal PONV prophylaxis,15 this practice is now considered a standard of care.
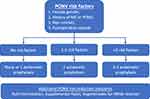 |
Figure 1 Summary of the expert consensus guidelines on postoperative nausea and vomiting (PONV) management.2 Abbreviations: MS, motion sickness; NMJb, neuromuscular junction blocker; N2O, nitrous oxide. |
Novel Risk Reduction Measures
In addition to the well-known PONV risk associated with volatile inhalational agents and opioid use, recent literature has highlighted several other potentially modifiable perioperative risk factors. These are discussed below.
Propofol
Propofol is an intravenous general anesthesia agent. Clinical trials and meta-analyses have shown that intravenous anesthesia (TIVA) with propofol is associated with significantly lower risk of PONV than volatile anesthesia.16,17 On the other hand, it is not clear if propofol, when used as an induction only, is less emetogenic when compared with other intravenous agents.18,19 Interestingly, sub-hypnotic doses of propofol (20–40 mg) is also effective as a rescue treatment for PONV.20,21 However, this should be done with caution considering the sedative effects of propofol.
Nitrous Oxide Sparing Anesthesia
Nitrous oxide is a gaseous anesthetic agent with mild analgesic effect. On the other hand, it is also commonly associated with PONV risk. Peyton and Wu12 conducted a meta-analysis and meta-regression on the efficacy of nitrous oxide avoidance for PONV prevention; they found that the number needed to treat (NNT) in anesthesia lasting more than 2 hours was nine; whereas the NNT in anesthesia less than 1 hour is 128. This suggests that nitrous oxide avoidance may not be an effective strategy in shorter surgeries.
Reversal of Neuromuscular Blockade with Sugammadex
Prior to the introduction of sugammadex for the reversal of amino-steroid neuromuscular blockers (NMJB), neostigmine was routinely used. Neostigmine is an acetylcholine esterase inhibitor, which has a parasympathomimetic effect on the gastrointestinal tract, such as increasing gastrointestinal motility and secretion. Cheng et al22 conducted a meta-analysis of clinical trials comparing NMJB reversal with neostigmine and those who did not receive NMJB reversal, and found while the incidence of nausea was slightly higher on the neostigmine arm with a trend towards dose correlation, neither were statistically significant.
With the introduction with sugammadex for NMJB reversal, several clinical trials and a recent Cochrane meta-analysis have investigated the effect of sugammadex vs neostigmine on PONV risk. They found that PONV risk is lower with sugammadex compared to neostigmine reversal [numbers needed to treat (NNT)=16]. The quality of evidence was deemed low due to the risk of study bias and, therefore, further research is warranted.23
Lidocaine Infusion
Studies have demonstrated that intravenous lidocaine is an effective analgesic in several major abdominal procedures.24,25 It has been proposed that the opioid sparing effect of lidocaine infusion may also result in a lower incidence of PONV. Weibel et al26 conducted a recent systematic review and meta-analysis (SRMA) on the use of intravenous lidocaine and included PONV as a secondary outcome; the PONV analysis included a total of 35 studies and 1,903 patients. The authors reported that lidocaine infusion reduced the incidence of postoperative nausea, but there was no significant difference in the incidence of postoperative vomiting; there was also a significant risk of publication bias.
The meta-analysis included a range of procedures, included laparoscopic abdominal and pelvic surgeries, as well as thyroid, breast, and spinal surgeries; and did not conduct a subgroup analysis according to the procedure type. In a previous iteration of the meta-analysis, lidocaine significantly reduced the risk of PONV in patients undergoing laparoscopic abdominal procedures only.27 More surgery-specific data are needed to assess if a lidocaine infusion may be beneficial in certain procedure types.
Dexmedetomidine
Dexmedetomidine is a highly selective α-2 adrenergic agonist with sedative and analgesic properties. It can be administered intravenously or as a regional anesthesia adjunct.28,29 When administered intravenously, dexmedetomidine is thought to reduce postoperative pain and opioid requirement. Jin et al30 conducted a meta-analysis of 24 clinical trials, and reported that single bolus and continuous infusion of dexmedetomidine both reduced the risk of PONV. As a regional anesthesia adjunct, dexmedetomidine have been shown to prolong the duration of analgesia, which may translate to an opioid-sparing effect.31
Supplemental Fluids
It has been suggested that perioperative fluid status is an important risk factor for the development of PONV. The role of preoperative carbohydrate solution is currently unclear. Awad et al32 conducted a meta-analysis which included abdominal, thyroid, and cardiac surgeries, and found limited evidence that preoperative carbohydrate alters the risk of PONV. A more recent meta-analysis by Xu et al33 looking into laparoscopic cholecystectomy reported that carbohydrate beverage before surgery was associated with significantly lower risk of postoperative vomiting.
Intraoperative fluid administration may affect the risk of PONV. A recent Cochrane review found that a 10–30 mL/kg intraoperative crystalloids infusion significantly reduces the risk of both early and late PONV and the need for rescue antiemetics.34 Due to the heterogeneity of the surgical procedures included, there was no consensus on the optimal volume of intravenous fluid administration. Colloid solutions (such as hydroxyethyl starch) contain macromolecules which are thought to remain in the intravascular component for a longer period of time. A recent meta-analysis by Kim et al35 reported that, compared to fluid supplementation with crystalloids, colloids significantly reduced the risk of PONV in longer surgeries (>3 hours) when compared to shorter surgeries (<3 hours).
Opioid Free Anesthesia/Analgesia
With the advances in regional anesthesia techniques and non-opioid analgesia options, several authors have discussed the feasibility of opioid free anesthesia or analgesia.36,37 While the two terms are often used interchangeably, the American Society for Enhanced Recovery and Perioperative Quality Initiative joint consensus defined opioid free anesthesia as “the absolute avoidance of opioids from induction of anesthesia until complete emergence”; and opioid free analgesia as “the absolute avoidance of opioids in the pre and postoperative periods“.38 Avoidance of opioids in the perioperative period eliminates the risk of any opioid related adverse events, this includes PONV, as well as respiratory depression and ileus.39 Bakan et al40 conducted a clinical trial of patients undergoing laparoscopic cholecystectomy, and compared propofol/remifentanil anesthesia to propofol/lidocaine/dexmedetomidine anesthesia, and reported that the latter technique had significantly lower PONV and pain. It should be noted, however, that fentanyl PCA was used for postoperative pain control. Hakim and Wahba41 conducted a similar study, and found that propofol/dexmedetomidine anesthesia was associated with significantly less antiemetic requirement than propofol/fentanyl anesthesia. Again, this did not eliminate the need for postoperative opioid (tramadol) analgesia. Opioid-free postoperative analgesia is also possible with the use of regional anesthesia, Becchi et al42 conducted a clinical trial comparing the use of continuous Psoas compartment block for postoperative analgesia to continuous morphine infusion, and reported that the opioid free continuous block was associated with comparable analgesia but significantly less PONV. As the study did not employ a placebo catheter technique, patients and clinicians were not blinded. It is worth noting that opioid free anesthesia and analgesia are only suitable in selected patient/surgery combinations, and often rely on the use of regional anesthesia. The benefit of absolute opioid avoidance will need to be balanced with issues such as risk of block failure and undesired motor block. In addition, most available literature did not compare opioid free anesthesia/analgesia to an opioid sparing approach. In their consensus statement, the American Society for Enhanced Recovery and Perioperative Quality Initiative concluded that there is limited evidence that an opioid free approach is superior to an opioid minimizing approach.38 As such, the routine adoption of opioid free anesthesia/analgesia require further studies.
Novel Chemoprophylaxis
The combination of ondansetron and dexamethasone is one of the most studied and utilized multimodal PONV prophylaxis.15 In recent years, evidence has emerged for novel therapeutic options for PONV prophylaxis, as summarized below.
Palonosetron
Palonosetron is a second generation 5-HT3 receptor antagonist, first licensed in 2003, for use in acute and delayed chemotherapy-induced nausea and vomiting. It is only available as a solution for intravenous administration. Palonosetron has 100-fold higher affinity to the 5-HT3 receptor, when compared to ondansetron, and a terminal half-life of 40 hours, which is ten-times longer than ondansetron.43 It has been postulated that palonosetron causes irreversible, allosteric inhibition of the 5-HT3 receptor,44 and receptor internalization.45
Candiotti et al46 and Kovac et al47 reported in moderate-to-high risk female patients undergoing high risk surgeries that 0.075 mg palonosetron prophylaxis was associated with a significantly lower incidence of PONV for 72 hours after surgery.
Palonosetron monotherapy for PONV prophylaxis is more effective than other 5-HT3 antagonists, including ondansetron, granisetron, ramosetron;48–50 it is also more effective than dexamethasone.51,52 Palonosetron has comparable efficacy to aprepitant.53
Palonosetron has additional advantages in ambulatory surgeries. Post-discharge nausea and vomiting is a common complication after ambulatory surgeries, and often patients will not have access to rescue anti-emetics. The long duration of action means that intraoperative palonosetron may reduce the risk of nausea and vomiting for an extended period of time after surgery, and patients are less likely to experience post-discharge nausea and vomiting.47
One of the factors which likely limited the use of palonosetron was its cost. However, since 2018, generic versions of palonosetron have been approved for use by the FDA. This will likely make palonosetron more cost-effective.
Aprepitant
Aprepitant is a competitive Neurokinin (NK)-1 receptor antagonist which was also initially approved for the treatment of chemotherapy-induced nausea and vomiting. It is administered orally, although an intravenous equivalent is also available, in the form of a pro-drug Fosaprepitant. It has a half-life of 9–13 hours,54 and it has been suggested that its duration of action may be as long as 40 hours.55 Fosaprepitant is approved only for chemotherapy-induced nausea and vomiting.
Singh et al56 conducted a meta-analysis, and included trials of various design, including aprepitant compared to placebo, and aprepitant compared to other anti-emetics as a part of multimodal prophylaxis. While they concluded that 40–125 mg aprepitant had significantly lower incidence of vomiting on both postoperative days 1 and 2, the clinical significance of the findings are not clear when considering the heterogeneity in study design.
As a single agent prophylaxis, 40 mg aprepitant has similar efficacy as 0.075 mg palonosetron.53 Clinical trials and meta-analyses have reported that aprepitant is more effective in preventing PONV when compared to ondansetron.57,58
Similar to palonosetron, the aprepitant is also shown to be beneficial in ambulatory surgery due to its long duration of action and lower risk of postdischarge nausea and vomiting. Vallejo et al59 conducted a clinical trial of 150 patients with moderate-to-high risk undergoing ambulatory plastic surgery, and found that aprepitant plus ondansetron was associated with significantly lower incidence of postdischarge nausea and vomiting than ondansetron alone.
Amisulpride
Amisulpride is a dopamine receptors antagonist. While initially licensed as an antipsychotic, in February 2020 the FDA approved its IV formulation for prophylactic and rescue therapy of PONV. The anti-emetic dose for prophylaxis is 5 mg IV, 10 mg IV for rescue treatment, whereas its antipsychotic dose is 50–1,200 mg/day orally.
Several clinical trials have reported that, when compared to placebo, amisulpride significantly reduces the incidence of PONV as well as rescue anti-emetic requirement.60,61 In addition, at the dosage used for PONV prophylaxis, amisulpride is not associated with significant risk of prolonged QT interval or extrapyramidal side-effects.61 There are currently limited head to head studies comparing amisulpride to other anti-emetics.
Midazolam
Midazolam is a short acting benzodiazepine primarily used as an anxiolytic premedication. Meta-analysis showed that midazolam administration at induction reduces the risk of PONV,62 the efficacy is comparable to ondansetron prophylaxis.63,64 Again, it is not recommended to use midazolam solely for its antiemetic effect due to the risk of sedation.
Acupressure/Acupuncture
Pericardium 6 (PC6) is an acupoint located on the palmar aspect of the forearm, between the palmaris longus and flexor carpi radialis tendons, approximately 6 cm proximal to the wrist. Clinical trials and a Cochrane review have concluded that stimulation of the acupoint with a variety of instruments (including needle acupuncture, acupressure devices, nerve stimulator, electrical stimulation needles, and laser) are effective in reducing the risk of PONV and antiemetic requirement.65 Trial sequential analysis indicates that currently data has exceeded the information required for moderate strength evidence (defined as type 1 error of <5%, power at >80%).
The study also investigated the use of PC6 stimulation in combination with pharmacoprophylaxis (ondansetron, droperidol, or ondansetron plus dexamethasone), compared to pharmacoprophylaxis alone. The addition of PC6 stimulation was associated with lower risk of vomiting and rescue anti-emetic requirement. However, the reliability of the findings were limited by the heterogeneity of the included studies.65
Several other acupoints have also been investigated for PONV prophylaxis. Large intestine 4 (LI4) is an acupoint on the dorsal aspect of the hand between the first and second metacarpal. In an RCT of patients undergoing high emetogenic surgeries, acupuncture at the LI4 point in additional to PC6 point significantly reduced the incidence of PONV compared to PC6 acupuncture alone.66 Stomach 36 (ST36) is another acupoint infero-lateral to the tibial tuberosity. A RCT of patients undergoing laparoscopic surgeries, bilateral ST36 acupoint injection of vitamin B1 was associated with significantly lower incidence of PONV.67
Use of Novel Therapy as Part of a Multimodal Prophylaxis Regimen
With the implementation of the general multimodal prophylaxis, the pressing clinical question is whether the novel therapies are effective when used in combination with other prophylactic agents. While it is well established that multimodal prophylaxis is more effective than monotherapy, questions remain as to what the margin of gain from each additional antiemetic is.15
As a NK-1 receptor antagonist, aprepitant can be used in combination with 5-HT3 antagonists as well as other antiemetics. Vallejo et al59 conducted a clinical trial of 150 patients with moderate-to-high risk undergoing ambulatory plastic surgery, and found that aprepitant plus ondansetron was associated with significantly lower incidence and severity of PONV than ondansetron alone. Similarly, Lee et al68 conducted a clinical trial of 84 female patients with low-to-moderate risk undergoing gynecological surgeries, and found that aprepitant plus ramosetron was associated with significantly lower incidence and severity of PONV than ramosetron alone.
On the other hand, Yoo et al69 conducted a clinical trial of 100 moderate risk female patients undergoing moderate-to-high risk surgeries, and reported that aprepitant plus palonosetron did not significantly reduce the incidence of PONV or the rescue anti-emetic requirement when compared to palonosetron alone. One possible explanation is that, as palonosetron is intrinsically more effective than the other 5-HT3 antagonists, the marginal gain of adding aprepitant is diminished.
Aprepitant could also be used in combination with dexamethasone. While Aprepitant monotherapy is more effective than ondansetron, its benefit as a part of the combination therapy is unclear. Habib et al70 conducted a clinical trial of 104 low-to-moderate risk patients undergoing craniotomy, and reported that aprepitant plus dexamethasone significantly reduced the incidence of PONV compared to ondansetron plus dexamethasone. On the other hand, Bilgen et al71 conducted a clinical trial of 67 moderate-to-high risk patients undergoing laparoscopic surgeries, and reported that aprepitant plus dexamethasone did not significantly reduce the incidence of PONV or the rescue anti-emetic requirement when compared to ondansetron and dexamethasone.
In addition, there is limited evidence that aprepitant is effective as a third additional agent. Holder-Murray et al72 conducted a clinical trial of 498 patients with low-to-moderate PONV risk undergoing colorectal surgeries, and found that when used in addition to ondansetron and dexamethasone prophylaxis, aprepitant did not significantly reduce the incidence of PONV or the rescue anti-emetic requirement when compared to perphenazine. Bergese et al73 conducted a clinical trial of 95 patients with low-to-moderate PONV risk undergoing craniotomy, and found that when used in addition to dexamethasone and promethazine prophylaxis, aprepitant did not significantly reduce the incidence of PONV or the rescue anti-emetic requirement when compared to ondansetron.
The diminished return associated with multimodal prophylaxis regimens is also seen with palonosetron. While multimodal prophylaxis 5-HT3 antagonist and dexamethasone is used extensively in clinical practice, the efficacy of palonosetron plus dexamethasone combination is unclear. Two clinical trials have reported that palonosetron plus dexamethasone prophylaxis was associated with lower risk of PONV.74,75 Most other clinical trials reported trends favoring the combination prophylaxis, but the results were not statistically significant.76–81
While palonosetron monotherapy is more effective than other 5-HT3 antagonists, the advantage of palonosetron as a part of the multimodal prophylaxis is also not clear. Choi et al82 conducted a clinical trial of 88 female patients with moderate-to-high PONV risk undergoing laparoscopic cholecystectomy, and found that palonosetron plus aprepitant was associated with significantly lower risk of PONV than ramosetron plus aprepitant. On the other hand, several studies have compared the efficacy of palonosetron plus dexamethasone to ondansetron plus dexamethasone, while the results appear to favor palonosetron plus dexamethasone, and the difference was not statistically significant.83–88
In summary, while the novel therapies are more effective as monotherapy, it appears that the benefit is diminished when used as a part of multimodal prophylaxis regimens. In their consensus guidelines, Gan et al2 suggested that the use of multimodal prophylaxis may allow for a lower dose of the individual anti-emetics, thereby further reducing the risk of adverse reactions. This is an area which requires further study.
Novel Rescue Treatment
In patients with established PONV (with or without prior PONV prophylaxis), common rescue treatment includes ondansetron, promethazine, and droperidol.89–91 Several additional rescue treatments have been proposed, as summarized below.
Palonosetron administration resulted in a higher rate of PONV resolution when compared to placebo.92 In patients who received ondansetron prophylaxis, administration of palonosetron resulted in complete response in 25% of the patients, this was not significantly different from administration of additional ondansetron dose as rescue.93 Hence, we do not recommend redosing of 5-HT3 receptor antagonists if a previous dose was administered within 6 hours.
Intravenous vestipitant is also an effective rescue treatment for established PONV. In patients who developed PONV despite ondansetron prophylaxis, rescue IV vestipitant resulted in a comparable complete response rate to IV ondansetron, and significantly lower incidence of further vomiting episodes.94
Amisulpride may also be effective for treating established PONV. In patients who did not receive PONV prophylaxis, 5 mg (and 10 mg) amisulpride resulted in a significantly higher complete response rate compared to placebo.64 A further multicenter study reported that, compared to placebo, 5 mg amisulpride resulted in a significantly lower rate of further vomiting episode; however, the overall complete response rate was not statistically different.95 Hence, a 10 mg dose is recommended.
PC6 acupoint stimulation may also be effective as a rescue treatment for PONV. Coloma et al96 conducted a clinical trial of moderate risk patients undergoing laparoscopic surgeries, who developed PONV despite droperidol or metoclopramide prophylaxis. Electrical stimulation of PC6 resulted in a comparable complete response rate to ondansetron rescue, and addition of PC6 acu-stimulation to ondansetron rescue resulted in a significantly better complete response rate.
A wide range of aromatherapy treatments have been proposed for the treatment of PONV, including peppermint, ginger, isopropyl alcohol, and various aromatherapy blends. Hines et al97 conducted a Cochrane review on the use of aromatherapy, and reported that isopropyl alcohol reduced the duration as well as the severity of nausea. No benefits were observed with aromatherapy blends. Another meta-analysis by Tóth et al98 investigated the use of ginger for the treatment of PONV, that ginger aromatherapy was associated with slightly reduced nausea severity. Further studies are warranted.
Application of PONV Guidelines to Enhanced Recovery Pathways
PONV management is becoming an increasingly integral aspect of enhanced recovery pathways. This is reflected in the American Society for Enhanced Recovery (ASER) Expert Opinion Statement that all patients should receive PONV prophylaxis during the perioperative period. The numbers of medications used for treatment and prophylaxis should be determined by the number of modifiable and non-modifiable risk factors; medications used should be from different pharmacological classes, with different mechanisms of action, in an attempt to achieve multimodal benefit.99 The approach to managing PONV as part of the enhanced recovery pathway is similar to the multimodal approach discussed above, and should include measures to reduce baseline emetogenic risks, and the use of general multimodal prophylaxis with at least two agents. In patients who develop PONV, prompt rescue treatment should be started.100 The specific components can vary between different surgeries due to factors such as the emetogenic risk of the surgical procedure, special anesthesia considerations (such as in neurosurgery), viability of regional anesthesia techniques, as well as any special considerations for postoperative recovery.
In colorectal surgeries, postoperative pain can be significant, which is associated with high opioid requirement; in addition, postoperative ileus is also a common adverse event. Postoperative pain can be managed effectively through the use of techniques such as epidural analgesia, transverse abdominis plane (TAP) block and bupivacaine infiltration. Postoperative ileus risk can be managed using minimally invasive surgical technique whenever possible, as well as maintaining euvolemia and early mobilization.101,102 General multimodal prophylaxis for PONV is recommended in several enhanced recovery consensus guidelines.103 The principles of colorectal enhanced recovery pathway could also be adapted to other abdominal or gastrointestinal procedures, such as esophageal, gastric, pancreatic, and hepatic procedures.104–106
Similar to major abdominal surgeries, major pelvic surgeries are also associated with significant emetogenic risk due to pain and ileus. The enhanced recovery guideline for radical cystectomy recommends the use of minimally invasive surgery, early oral intake, liberal use of antiemetics, chewing gum, prokinetic agents and opioid sparing analgesia to minimize PONV and postoperative ileus.107 In addition, the stenting of the uretero-ileal anastomosis have also been shown to reduce the risk of PONV.108–110 For gynecologic/oncologic surgery, general multimodal PONV prophylaxis is again recommended; regional interventions (e.g., TAP blocks) may decrease opioid use and postoperative pain, but this may not directly translate into a PONV advantage in all cases.111,112
For cesarean delivery, specific risk factors include neuraxial anesthesia associated hypotension, reduced cardiac output from aortocaval compression, surgical stimulation, use of uterotonics, and the use of neuraxial opioids.113 PONV risk reducing measures specific to cesarean delivery include intravenous fluid loading, lower limb compression stocking, and the use of phenylephrine and ephedrine to prevent hypotension, and should be administered in additional to general multimodal PONV prophylaxis.113
In orthopedic surgery, pain is the main postoperative adverse event and can result in high opioid requirement. Effective analgesic techniques are available for most procedures, including spinal anesthesia, peripheral nerve block, and liposomal bupivacaine infiltration for the joint capsule.114 General multimodal PONV prophylaxis is again recommended.115 In a prospective before-and-after study, introduction of standardized multimodal, opioid sparing analgesia, and general PONV prophylaxis significantly decreased the risk PONV.116
Similarly in breast surgeries, effective postoperative analgesia techniques including paravertebral block (PVB) or pectoral nerves block (PECs) can reduce the risk of PONV;117–119 and should be used in addition to nonopioid analgesia and multimodal PONV prophylaxis.120–122
Head and neck surgeries are considered high risk for the development of PONV, and a recent clinical trial has demonstrated that preoperative assessment and multimodal prophylaxis are effective in reducing the risk of PONV.123 A expert consensus statement on enhanced recovery for head and neck surgeries also supported the use of multimodal PONV prophylaxis.124
Enhanced recovery pathways for several other surgical procedures have also included general multimodal PONV prophylaxis as part of their PONV management component.125–127 It could therefore be summarized that multimodal PONV prophylaxis is applicable to most enhanced recovery pathways; while surgery specific considerations include the emetogenicity of the procedure, risk of postoperative ileus, applicability of regional anesthesia techniques, and whether PONV is associated with any procedure specific risks (such as with neurosurgical procedures).
Conclusions
In recent years, the approach to PONV management has shifted from administering none or one PONV prophylaxis to low risk patients to administering multimodal PONV prophylaxis as a standard of care. The introduction of novel therapies will allow for a greater number of prophylaxis and rescue anti-emetic combinations. There are also emerging evidence for several non-pharmacological risk management strategies, such as the minimizing fasting time, use of supplemental IV fluids and acupressure/acupuncture. As such, there is a greater number of potential therapeutic options for PONV than ever before. However, the efficacy of the different therapy combinations will require further studies.
Disclosure
Tong J Gan received honoraria from Acacia and Merck. Sergio Bergese received Funding for clinical trial from Acacia. The authors report no other potential conflicts of interest for this work.
References
1. Gan TJ, Diemunsch P, Habib AS, et al. Consensus guidelines for the management of postoperative nausea and vomiting. Anesth Analg. 2014;118(1):85–113. doi:10.1213/ANE.0000000000000002
2. Gan TJ, Belani KG, Bergese S, et al. Fourth consensus guidelines for the management of postoperative nausea and vomiting. Anesth Analg. 2020;131(2):411–448. doi:10.1213/ANE.0000000000004833
3. Apfel CC, Laara E, Koivuranta M, Greim CA, Roewer N. A simplified risk score for predicting postoperative nausea and vomiting: conclusions from cross-validations between two centers. Anesthesiology. 1999;91(3):693–700. doi:10.1097/00000542-199909000-00022
4. Eberhart LH, Mauch M, Morin AM, Wulf H, Geldner G. Impact of a multimodal anti-emetic prophylaxis on patient satisfaction in high-risk patients for postoperative nausea and vomiting. Anaesthesia. 2002;57(10):1022–1027. doi:10.1046/j.1365-2044.2002.02822.x
5. Myles PS, Williams DL, Hendrata M, Anderson H, Weeks AM. Patient satisfaction after anaesthesia and surgery: results of a prospective survey of 10,811 patients. Br J Anaesth. 2000;84(1):6–10. doi:10.1093/oxfordjournals.bja.a013383
6. Parra-Sanchez I, Abdallah R, You J, et al. A time-motion economic analysis of postoperative nausea and vomiting in ambulatory surgery. Can J Anaesth. 2012;59(4):366–375. doi:10.1007/s12630-011-9660-x
7. Berger ER, Huffman KM, Fraker T, et al. Prevalence and risk factors for bariatric surgery readmissions: findings from 130,007 admissions in the metabolic and bariatric surgery accreditation and quality improvement program. Ann Surg. 2018;267(1):122–131. doi:10.1097/SLA.0000000000002079
8. Hardin L, Kilian A, Murphy E. Bundled payments for care improvement: preparing for the medical diagnosis-related groups. J Nurs Adm. 2017;47(6):313–319. doi:10.1097/NNA.0000000000000492
9. Merit-based Incentive Payment System (MIPS) overview – QPP; 2019. Available from: https://qpp.cms.gov/mips/overview.
10. Koivuranta M, Laara E, Snare L, Alahuhta S. A survey of postoperative nausea and vomiting. Anaesthesia. 1997;52(5):443–449. doi:10.1111/j.1365-2044.1997.117-az0113.x
11. Apfel CC, Kranke P, Eberhart LH. Comparison of surgical site and patient’s history with a simplified risk score for the prediction of postoperative nausea and vomiting. Anaesthesia. 2004;59(11):1078–1082.
12. Peyton PJ, Wu CY. Nitrous oxide-related postoperative nausea and vomiting depends on duration of exposure. Anesthesiology. 2014;120(5):1137–1145. doi:10.1097/ALN.0000000000000122
13. Dewinter G, Staelens W, Veef E, Teunkens A, Van de Velde M, Rex S. Simplified algorithm for the prevention of postoperative nausea and vomiting: a before-and-after study. Br J Anaesth. 2018;120(1):156–163. doi:10.1016/j.bja.2017.08.003
14. Kranke P. General multimodal or scheduled risk-adopted postoperative nausea and vomiting prevention: just splitting hairs? Br J Anaesth. 2015;114(2):190–193. doi:10.1093/bja/aeu344
15. Som A, Bhattacharjee S, Maitra S, Arora MK, Baidya DK. Combination of 5-HT3 antagonist and dexamethasone is superior to 5-HT3 antagonist alone for PONV prophylaxis after laparoscopic surgeries: a meta-analysis. Anesth Analg. 2016;123(6):1418–1426. doi:10.1213/ANE.0000000000001617
16. Schraag S, Pradelli L, Alsaleh AJO, et al. Propofol vs. inhalational agents to maintain general anaesthesia in ambulatory and in-patient surgery: a systematic review and meta-analysis. BMC Anesthesiol. 2018;18(1):162. doi:10.1186/s12871-018-0632-3
17. Schaefer MS, Kranke P, Weibel S, Kreysing R, Kienbaum P. Total intravenous anaesthesia versus single-drug pharmacological antiemetic prophylaxis in adults: a systematic review and meta-analysis. Eur J Anaesthesiol. 2016;33(10):750–760. doi:10.1097/EJA.0000000000000520
18. Chen L, Liang X, Tan X, Wen H, Jiang J, Li Y. Safety and efficacy of combined use of propofol and etomidate for sedation during gastroscopy: systematic review and meta-analysis. Medicine. 2019;98(20).
19. St Pierre M, Dunkel M, Rutherford A, Hering W. Does etomidate increase postoperative nausea? A double-blind controlled comparison of etomidate in lipid emulsion with propofol for balanced anaesthesia. Eur J Anaesthesiol. 2000;17(10):634–641. doi:10.1097/00003643-200010000-00007
20. Gan TJ, Glass PS, Howell ST, Canada AT, Grant AP, Ginsberg B. Determination of plasma concentrations of propofol associated with 50% reduction in postoperative nausea. Anesthesiology. 1997;87(4):779–784. doi:10.1097/00000542-199710000-00010
21. Gan TJ, El-Molem H, Ray J, Glass PS. Patient-controlled antiemesis: a randomized, double-blind comparison of two doses of propofol versus placebo. Anesthesiology. 1999;90(6):1564–1570.
22. Cheng CR, Sessler DI, Apfel CC. Does neostigmine administration produce a clinically important increase in postoperative nausea and vomiting? Anesth Analg. 2005;101(5):1349–1355. doi:10.1213/01.ANE.0000180992.76743.C9
23. Hristovska AM, Duch P, Allingstrup M, Afshari A. Efficacy and safety of sugammadex versus neostigmine in reversing neuromuscular blockade in adults. Cochrane Database Syst Rev. 2017;14.
24. Kaba A, Laurent SR, Detroz BJ, et al. Intravenous lidocaine infusion facilitates acute rehabilitation after laparoscopic colectomy. Anesthesiology. 2007;106(1):11–16. doi:10.1097/00000542-200701000-00007
25. Zhao JB, Li YL, Wang YM, et al. Intravenous lidocaine infusion for pain control after laparoscopic cholecystectomy: a meta-analysis of randomized controlled trials. Medicine (Baltimore). 2018;97(5):e9771. doi:10.1097/MD.0000000000009771
26. Weibel S, Jelting Y, Pace NL, et al. Continuous intravenous perioperative lidocaine infusion for postoperative pain and recovery in adults. Cochrane Database Syst Rev. 2018;6:Cd009642.
27. Weibel S, Jokinen J, Pace NL, et al. Efficacy and safety of intravenous lidocaine for postoperative analgesia and recovery after surgery: a systematic review with trial sequential analysis. Br J Anaesth. 2016;116(6):770–783. doi:10.1093/bja/aew101
28. El-Boghdadly K, Brull R, Sehmbi H, Abdallah F. Perineural dexmedetomidine is more effective than clonidine when added to local anesthetic for supraclavicular brachial plexus block: a systematic review and meta-analysis. Anesth Analg. 2017;124(6):2008–2020. doi:10.1213/ANE.0000000000002014
29. Zeng H, Li Z, He J, Fu W, Cheungpasitporn W. Dexmedetomidine for the prevention of postoperative delirium in elderly patients undergoing noncardiac surgery: a meta-analysis of randomized controlled trials. PLoS One. 2019;14(8):e0218088. doi:10.1371/journal.pone.0218088
30. Jin S, Liang D, Chen C, Zhang M, Wang J. Dexmedetomidine prevent postoperative nausea and vomiting on patients during general anesthesia: a PRISMA-compliant meta analysis of randomized controlled trials. Medicine. 2017;96(1).
31. Hussain N, Grzywacz V, Ferreri C, et al. Investigating the efficacy of dexmedetomidine as an adjuvant to local anesthesia in brachial plexus block: a systematic review and meta-analysis of 18 randomized controlled trials. Reg Anesth Pain Med. 2017;42(2):184–196. doi:10.1097/AAP.0000000000000564
32. Awad S, Varadhan KK, Ljungqvist O, Lobo DN. A meta-analysis of randomised controlled trials on preoperative oral carbohydrate treatment in elective surgery. Clin Nutr. 2013;32(1):34–44. doi:10.1016/j.clnu.2012.10.011
33. Xu D, Zhu X, Xu Y, Zhang L. Shortened preoperative fasting for prevention of complications associated with laparoscopic cholecystectomy: a meta-analysis. J Int Med Res. 2017;45(1):22–37. doi:10.1177/0300060516676411
34. Jewer JK, Wong MJ, Bird SJ, Habib AS, Parker R, George RB. Supplemental perioperative intravenous crystalloids for postoperative nausea and vomiting. Cochrane Database Syst Rev. 2019;3:Cd012212.
35. Kim HJ, Choi SH, Eum D, Kim SH. Is perioperative colloid infusion more effective than crystalloid in preventing postoperative nausea and vomiting?: a systematic review and meta-analysis. Medicine (Baltimore). 2019;98(7):e14339. doi:10.1097/MD.0000000000014339
36. Brandal D, Keller M, Lee C, et al. Impact of enhanced recovery after surgery and opioid-free anesthesia on opioid prescriptions at discharge from the hospital: a historical-prospective study. Anesth Analg. 2017;125(5):1784–1792. doi:10.1213/ANE.0000000000002510
37. Ziemann-Gimmel P, Goldfarb A, Koppman J, Marema R. Opioid-free total intravenous anaesthesia reduces postoperative nausea and vomiting in bariatric surgery beyond triple prophylaxis. Br J Anaesth. 2014;112(5):906–911. doi:10.1093/bja/aet551
38. Wu CL, King AB, Geiger TM, et al. American Society for Enhanced Recovery and Perioperative Quality initiative joint consensus statement on perioperative opioid minimization in opioid-naive patients. Anesth Analg. 2019;129(2):567–577. doi:10.1213/ANE.0000000000004194
39. Mauermann E, Ruppen W, Bandschapp O. Different protocols used today to achieve total opioid-free general anesthesia without locoregional blocks. Best Pract Res Clin Anaesthesiol. 2017;31(4):533–545. doi:10.1016/j.bpa.2017.11.003
40. Bakan M, Umutoglu T, Topuz U, et al. Opioid-free total intravenous anesthesia with propofol, dexmedetomidine and lidocaine infusions for laparoscopic cholecystectomy: a prospective, randomized, double-blinded study. Braz J Anesthesiol (Elsevier). 2015;65(3).
41. Hakim K, Wahba W. Opioid-free total intravenous anesthesia improves postoperative quality of recovery after ambulatory gynecologic laparoscopy. Anesthes Essays Res. 2019;13(2):199. doi:10.4103/aer.AER_74_19
42. Becchi C, Al Malyan M, Coppini R, Campolo M, Magherini M, Boncinelli S. Opioid-free analgesia by continuous psoas compartment block after total hip arthroplasty. A randomized study. Eur J Anaesthesiol. 2008;25(5):418–423. doi:10.1017/S026502150700302X
43. Zabirowicz ES, Gan TJ. 34 - pharmacology of postoperative nausea and vomiting. In: Hemmings HC, Egan TD, editors. Pharmacology and Physiology for Anesthesia (Second Edition). Philadelphia: Elsevier; 2019:671–692.
44. Hothersall JD, Moffat C, Connolly CN. Prolonged inhibition of 5-HT(3) receptors by palonosetron results from surface receptor inhibition rather than inducing receptor internalization. Br J Pharmacol. 2013;169(6):1252–1262. doi:10.1111/bph.12204
45. Rojas C, Thomas AG, Alt J, et al. Palonosetron triggers 5-HT(3) receptor internalization and causes prolonged inhibition of receptor function. Eur J Pharmacol. 2010;626(2–3):193–199. doi:10.1016/j.ejphar.2009.10.002
46. Candiotti KA, Kovac AL, Melson TI, Clerici G, Joo Gan T. A randomized, double-blind study to evaluate the efficacy and safety of three different doses of palonosetron versus placebo for preventing postoperative nausea and vomiting. Anesth Analg. 2008;107(2):445–451. doi:10.1213/ane.0b013e31817b5ebb
47. Kovac AL, Eberhart L, Kotarski J, Clerici G, Apfel C. A randomized, double-blind study to evaluate the efficacy and safety of three different doses of palonosetron versus placebo in preventing postoperative nausea and vomiting over a 72-hour period. Anesth Analg. 2008;107(2):439–444. doi:10.1213/ane.0b013e31817abcd3
48. Singh PM, Borle A, Gouda D, et al. Efficacy of palonosetron in postoperative nausea and vomiting (PONV)-a meta-analysis. J Clin Anesth. 2016;34:459–482. doi:10.1016/j.jclinane.2016.05.018
49. Xiong C, Liu G, Ma R, Xue J, Wu A. Efficacy of palonosetron for preventing postoperative nausea and vomiting: a systematic review and meta-analysis. Can J Anaesth. 2015;62(12):1268–1278. doi:10.1007/s12630-015-0457-1
50. Li Y, Wei X, Zhang S, Zhou L, Zhang J. A meta-analysis of palonosetron for the prevention of postoperative nausea and vomiting in adults. J Perianesth Nurs. 2015;30(5):398–405. doi:10.1016/j.jopan.2015.05.116
51. Paul A, George S, Ranjan R, et al. Randomised control study of palonosetron versus dexamethasone in preventing postoperative nausea and vomiting following ear and nose surgeries under general anesthesia. J Clin Diagn Res. 2018;12(11):UC10–UC13.
52. Kim BG, Kim H, Lim HK, Yang C, Oh S, Lee BW. A comparison of palonosetron and dexamethasone for postoperative nausea and vomiting in orthopedic patients receiving patient-controlled epidural analgesia. Korean J Anesthesiol. 2017;70(5):520–526. doi:10.4097/kjae.2017.70.5.520
53. Moon HY, Baek CW, Choi GJ, et al. Palonosetron and aprepitant for the prevention of postoperative nausea and vomiting in patients indicated for laparoscopic gynaecologic surgery: a double-blind randomised trial. BMC Anesthesiol. 2014;14:68. doi:10.1186/1471-2253-14-68
54. Majumdar AK, Howard L, Goldberg MR, et al. Pharmacokinetics of aprepitant after single and multiple oral doses in healthy volunteers. J Clin Pharmacol. 2006;46(3):291–300. doi:10.1177/0091270005283467
55. Ibrahim MA, Preuss CV. Antiemetic neurokinin-1 receptor blockers. In: StatPearls. Treasure Island (FL): StatPearls PublishingStatPearls Publishing LLC; 2020. ed: Charles Preuss.
56. Singh PM, Borle A, Rewari V, et al. Aprepitant for postoperative nausea and vomiting: a systematic review and meta-analysis. Postgrad Med J. 2016;92(1084):87–98. doi:10.1136/postgradmedj-2015-133515
57. Gan TJ, Apfel CC, Kovac A, et al. A randomized, double-blind comparison of the NK1 antagonist, aprepitant, versus ondansetron for the prevention of postoperative nausea and vomiting. Anesth Analg. 2007;104(5):1082–1089, tables of contents. doi:10.1213/01.ane.0000263277.35140.a3
58. Liu M, Zhang H, Du BX, et al. Neurokinin-1 receptor antagonists in preventing postoperative nausea and vomiting: a systematic review and meta-analysis. Medicine (Baltimore). 2015;94(19):e762. doi:10.1097/MD.0000000000000762
59. Vallejo MC, Phelps AL, Ibinson JW, et al. Aprepitant plus ondansetron compared with ondansetron alone in reducing postoperative nausea and vomiting in ambulatory patients undergoing plastic surgery. Plast Reconstr Surg. 2012;129(2):519–526. doi:10.1097/PRS.0b013e31822b6932
60. Kranke P, Eberhart L, Motsch J, et al. I.V. APD421 (amisulpride) prevents postoperative nausea and vomiting: a randomized, double-blind, placebo-controlled, multicentre trial. Br J Anaesth. 2013;111(6):938–945. doi:10.1093/bja/aet251
61. Gan TJ, Kranke P, Minkowitz HS, et al. Intravenous amisulpride for the prevention of postoperative nausea and vomiting: two concurrent, randomized, double-blind, placebo-controlled trials. Anesthesiology. 2017;126(2):268–275. doi:10.1097/ALN.0000000000001458
62. Ahn EJ, Kang H, Choi GJ, Baek CW, Jung YH, Woo YC. The effectiveness of midazolam for preventing postoperative nausea and vomiting: a systematic review and meta-analysis. Anesth Analg. 2016;122(3):664–676. doi:10.1213/ANE.0000000000001062
63. Lee Y, Wang JJ, Yang YL, Chen A, Lai HY. Midazolam vs ondansetron for preventing postoperative nausea and vomiting: a randomised controlled trial. Anaesthesia. 2007;62(1):18–22. doi:10.1111/j.1365-2044.2006.04895.x
64. Candiotti KA, Kranke P, Bergese SD, et al. Randomized, double-blind, placebo-controlled study of intravenous amisulpride as treatment of established postoperative nausea and vomiting in patients who have had no prior prophylaxis. Anesth Analg. 2019;128(6):1098–1105. doi:10.1213/ANE.0000000000003733
65. Lee A, Chan SK, Fan LT. Stimulation of the wrist acupuncture point PC6 for preventing postoperative nausea and vomiting. Cochrane Database Syst Rev. 2015;(11):CD003281.
66. Alizadeh R, Esmaeili S, Shoar S, Bagheri-Hariri S, Shoar N. Acupuncture in preventing postoperative nausea and vomiting: efficacy of two acupuncture points versus a single one. J Acupunct Meridian Stud. 2014;7(2):71–75. doi:10.1016/j.jams.2013.04.005
67. Chen ZY, Lin L, Wang HH, et al. Ondansetron combined with ST36 (Zusanli) acupuncture point injection for postoperative vomiting. Acupunct Med. 2014;32(2):124–131. doi:10.1136/acupmed-2013-010340
68. Lee SJ, Lee SM, Kim SI, et al. The effect of aprepitant for the prevention of postoperative nausea and vomiting in patients undergoing gynecologic surgery with intravenous patient controlled analgesia using fentanyl: aprepitant plus ramosetron vs ramosetron alone. Korean J Anesthesiol. 2012;63(3):221–226. doi:10.4097/kjae.2012.63.3.221
69. Yoo JH, Kim SI, Chung JW, Jun MR, Han YM, Kim YJ. Aprepitant in combination with palonosetron for the prevention of postoperative nausea and vomiting in female patients using intravenous patient-controlled analgesia. Korean J Anesthesiol. 2018;71(6):440–446. doi:10.4097/kja.d.18.00011
70. Habib AS, Keifer JC, Borel CO, White WD, Gan TJ. A comparison of the combination of aprepitant and dexamethasone versus the combination of ondansetron and dexamethasone for the prevention of postoperative nausea and vomiting in patients undergoing craniotomy. Anesth Analg. 2011;112(4):813–818. doi:10.1213/ANE.0b013e3181ff47e2
71. Bilgen S, Kizilcik N, Haliloglu M, Yildirim G, Kaspar EC, Koner O. Effect of the dexamethasone-ondansetron combination versus dexamethasone-aprepitant combination to prevent postoperative nausea and vomiting. Turk J Anaesthesiol Reanim. 2018;46(5):373–380. doi:10.5152/TJAR.2018.53179
72. Holder-Murray J, Esper SA, Boisen ML, et al. Postoperative nausea and vomiting in patients undergoing colorectal surgery within an institutional enhanced recovery after surgery protocol: comparison of two prophylactic antiemetic regimens. Korean J Anesthesiol. 2019;72(4):344–350. doi:10.4097/kja.d.18.00355
73. Bergese SD, Puente EG, Antor MA, et al. A prospective, randomized, double-blinded, double-dummy pilot study to assess the preemptive effect of triple therapy with aprepitant, dexamethasone, and promethazine versus ondansetron, dexamethasone and promethazine on reducing the incidence of postoperative nausea and vomiting experienced by patients undergoing craniotomy under general anesthesia. Front Med (Lausanne). 2016;3:29.
74. Tiwari S, Katiyar S, Jain R. To compare antiemetic efficacy of palonosetron alone versus palonosetron combined with dexamethasone as a prophylactic regimen for the prevention of postoperative nausea and vomiting in patients undergoing laparoscopic surgery under general anesthesia. Int J Contemp Med Res. 2017;4(5):1186–1189.
75. Bala I, Bharti N, Murugesan S, Gupta R. Comparison of palonosetron with palonosetron-dexamethasone combination for prevention of postoperative nausea and vomiting in patients undergoing laparoscopic cholecystectomy. Minerva Anestesiol. 2014;80(7):779–784.
76. Ghosh S, Pal A, Biswas C, Biswas C, Ghosh TR, Ghosh T. Palonosetron and palonosetron plus dexamethasone to prevent postoperative nausea and vomiting in patients undergoing laparoscopic cholecystectomy: a prospective, randomized, double-blind comparative study. Anesth Essays Res. 2011;5(2):134–137. doi:10.4103/0259-1162.94751
77. Park JW, Jun JW, Lim YH, et al. The comparative study to evaluate the effect of palonosetron monotherapy versus palonosetron with dexamethasone combination therapy for prevention of postoperative nausea and vomiting. Korean J Anesthesiol. 2012;63(4):334–339. doi:10.4097/kjae.2012.63.4.334
78. Swaro S, Karan D, Banerjee A. Comparison of palonosetron, dexamethasone, and palonosetron plus dexamethasone as prophylactic antiemetic and antipruritic drug in patients receiving intrathecal morphine for lower segment cesarean section. Anesth Essays Res. 2018;12(2):322–327. doi:10.4103/aer.AER_183_17
79. Blitz JD, Haile M, Kline R, et al. A randomized double blind study to evaluate efficacy of palonosetron with dexamethasone versus palonosetron alone for prevention of postoperative and postdischarge nausea and vomiting in subjects undergoing laparoscopic surgeries with high emetogenic risk. Am J Ther. 2012;19(5):324–329. doi:10.1097/MJT.0b013e318209dff1
80. Didehvar S, Viola-Blitz JD, Haile M, et al. A randomized, double blind study to evaluate the efficacy of palonosetron with dexamethasone versus palonosetron alone for prevention of post-operative nausea and vomiting in subjects undergoing bariatric surgeries with high emetogenic risk. Open Anesthesiol J. 2013;7(1):30–36. doi:10.2174/1874321801307010030
81. Cho E, Kim DH, Shin S, Kim SH, Oh YJ, Choi YS. Efficacy of palonosetron-dexamethasone combination versus palonosetron alone for preventing nausea and vomiting related to opioid-based analgesia: a prospective, randomized, double-blind trial. Int J Med Sci. 2018;15(10):961–968. doi:10.7150/ijms.24230
82. Choi EK, Kim DG, Jeon Y. Comparison of the prophylactic antiemetic efficacy of aprepitant plus palonosetron versus aprepitant plus ramosetron in patients at high risk for postoperative nausea and vomiting after laparoscopic cholecystectomy: a prospective randomized-controlled trial. Surg Laparosc Endosc Percutan Tech. 2016;26(5):354–357.
83. Campos GO, de Jesus Martins M, Jesus GN, et al. Palonosetron versus ondansetron for prevention of nausea and vomiting after total abdominal hysterectomy under spinal anesthesia with intrathecal morphine: a double-blind, randomized controlled trial. BMC Anesthesiol. 2019;19(1):159. doi:10.1186/s12871-019-0830-7
84. Kaloria N, Sen I, Vikas S, Gupta R, Walia R. Palonosetron-dexamethasone versus ondansetron-dexamethasone to prevent postoperative nausea and vomiting undergoing laparoscopic sleeve gastrectomy: a preliminary, randomized, double blinded study. Indian J Clin Anaesth. 2017;4(4):523–529.
85. Kumar A, Solanki SL, Gangakhedkar GR, Shylasree TS, Sharma KS. Comparison of palonosetron and dexamethasone with ondansetron and dexamethasone for postoperative nausea and vomiting in postchemotherapy ovarian cancer surgeries requiring opioid-based patient-controlled analgesia: a randomised, double-blind, active controlled study. Indian J Anaesth. 2018;62(10):773–779.
86. Prajapat G, Pareek A, Sethia S, Bhati K, Meena S. Role of ondansetron with dexamethasone and palonosetron with dexamethasone as antiemetic in laproscopic surgery under general anaesthesia: a comparative study. J Med Sci Clin Res. 2018;6(4):555–560. doi:10.18535/jmscr/v6i4.91
87. Rajnikant K, Bhukal I, Kaloria N, Soni SL, Kajal K. Comparison of palonosetron and dexamethasone with ondansetron and dexamethasone to prevent postoperative nausea and vomiting in patients undergoing laparoscopic cholecystectomy. Anesth Essays Res. 2019;13(2):317–322. doi:10.4103/aer.AER_21_19
88. Sharma AN, Shankaranarayana P. Postoperative nausea and vomiting: palonosetron with dexamethasone vs. ondansetron with dexamethasone in laparoscopic hysterectomies. Oman Med J. 2015;30(4):252–256. doi:10.5001/omj.2015.51
89. Choi YS, Sohn HM, Do SH, Min KT, Woo JH, Baik HJ. Comparison of ramosetron and ondansetron for the treatment of established postoperative nausea and vomiting after laparoscopic surgery: a prospective, randomized, double-blinded multicenter trial. Ther Clin Risk Manag. 2018;14:601–606. doi:10.2147/TCRM.S159211
90. Habib AS, Reuveni J, Taguchi A, White WD, Gan TJ. A comparison of ondansetron with promethazine for treating postoperative nausea and vomiting in patients who received prophylaxis with ondansetron: a retrospective database analysis. Anesth Analg. 2007;104(3):548–551. doi:10.1213/01.ane.0000252433.73485.be
91. Habib AS, Gan TJ. The effectiveness of rescue antiemetics after failure of prophylaxis with ondansetron or droperidol: a preliminary report. J Clin Anesth. 2005;17(1):62–65. doi:10.1016/j.jclinane.2004.04.004
92. Hahm TS, Hwang JW, Kim WH, et al. A prospective, randomized, double-blind, multicenter trial to evaluate the therapeutic efficacy and safety of palonosetron in the treatment of postoperative nausea and vomiting over a 72-h period. J Anesth. 2015;29(1):21–28. doi:10.1007/s00540-014-1884-9
93. Candiotti KA, Ahmed SR, Cox D, Gan TJ. Palonosetron versus ondansetron as rescue medication for postoperative nausea and vomiting: a randomized, multicenter, open-label study. BMC Pharmacol Toxicol. 2014;15:45. doi:10.1186/2050-6511-15-45
94. Kranke P, Thompson JP, Dalby PL, et al. Comparison of vestipitant with ondansetron for the treatment of breakthrough postoperative nausea and vomiting after failed prophylaxis with ondansetron. Br J Anaesth. 2015;114(3):423–429. doi:10.1093/bja/aeu376
95. Habib AS, Kranke P, Bergese SD, et al. Amisulpride for the rescue treatment of postoperative nausea or vomiting in patients failing prophylaxis: a randomized, placebo-controlled phase iii trial. Anesthesiology. 2019;130(2):203–212. doi:10.1097/ALN.0000000000002509
96. Coloma M, White PF, Ogunnaike BO, et al. Comparison of acustimulation and ondansetron for the treatment of established postoperative nausea and vomiting. Anesthesiology. 2002;97(6):1387–1392. doi:10.1097/00000542-200212000-00009
97. Hines S, Steels E, Chang A, Gibbons K. Aromatherapy for treatment of postoperative nausea and vomiting. Cochrane Database Syst Rev. 2018;3.
98. Toth B, Lantos T, Hegyi P, et al. Ginger (Zingiber officinale): an alternative for the prevention of postoperative nausea and vomiting. A meta-analysis. Phytomedicine. 2018;50:8–18. doi:10.1016/j.phymed.2018.09.007
99. Gupta R, Soto R. Prophylaxis and management of postoperative nausea and vomiting in enhanced recovery protocols: expert opinion statement from the American Society for Enhanced Recovery (ASER). Perioper Med (Lond). 2016;5:4. doi:10.1186/s13741-016-0029-0
100. Nygren J, Thacker J, Carli F, et al. Guidelines for perioperative care in elective rectal/pelvic surgery: Enhanced Recovery After Surgery (ERAS(R)) society recommendations. Clin Nutr. 2012;31(6):801–816. doi:10.1016/j.clnu.2012.08.012
101. Feldheiser A, Aziz O, Baldini G, et al. Enhanced Recovery After Surgery (ERAS) for gastrointestinal surgery, part 2: consensus statement for anaesthesia practice. Acta Anaesthesiol Scand. 2016;60(3):289–334. doi:10.1111/aas.12651
102. Hedrick TL, McEvoy MD, Mythen MMG, et al. American Society for Enhanced Recovery and Perioperative Quality initiative joint consensus statement on postoperative gastrointestinal dysfunction within an enhanced recovery pathway for elective colorectal surgery. Anesth Analg. 2018;126(6):1896–1907. doi:10.1213/ANE.0000000000002742
103. Sarin A, Litonius ES, Naidu R, Yost CS, Varma MG, Chen LL. Successful implementation of an enhanced recovery after surgery program shortens length of stay and improves postoperative pain, and bowel and bladder function after colorectal surgery. BMC Anesthesiol. 2016;16(1):55. doi:10.1186/s12871-016-0223-0
104. Lassen K, Coolsen MM, Slim K, et al. Guidelines for perioperative care for pancreaticoduodenectomy: Enhanced Recovery After Surgery (ERAS(R)) society recommendations. Clin Nutr. 2012;31(6):817–830. doi:10.1016/j.clnu.2012.08.011
105. Pecorelli N, Nobile S, Partelli S, et al. Enhanced recovery pathways in pancreatic surgery: state of the art. World J Gastroenterol. 2016;22(28):6456–6468. doi:10.3748/wjg.v22.i28.6456
106. Dorcaratto D, Grande L, Pera M. Enhanced recovery in gastrointestinal surgery: upper gastrointestinal surgery. Dig Surg. 2013;30(1):70–78. doi:10.1159/000350701
107. Cerantola Y, Valerio M, Persson B, et al. Guidelines for perioperative care after radical cystectomy for bladder cancer: Enhanced Recovery After Surgery (ERAS((R))) society recommendations. Clin Nutr. 2013;32(6):879–887. doi:10.1016/j.clnu.2013.09.014
108. Mattei A, Birkhaeuser FD, Baermann C, Warncke SH, Studer UE. To stent or not to stent perioperatively the ureteroileal anastomosis of ileal orthotopic bladder substitutes and ileal conduits? Results of a prospective randomized trial. J Urol. 2008;179(2):582–586. doi:10.1016/j.juro.2007.09.066
109. Azhar RA, Bochner B, Catto J, et al. Enhanced recovery after urological surgery: a contemporary systematic review of outcomes, key elements, and research needs. Eur Urol. 2016;70(1):176–187. doi:10.1016/j.eururo.2016.02.051
110. Maloney I, Parker DC, Cookson MS, Patel S. Bladder cancer recovery pathways: a systematic review. Bladder Cancer. 2017;3(4):269–281. doi:10.3233/BLC-170136
111. Nelson G, Altman AD, Nick A, et al. Guidelines for pre- and intra-operative care in gynecologic/oncology surgery: Enhanced Recovery After Surgery (ERAS(R)) society recommendations–part I. Gynecol Oncol. 2016;140(2):313–322. doi:10.1016/j.ygyno.2015.11.015
112. Yoo JE, Oh DS. Potential benefits of acupuncture for enhanced recovery in gynaecological surgery. Forsch Komplementmed. 2015;22(2):111–116.
113. Macones GA, Caughey AB, Wood SL, et al. Guidelines for postoperative care in cesarean delivery: Enhanced Recovery After Surgery (ERAS) society recommendations (part 3). Am J Obstet Gynecol. 2019;221:
114. Pham Dang C, Gautheron E, Guilley J, et al. The value of adding sciatic block to continuous femoral block for analgesia after total knee replacement. Reg Anesth Pain Med. 2005;30(2):128–133.
115. Soffin EM, YaDeau JT. Enhanced recovery after surgery for primary hip and knee arthroplasty: a review of the evidence. Br J Anaesth. 2016;117(suppl3):iii62–iii72. doi:10.1093/bja/aew362
116. Tan NLT, Hunt JL, Gwini SM. Does implementation of an enhanced recovery after surgery program for hip replacement improve quality of recovery in an Australian private hospital: a quality improvement study. BMC Anesthesiol. 2018;18(1):64. doi:10.1186/s12871-018-0525-5
117. Amaya F, Hosokawa T, Okamoto A, et al. Can acute pain treatment reduce postsurgical comorbidity after breast cancer surgery? A literature review. Biomed Res Int. 2015;2015:641508. doi:10.1155/2015/641508
118. Abdallah FW, Morgan PJ, Cil T, et al. Ultrasound-guided multilevel paravertebral blocks and total intravenous anesthesia improve the quality of recovery after ambulatory breast tumor resection. Anesthesiology. 2014;120(3):703–713. doi:10.1097/ALN.0000436117.52143.bc
119. Bashandy GM, Abbas DN. Pectoral nerves I and II blocks in multimodal analgesia for breast cancer surgery: a randomized clinical trial. Reg Anesth Pain Med. 2015;40(1):68–74. doi:10.1097/AAP.0000000000000163
120. Olanders KJ, Lundgren GAE, Johansson AMG. Betamethasone in prevention of postoperative nausea and vomiting following breast surgery. J Clin Anesth. 2014;26(6):461–465. doi:10.1016/j.jclinane.2014.02.006
121. Kim SH, Oh YJ, Park BW, Sim J, Choi YS. Effects of single-dose dexmedetomidine on the quality of recovery after modified radical mastectomy: a randomised controlled trial. Minerva Anestesiol. 2013;79(11):1248–1258.
122. Chiu C, Aleshi P, Esserman LJ, et al. Improved analgesia and reduced post-operative nausea and vomiting after implementation of an enhanced recovery after surgery (ERAS) pathway for total mastectomy. BMC Anesthesiol. 2018;18(1):41. doi:10.1186/s12871-018-0505-9
123. Lu D, Wang Y, Zhao T, et al. Successful implementation of an enhanced recovery after surgery (ERAS) protocol reduces nausea and vomiting after infratentorial craniotomy for tumour resection: a randomized controlled trial. BMC Neurol. 2020;20(1). doi:10.1186/s12883-020-01699-z.
124. Dort J, Farwell D, Findlay M, et al. Optimal perioperative care in major head and neck cancer surgery with free flap reconstruction: a consensus review and recommendations from the enhanced recovery after surgery society. JAMA Otolaryngol. 2017;143(3):292. doi:10.1001/jamaoto.2016.2981
125. Fleming IO, Garratt C, Guha R, et al. Aggregation of marginal gains in cardiac surgery: feasibility of a perioperative care bundle for enhanced recovery in cardiac surgical patients. J Cardiothorac Vasc Anesth. 2016;30(3):665–670. doi:10.1053/j.jvca.2016.01.017
126. Gemma M, Toma S, Lira Luce F, Beretta L, Braga M, Bussi M. Enhanced recovery program (ERP) in major laryngeal surgery: building a protocol and testing its feasibility. Acta Otorhinolaryngol Ital. 2017;37(6):475–478.
127. Mihara T, Ishii T, Ka K, Goto T, Gluud C. Effects of steroids on quality of recovery and adverse events after general anesthesia: meta-analysis and trial sequential analysis of randomized clinical trials. PLoS One. 2016;11(9):e0162961. doi:10.1371/journal.pone.0162961
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
