Back to Journals » Journal of Pain Research » Volume 15
Prevention and Treatment of Neuraxial Morphine-Induced Pruritus: A Scoping Review
Authors Becker LM , Teunissen AJW , Koopman JSHA
Received 23 February 2022
Accepted for publication 21 May 2022
Published 4 June 2022 Volume 2022:15 Pages 1633—1645
DOI https://doi.org/10.2147/JPR.S361225
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jinlei Li
Leonie M Becker,1,2 Aart Jan W Teunissen,2 Joseph SHA Koopman2
1Department of Cardiology, St. Antonius Ziekenhuis, Nieuwegein, The Netherlands; 2Department of Anaesthesiology, Maasstad Ziekenhuis, Rotterdam, The Netherlands
Correspondence: Leonie M Becker, Department of Cardiology, St. Antonius Hospital, Koekoekslaan 1, Nieuwegein, 3435 CM, The Netherlands, Tel +31 88 320 09 22, Email [email protected]
Abstract: The addition of morphine to neuraxial anaesthesia leads to improved postoperative analgesia and lower opioid consumption, but is often accompanied by pruritus. Studies on preventing or treating pruritus show contradictory results. Our objective was to identify effective drugs for the prevention or treatment of pruritus by a scoping review of clinical trials. A systematic literature search was conducted in PubMed, Embase and Web of Science. We identified clinical trials investigating the prevention or treatment of neuraxial morphine-induced pruritus in adults. Systematic reviews and meta-analyses were screened for eligible studies. One-hundred-and-four articles were included covering 13 pharmacological groups. We conclude that dopamine antagonists, μ-opioid agonist/antagonists and neuraxial or orally administered μ-opioid antagonists prevent pruritus caused by neuraxial morphine regardless of the timing of administration. In the reviewed literature, 5HT3-antagonists prevent neuraxial morphine-induced pruritus when administered before morphine administration. For the treatment of neuraxial morphine-induced pruritus, only nalbuphine appears to be consistently effective. More research is needed to find the most effective doses and the optimal timing of the effective medication.
Keywords: pruritus, prevention, treatment, morphine, epidural, spinal
Introduction
Adding morphine to neuraxial (NA) anaesthesia improves postoperative analgesia and lowers the opioid-consumption.1–3 These benefits enhance recovery speed and shorten the length of hospital stay due to earlier mobilization. Although the systemic uptake of neuraxial morphine is relatively low, side effects do occur. A frequently reported side-effect is pruritus. Depending on surgical and patient characteristics, an estimated 30 to 60% of general surgery patients and up to 100% of parturients (women undergoing a caesarean section) experience pruritus after neuraxial opioid administration.4,5 Pruritus can be perceived as worse than pain, leading to severe discomfort.6 The peak severity is 6 to 9 hours after morphine administration and varies from mild to severe with patients requesting treatment.6
The mechanism causing neuraxial opioid-induced pruritus is not yet fully understood. Multiple pathways are suggested. One is the modulation of serotonergic pathways. Serotonin contributes to peripheral sensitization and hyperalgesia.7 Five-hydroxytryptamine type 3-receptor (5HT3-) antagonists have an anti-serotonergic effect and are already frequently used to prevent postoperative nausea and vomiting (PONV).8–10 Another pathway might be through central and peripheral acting opioid receptors, especially µ-opioid receptors.11 Therefore, opioid antagonists and agonist/antagonists have been studied. On the downside, these drugs may reverse analgesic effects which are undesirable.12–14 Another pathway which has been hypothesized is that PONV and pruritus are caused by an allergic reaction to morphine or histamine release.15–17 Corticosteroids lessen allergic reactions and are already used frequently to prevent PONV.15–17 Systemic morphine leads to histamine release through enhanced c-fibre transmission by prostaglandins.18 Prostaglandins also lower the threshold for serotonin-induced pain sensitization.18 Non-steroid anti-inflammatory drugs (NSAID’s) decrease the formation of prostaglandins by inhibiting cyclo-oxygenases (COX), modulating both histaminergic and serotonergic pathways.19 Evidence suggests pruritus after neuraxial morphine is not caused by an allergic reaction or histamine release, but antihistamines are still frequently used as treatment.20
Opioid-induced pruritus is a dose-related side effect.5 Various drugs have been used to lower opioid consumption by improving analgesia and decreasing central sensitization and hyperalgesia. N-methyl-D-aspartate (NMDA)-receptor antagonists affect central sensitization, enhancing the analgesic effect of opioids.21,22 The use of α2-agonists leads to intensified analgesia by affecting synaptic adrenergic receptors in the dorsal horn of the spinal cord and the locus coeruleus.23,24 Gamma-aminobutyric acid (GABA) reduces hyperalgesia and central sensitization by binding to the α2-δ-subunit of voltage-dependent calcium channels.25 Propofol enhances the function of GABA, and gabapentin, an anticonvulsant, is a structural analogue of GABA.25–27 Furthermore, dopamine antagonists have been studied. The involvement of the midbrain in the reward circuit of scratching an itch suggests a role for the dopaminergic system, but the mechanism of action regarding pruritus is unclear.28–31 Finally, in some practices, acupuncture is used as an antipruritic treatment. It has been used, for example, in chronic kidney disease.32
Results of studies on this topic are contradictory, and the best remedy has not yet been found.6,33,34 Therefore, the objectives of this review were to identify effective medications to prevent and treat neuraxial morphine-induced pruritus and find good insights for future research.
Methods
This systematic review was written according to the PRISMA 2018 Guidelines for Scoping Reviews.
Database Searching
PubMed, Embase and Web of Science were searched (14–01-2021) using a systematic search strategy. We limited our search to articles in English or Dutch involving adult humans. For PubMed and Web of Science, a search was conducted combining the MeSH-term pruritus with either spinal injections, epidural anaesthesia, spinal anaesthesia, opioid analgesics, or morphine.
For Embase, a search was conducted combining the search terms pruritus with either opiate or morphine and either intraspinal drug administration, epidural anaesthesia, or spinal anaesthesia.
The database search was performed in Duplo by the first author (LB) and checked by the second author (AJT). Then, on 26–01-2021, the final free text search was conducted in all three databases combining morphine and pruritus with either spinal, epidural or intrathecal. Results were screened until the most recently published article was already included.
Inclusion and Exclusion Criteria
Articles describing original clinical trials assessing pruritus after neuraxial morphine were eligible for inclusion. The study population had to have at least two treatment groups receiving the same dose of neuraxial morphine. Articles were excluded if the full text was unavailable or if the neuraxial anaesthesia protocol differed between the intervention and control groups. For example, a comparison between bupivacaine with morphine in the control group versus morphine alone in the intervention group was excluded. If the opioid treatment protocols differed between the intervention and control groups, studies were also excluded. For example, a comparison was excluded between neuraxial sufentanil with morphine in the intervention group versus morphine alone in the control group. Articles comparing pruritus between two groups of patients who either did or did not receive morphine were also excluded.
Systematic reviews and meta-analyses found with the database search that mentioned pruritus in their abstract were screened for eligible articles not yet included using the same in- and exclusion criteria mentioned above.
The first and second author independently assessed all articles. The same authors included articles fulfilling all inclusion criteria and no exclusion criteria. Discrepancies were discussed with the other authors until a decision was reached.
Assessment of Risk of Bias
To assess the risk of bias, the Cochrane Risk of Bias Tool for Randomised Controlled Trials version 1 was used. The bias assessment was performed in Duplo by two different authors (LB and AJT).
Data-Extraction
Data were extracted by the first and second author using a standardized extraction form constructed in consultation with the other authors.
Study Characteristics
Included articles, first author, year of publication, type of study and surgical population were noted. The study groups, the number of patients and neuraxial procedure (spinal anaesthesia (SA), epidural anaesthesia (EA), combined spinal and epidural anaesthesia (CSEA)) were registered. For all study medication and neuraxial medication, including morphine, administration, dose, and timing were noted. The timing of study medication was either prophylactic (administered before the patient experiences pruritus) or therapeutic (administered to treat pruritus). Prophylactic use can be further sorted in administration before, after, or simultaneously with morphine administration. The way of pruritus assessment (incidence/severity/visual analogue score (VAS)) and duration of follow-up was registered. Whether pruritus was a primary or secondary outcome was also noted. Finally, the use of peri-operative opiates was documented since these might cause pruritus through different pathways.
Study Results
Of the included articles, the incidence and severity of pruritus and treatment effect were noted with recurrence rate and significant changes in analgesia if reported. The primary conclusion of the article authors was stated and compared with the data provided.
Results
Database Searching
A total of 104 articles were included in this scoping review. A flow diagram of the literature search is shown in Figure 1. The free text search yielded no new articles that were not (yet) indexed for MeSH-terms or keywords. Eighteen articles met the inclusion criteria, but also one or more exclusion criteria and were subsequently excluded. Supplemental Table 1 contains the list of these articles with reasons for exclusion. The list of all included articles and their bias assessments is shown in Supplemental Table 2.
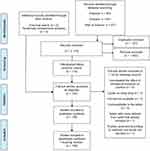 |
Figure 1 Flow diagram of the database search. Notes: The PRISMA diagram details the search and selection process applied during our scoping literature search and critical review. Values are presented as n = number of articles. PRISMA figure adapted from Moher D, Liberati A, Altman D, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Journal of clinical epidemiology. 2009;62(10). Creative Commons.45 |
Subgroup Analysis
The included studies were divided into pharmacological groups based on the study medication. Pruritus incidence and treatment success varied greatly among both treatment and control groups. Pruritus severity was usually assessed as a score from 0–10 or 0–100 or a rating scale from mild to severe. An overview of all included studies and their individual outcomes for each pharmacological group is included in Supplemental Tables 3–14. In addition, available data were analysed for possible relations between pruritus and pregnancy; timing, route, dose, and mechanism of medication administration and use of placebo in control groups. After this, the study medication regiments were divided into three groups. Prophylactic effectiveness when study medication was administered before or at the same time as NA morphine is shown in Table 1. Prophylactic effectiveness when study medication was administered after NA morphine is shown in Table 2. Lastly, therapeutic effectiveness is shown in Table 3.
 |
Table 1 Prophylactic Use: Overview of Results of Administrating Study Medicine Before or at the Same Time as Spinal Morphine |
 |
Table 2 Prophylactic Use: Administration After Administration of Spinal Morphine but Before Occurrence of Pruritis |
 |
Table 3 Therapeutic Use |
5HT3-Antagonists
Twenty-two studies investigated the effect of 5HT3-antagonists, most frequently ondansetron 4mg intravenous (IV). In ten studies, 5HT3-antagonists were superior to at least one other study group. Of the fourteen studies conducted among parturients, 5HT3-antagonists were superior in five studies. The most evidence existed for a prophylactic use of 5HT3-antagonists (before morphine administration). Mirtazapine showed a significant reduction in pruritus incidence in two studies when used as prophylactic.35,36
Studies that reported a significant reduction of pruritus incidence found a reduction between 7 and 71%, with an average of 50%.
α2-adrenergic (α2-) agonists
Four studies investigated an α2-agonist. In all studies, the α2-agonist clonidine was administered prophylactically. No study reported effectiveness in reduction of either incidence or severity of pruritus.
µ-opioid antagonists
A total of 40 studies were found investigating µ-opioid antagonists. These can be divided into full opioid antagonists and mixed opioid agonists/antagonists.
Opioid Antagonists
Twenty-two studies used opioid antagonists, of which three in therapeutic setting. A significant reduction in pruritus was reported in fourteen studies, all using opioid antagonists prophylactically. Seven of thirteen studies conducted among parturients showed a substantial reduction of pruritus. A significant decrease in quality of analgesia was reported in six studies using naloxone (either prophylactically or therapeutically) and three studies using naltrexone prophylactically.
The average reduction of pruritus incidence compared to control groups was 66% for naloxone and 76% for naltrexone respectively.
Opioid Agonist/Antagonists
Twenty-four studies used opioid agonist/antagonists, of which 18 were conducted among parturients. A significant reduction of pruritus was reported in 21 studies, of which 15 studies were conducted among parturients. Of the 19 studies using opioid agonist/antagonists prophylactically, 17 showed a significant reduction of pruritus. Of the five studies using opioid agonist/antagonists therapeutically, four studies showed a significant reduction of pruritus.
The average reduction in pruritus incidence was 63% for nalbuphine, 81% for butorphanol and 31% for pentazocine. A significant decrease in quality of analgesia was reported in three studies using nalbuphine, two of which used it in a prophylactic setting.
Antihistamines
Seven studies investigated the effect of antihistamines. Study medication was administered prophylactically in four and therapeutically in three studies. One prophylactic study showed a significant reduction in pruritus incidence.37
Corticosteroids
Four studies investigated the prophylactic use of corticosteroids. Only one study showed a reduction of 39% in the incidence of pruritus.17
Dopamine Antagonists
Nine studies investigated the prophylactic effect of the dopamine antagonists droperidol and alizapride. All but one reported a significant reduction of pruritus. No study used dopamine antagonists in therapeutic settings. The average reduction in pruritus incidence was 39% for droperidol and 45% for alizapride.
Neuraxial Epinephrine
Four studies used neuraxial epinephrine before or together with neuraxial morphine. Two studies found an increase in pruritus when epinephrine and morphine were administered epidurally at the same time. A dose dependent increase was suggested.
NMDA-Receptor Antagonists
Five studies investigated NMDA-receptor antagonists. Four studies used prophylactic ketamine, one study used epidural magnesium sulphate. Kararmaz et al reported a 67% reduction of pruritus using ketamine 0.5mg/kg/hour iv compared with control.22 Farzanegan et al reported no pruritus in patients receiving epidural magnesium sulphate and 10% pruritus in patients without magnesium sulphate epidurally.38
Non-Steroid Anti-Inflammatory Drugs (NSAID’s)
Six studies used prophylactic NSAID’s. Colbert et al mentioned a significant decrease of pruritus using rectal diclofenac 100mg but did not provide data.19 Other studies showed no effect.
Propofol
Ten studies investigated propofol. Six studies used propofol prophylactically, of which four studies showed a significant reduction in pruritus. Five studies used therapeutic propofol with only one study showing a significant reduction in pruritus incidence. The average reduction was 65%.
Other Study Treatments
Six studies investigated other treatment protocols. Jiang et al showed a reduction in pruritus incidence between 31 and 63% for acupuncture.39 In the study of Mazda et al over half of patients were excluded because the acupuncturists were not available.32
Three studies investigated prophylactic use of gabapentin. The pruritus incidence was lower for gabapentin 1200mg compared with placebo. There was no significant difference between gabapentin 600mg and placebo.
Two other drugs were studied in a prophylactic setting, namely midazolam and spinal chloroprocaine 150mg. Elhakim et al reported a significant reduction in pruritus incidence of 48% using midazolam.40 Hess et al reported no improvement using spinal chloroprocaine 150mg.41
Discussion
After our extensive scoping review, we conclude that dopamine antagonists and (mixed) opioid agonists/antagonists effectively prevent pruritus caused by neuraxial morphine. When administered before morphine, 5HT3-antagonists also show an excellent prophylactic effect. In therapeutic settings, the opioid agonist/antagonist nalbuphine was effective in most studies. Other pharmacological groups were not consistently effective and are not recommended to prevent or treat neuraxial morphine-induced pruritus. In general, the effect of study medication varied greatly among all subgroups. No difference was identified between the effect of study medication on pregnant and non-pregnant patients.
The effectiveness of 5HT3-antagonists was observed in most studies when administered before morphine administration. Less than a third of studies administering 5HT3-antagonists at the same time or after morphine showed effectiveness. This suggests a non-reversible modulation of serotonergic pathways caused by neuraxial morphine at the time of injection. Given the time from morphine administration to peak pruritus incidence (6–9 hours), the antipruritic effect of 5HT3-antagonists seems to extend beyond their half-life. 5HT3-antagonists are cheap, have minor side effects, and are frequently used in peri-operative settings to prevent PONV. The use of 5HT3-antagonists with a longer half-life could lead to superior pruritus prophylaxis. The same might be true when using a higher dose due to increased modulation of serotonergic pathways. In the existing literature, ondansetron 4mg IV is studied most frequently. Various 5HT3-antagonists or different doses are used in one or two studies. There is insufficient data to draw any conclusions on the preferred 5HT3-antagonist or dose. Mirtazapine, an antidepressant drug, also has 5HT3-antagonist properties and also showed potential to prevent pruritus.35,36 A recent study showing a dose-dependent increase of serum serotonin and pruritus after intrathecal injection of morphine supports the hypothesis that the serotonergic pathway is essential in intrathecal morphine-induced pruritus.42
As mentioned before, the involvement of dopaminergic pathways in the modulation and prevention of pruritus is unclear. The prophylactic effect of dopamine antagonists appears independent from the timing and administration route. It also seems unrelated to their half-life. Droperidol, the most frequently used dopamine antagonist, has a half-life of approximately two hours.43 This half-life suggests that modulation of dopaminergic pathways by morphine, if present, can be prevented by dopamine antagonists. It also indicates that modulation of dopaminergic pathways by neuraxial morphine, if present, is not a lasting effect.
When administered after morphine, the use of prophylactic droperidol still leads to reduced pruritus rates. This means droperidol might also be effective in therapeutic settings which has not been studied.
The most frequently tested and most effective dose of droperidol in preventing pruritus is 2.5mg IV or epidurally. Epidural droperidol is not approved by the US Food and Drug Administration (FDA). A higher dose of 5 mg was less effective than 2.5 mg in one study and equally effective in others. Two studies reported effectiveness of a lower dose (1.25 mg). More research is needed to estimate the therapeutic effect of droperidol and find the lowest effective dose. This is especially important for dopamine antagonists, given the side effects such as somnolence (which makes early mobilization challenging) and cardiac arrhythmias.43,44
In all studies, µ-opioid antagonists are frequently used as a rescue medication when study medication failed to prevent or treat pruritus. An effect of µ-opioid antagonists on morphine side-effects is to be expected since they directly interfere with its mechanism of action. Opioid agonist/antagonists showed greater effectiveness than full opioid antagonists when studied for their antipruritic effect. This might be due to the greater reversal of analgesia by full opioid antagonists (leading to more pain) or the additional analgesic effect of mixed opioid agonist/antagonists on other opioid receptors. This makes mixed opioid agonist/antagonists the preferred choice over full opioid antagonists. There is no need for continuous medication, as both iv and epidural boluses were effective. This is beneficial for early mobilization. Because of the wide variety of dosages, the optimal amount has not yet been identified. Therefore, advice concerning the preferred dose and route of administration of these drugs cannot be given. Furthermore, it is of note that epidural opioid agonists/antagonists are not FDA approved.
Conclusion
Based on current evidence, droperidol (intravenously or epidurally), nalbuphine (intravenously or neuraxial) or ondansetron intravenously (before neuraxial morphine administration) can be used to prevent neuraxial morphine-induced pruritus. Intravenous nalbuphine can be used to treat neuraxial morphine-induced pruritus. Since doses and ways of administration varied greatly among studies, more research is needed to find the optimal dose, administration route and timing for all recommended medication. Finally, dopamine antagonists should be studied in a therapeutic setting.
Abbreviations
5HT3-receptor, 5-hydroxytryptamine type 3-receptor; COX, cyclo-oxygenases; CSEA, combined spinal and epidural anesthesia; EA, epidural anesthesia; FDA, US Food and Drug Administration; GABA, gamma-aminobutyric acid; IV, intravenous; NA, neuraxial; NMDA-receptor, N-methyl-D-aspartate-receptor; NSAID’s, non-steroid anti inflammatory drugs; PONV, postoperative nausea and vomiting; SA, spinal anaesthesia; VAS, visual analogue score.
Acknowledgments
The authors have no acknowledgements to make.
Disclosure
The authors declare no conflicts of interest. The authors have no sources of funding to declare for this manuscript
References
1. Koning MV, Teunissen AJW, van der Harst E, Ruijgrok EJ, Stolker RJ. Intrathecal Morphine for Laparoscopic Segmental Colonic Resection as Part of an Enhanced Recovery Protocol: a Randomized Controlled Trial. Reg Anesth Pain Med. 2018;43(2):166–173. doi:10.1097/AAP.0000000000000703
2. Koning MV, de Vlieger R, Teunissen AJW, et al. The effect of intrathecal bupivacaine/morphine on quality of recovery in robot-assisted radical prostatectomy: a randomised controlled trial. Anaesthesia. 2020;75(5):599–608. doi:10.1111/anae.14922
3. Horta ML, Morejon LCL, da Cruz AW, et al. Study of the prophylactic effect of droperidol, alizapride, propofol and promethazine on spinal morphine-induced pruritus. Br J Anaesth. 2006;96(6):796–800. doi:10.1093/bja/ael072
4. Koju RB, Gurung BS, Dongol Y. Prophylactic administration of ondansetron in prevention of intrathecal morphine-induced pruritus and post-operative nausea and vomiting in patients undergoing caesarean section. BMC Anesthesiol. 2015;15:1. doi:10.1186/1471-2253-15-18
5. Swaro S, Karan D, Banerjee A. Comparison of Palonosetron, Dexamethasone, and Palonosetron Plus Dexamethasone as Prophylactic Antiemetic and Antipruritic Drug in Patients Receiving Intrathecal Morphine for Lower Segment Cesarean Section. Anesth Essays Res. 2014;12(2):322–327. doi:10.4103/aer.AER_183_17
6. Paech M, Sng B, Ng L, Nathan E, Sia A, Carvalho B. Methylnaltrexone to prevent intrathecal morphine-induced pruritus after Caesarean delivery: a multicentre, randomized clinical trial. Br J Anaesth. 2015;114(3):469–476. doi:10.1093/bja/aeu410
7. Sommer C. Serotonin in pain and analgesia: actions in the periphery. Mol Neurobiol. 2004;30(2):117–125. doi:10.1385/MN:30:2:
8. Tan T, Ojo R, Immani S, Choroszczak P, Carey M. Reduction of severity of pruritus after elective caesarean section under spinal anaesthesia with subarachnoid morphine: a randomised comparison of prophylactic granisetron and ondansetron. Int J Obstet Anesth. 2010;19(1):56–60. doi:10.1016/j.ijoa.2009.05.005
9. Wang W, Zhou L, Sun L. Ondansetron for neuraxial morphine-induced pruritus: a meta-analysis of randomized controlled trials. J Clin Pharm Ther. 2017;42(4):383–393. doi:10.1111/jcpt.12539
10. Siddik-Sayyid SM, Yazbeck-Karam VG, Zahreddine BW, et al. Ondansetron is as effective as diphenhydramine for treatment of morphine-induced pruritus after cesarean delivery. Acta Anaesthesiol Scand. 2010;54(6):764–769. doi:10.1111/j.1399-6576.2010.02231.x
11. Moustafa MA, Saleh RS. Nalbuphine added to intrathecal morphine in total knee arthroplasty; effect on postoperative analgesic requirements and morphine related side effects. Alexandria J Med. 2012;48(2):175–178. doi:10.1016/j.ajme.2012.01.003
12. Wu Z, Kong M, Chen J, Wen L, Wang J, Tan J. Continous Epidural Butorphanol Decreases the Incidence of Intrathecal Morphine-Related Pruritus After Cesarean Section: a Randomized, Double-Blinded, Placebo-Controlled Trial: epidural Butorphanol Decreases the Incidence of Intrathecal Morphine-Related Pruritus. Cell Biochem Biophys. 2014;70(1):209–213. doi:10.1007/s12013-014-9884-9
13. Kung AT, Yang X, Li Y, Vasudevan A, Pratt S, Hess P. Prevention versus treatment of intrathecal morphine-induced pruritus with ondansetron. Int J Obstet Anesth. 2014;23(3):222–226. doi:10.1016/j.ijoa.2014.04.007
14. Gupta SD, Pareek A, Maji S, Kundu SB, Ray M. Influence of Naloxone Infusion on Quality and Duration of Analgesia Produced by Thoracic Epidural Morphine and Bupivacaine Associate Professor 1, Consultant Anaesthetist. J Anaesthesiol. 2014;22(2):548.
15. Etchin A, Perl A, Bider D, Ben Rafael Z. Prevention of a side effect of epidural morphine by epidural steroid administration in cesarean section. Gynecol Obstet Invest. 1990;29(4):305–306. doi:10.1159/000293341
16. Szarvas S, Chellapuri RS, Harmon DC, Owens J, Murphy D, Shorten GD. A comparison of dexamethasone, ondansetron, and dexamethasone plus ondansetron as prophylactic antiemetic and antipruritic therapy in patients receiving intrathecal morphine for major orthopedic surgery. Anesth Analg. 2003;97(1):259–263. doi:10.1213/01.ANE.0000066310.49139.2A
17. Abdel-Aleem M, Osman A, Morsy K. Effect of coadministration of dexamethasone with intrathecal morphine on postoperative outcomes after cesarean delivery. Int J Gynecol Obstetrics. 2012;116(2):158–161. doi:10.1016/j.ijgo.2011.10.002
18. Greaves MW, McDonald-Gibson W. Itch: role of prostaglandins. Br Med J. 1973;3(5881):608–609. doi:10.1136/bmj.3.5881.608
19. Colbert S, O’Hanlon DM, Galvin S, Chambers F, Moriarty DC. The effect of rectal diclofenac on pruritus in patients receiving intrathecal morphine. Anaesthesia. 1999;54(10):948–952. doi:10.1046/j.1365-2044.1999.01066.x
20. Liao CC, Chang CS, Tseng CH, et al. Efficacy of intramuscular nalbuphine versus diphenhydramine for the prevention of epidural morphine-induced pruritus after cesarean delivery. Chang Gung Med J. 2011;34(2):172–178.
21. Tan PH, Kuo MC, Kao PF, Chia YY, Liu K. Patient-Controlled Epidural Analgesia with Morphine or Morphine plus Ketamine for Post-Operative Pain Relief. Eur J Anaesthesiol. 1999;16:820.
22. Kararmaz A, Kaya S, Karaman H, Turhanoglu S, Ozyilmaz MA. Intraoperative intravenous ketamine in combination with epidural analgesia: postoperative analgesia after renal surgery. Anesth Analg. 2003;97(4):1092–1096. doi:10.1213/01.ANE.0000080205.24285.36
23. Neves JFNP, Monteiro GA, Almeida JR, et al. Postoperative analgesia for cesarean section: does the addiction of clonidine to subarachnoid morphine improve the quality of the analgesia? Rev Bras Anestesiol. 2006;56(4):370–376. doi:10.1590/s0034-70942006000400005
24. Goyagi T, Tanaka M, Nishikawa T. Oral Clonidine Premedication Enhances Postoperative Analgesia by Epidural Morphine. Anesthesia Analgesia. 1999;87(6):1487.
25. Sheen MJ, Ho ST, Lee CH, Tsung YC, Chang FL. Preoperative gabapentin prevents intrathecal morphine- induced pruritus after orthopedic surgery. Anesth Analg. 2008;106(6):1868–1872. doi:10.1213/ane.0b013e3181730130
26. Waxler B, Dadabhoy ZP, Stojiljkovic L, Rabito SF. Primer of postoperative pruritus for anesthesiologists. Anesthesiology. 2005;103(1):168–178. doi:10.1097/00000542-200507000-00025
27. Kenany S, Tahan MR. Effect of preoperative pregabalin on post-caesarean delivery analgesia: a dose-response study. Int J Obstet Anesth. 2016;26:24–31. doi:10.1016/j.ijoa.2015.11.001
28. Horta ML, Ramos L, Gonçalves Z, et al. Inhibition of epidural morphine-induced pruritus by intravenous droperidol. The effect of increasing the doses of morphine and of droperidol. Reg Anesth. 2000;21(4):312–317.
29. Nakata K, Mammoto T, Kita T, et al. Continuous Epidural, Not Intravenous, Droperidol Inhibits Pruritus, Nausea, and Vomiting During Epidural Morphine Analgesia. J Clin Anesthesia. 2002;14(2):121.
30. Sanansilp V, Areewatana S, Tonsukchai N. Droperidol and the Side Effects of Epidural Morphine After Cesarean Section. Anesthesia Analgesia. 1998;86(3):532.
31. Misery L, Dutray S, Chastaing M, Schollhammer M, Consoli SG, Consoli SM. Psychogenic itch. Transl Psychiatry. 2018;8(1):52. doi:10.1038/s41398-018-0097-7
32. Mazda Y, Kikuchi T, Yoshimatsu A, Kato A, Nagashima S, Terui K. Acupuncture for reducing pruritus induced by intrathecal morphine at elective cesarean delivery: a placebo-controlled, randomized, double-blind trial. Int J Obstet Anesth. 2018;36:66–76. doi:10.1016/j.ijoa.2018.07.001
33. Chiravanich W, Oofuvong M, Kovitwanawong N. Single Dose of Gabapentin for Prophylaxis Intrathecal Morphine-Induced Pruritus in Orthopedic Surgery: A Randomized Controlled Trial. J Med Assoc Thailand. 2012;95(2):186.
34. Wu Z, Kong M, Wang N, Finlayson RJ, de Tran QH. Intravenous butorphanol administration reduces intrathecal morphine-induced pruritus after cesarean delivery: a randomized, double-blind, placebo-controlled study. J Anesth. 2012;26(5):752–757. doi:10.1007/s00540-012-1421-7
35. Sheen MJ, Ho ST, Lee CH, Tsung YC, Chang FL, Huang ST. Prophylactic mirtazapine reduces intrathecal morphine-induced pruritus. Br J Anaesth. 2008;101:711–715. doi:10.1093/bja/aen241
36. Akhan A, Subasi FD, Bosna G, et al. Comparison of mirtazapine, gabapentin and ondansetron to prevent intrathecal morphine-induced pruritus. North Clin Istanb. 2016;3(1):53–59. doi:10.14744/nci.2016.38233
37. Juneja MM, Ackerman WE, Bellinger K. Epidural morphine pruritus reduction with hydroxyzine in parturients. J Ky Med Assoc. 1991;89(7):319–321.
38. Farzanegan B, Zangi M, Saeedi K, et al. Effect of Adding Magnesium Sulphate to Epidural Bupivacaine and Morphine on Post-Thoracotomy Pain Management: a Randomized, Double-Blind, Clinical Trial. Basic Clin Pharmacol Toxicol. 2018;123(5):602–606. doi:10.1111/bcpt.13047
39. Jiang YH, Jiang W, Jiang LM, et al. Clinical efficacy of acupuncture on the morphine-related side effects in patients undergoing spinal-epidural anesthesia and analgesia. Chin J Integr Med. 2010;16(1):71–74. doi:10.1007/s11655-010-0070-7
40. Elhakim M, Abd-Elfattah H, El-Din DN, El-Kabarity R, Atef A, El-Fakey A. Midazolam as an antiemetic in patients receiving epidural morphine for postoperative pain relief. J Opioid Manag. 2015;5(4):189–195. doi:10.5055/jom.2009.0020
41. Hess PE, Snowman CE, Hahn CJ, Kunze LJ, Ingold VJ, Pratt SD. Chloroprocaine may not affect epidural morphine for postcesarean delivery analgesia. J Clin Anesth. 2006;18(1):29–33. doi:10.1016/j.jclinane.2005.05.003
42. Aly M, Ibrahim A, Farrag W, Abdelsalam K, Mohamed H, Tawfik A. Pruritus after intrathecal morphine for cesarean delivery: incidence, severity and its relation to serum serotonin level. Int J Obstet Anesth. 2018;35:52–56. doi:10.1016/j.ijoa.2018.02.004
43. National Center for Biotechnology Information. PubChem Compound Summary for CID 3168, Droperidol. https://pubchem.ncbi.nlm.nih.gov/compound/Droperidol. Accessed May 31, 2022.
44. Medscape. Inapsine, (droperidol) dosing, indications, interactions, adverse effects, and more. https://reference.medscape.com/drug/inapsine-droperidol-342049#4. Published April 18, 2022. Accessed May 31, 2022.
45. Moher D, Liberati A, Altman D, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Journal of clinical epidemiology. 2009;62(10).
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
