Back to Journals » Veterinary Medicine: Research and Reports » Volume 14
Prevalence, Risk Factors and Control Practices of Livestock Hirudiniasis in Mirab Abaya District, Southern Ethiopia
Authors Seyoum W , Ejigu C, Tora E
Received 2 January 2023
Accepted for publication 7 April 2023
Published 22 April 2023 Volume 2023:14 Pages 79—90
DOI https://doi.org/10.2147/VMRR.S401079
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Young Lyoo
Wasihun Seyoum, Chilotaw Ejigu, Ephrem Tora
Department of Animal Science, College of Agricultural Sciences, Arba Minch University, Arba Minch, Ethiopia
Correspondence: Wasihun Seyoum, Email [email protected]
Purpose: Hirudiniasis is a disease in people and animals who have been infested by blood sucking leeches. In Ethiopia, it is a neglected disease, causing significant economic loss in the livestock industry. In the study area, information on livestock Hirudiniasis is very limited. Therefore, this study was conducted to assess livestock owners’ awareness, knowledge, and control practices toward leech infestation, and to estimate the prevalence and associated risk factors of livestock Hirudiniasis.
Methods: A cross-sectional study was carried out from February to September 2022 on domestic animals randomly selected from livestock owners of Mirab Abaya district, Southern Ethiopia. In total, 384 domestic animals were sampled and examined for the presence of leech infestation. A questionnaire survey was carried out on 150 livestock owners.
Results: This study discovered a 13.54% (52/384) overall prevalence of livestock Hirudiniasis in the study area and the highest prevalence was observed in cattle (22.14%), followed by sheep (10%), goats (8.65%), and equines (5%). Limnatis nilotica was the only leech species identified in the study area. The occurrence of livestock Hirudiniasis was significantly higher in the dry season (OR=3.16, p< 0.05), around bodies of water (OR=10.88, p< 0.01), in extensive production systems (OR=3.81, p< 0.05), and in adult (OR=2.58, p< 0.05) and poor body condition animals (OR=9, p< 0.01). However, the species and sex of domestic animals were not significantly associated with Hirudiniasis. The questionnaire showed 61.33% and 35.33% of respondents had knowledge about leech infestations in domestic animals and its zoonotic impacts, respectively. Manual removal and traditional medicine are common control measures taken by livestock owners against Hirudiniasis.
Conclusion: The present study showed that leeches were prevalent and significantly affects the health and productivity of livestock in the study area. Hence, cost-effective parasitic control measures need to be implemented to address the setback.
Keywords: Hirudiniasis, leech, livestock, prevalence, risk factors, Ethiopia
Introduction
Ethiopia has the largest livestock population in Africa, which plays a significant role in the national economies, livelihoods of poor farmers and pastoral communities.1 The subsector contributes 35.6% of the agricultural Gross Domestic Product (GDP) and 16.5% of the national GDP. Thus, it serves as a “living bank” or “living account” for urban poor communities and rural livestock owners.2 Despite the huge livestock population, productivity is low in Ethiopia due to the presence of many inter-related factors such as widespread diseases, poor genetic potential, shortage of feed supply and nutrition and inefficient livestock services.3–5
Animal health constraints such as water-borne diseases like fasciolosis, paramphistomosis, schistosomosis, and leech infestation (Hirudiniasis) are among the major health problems associated with poor livestock production and productivity in the country.4,6,7 Hirudiniasis is a disease in which people and wild animals become infested by terrestrial or aquatic blood sucking leeches. It is also a neglected water-associated parasitic disease that affects domestic animals and causes a serious economic impact to livestock owners’ due to losses in productivity, mortality, and treatment cost of diseased animals.7,8 Leeches are invertebrate blood-sucking hermaphroditic parasites which belongs to the phylum Annelida, class clitellata, order Arhynchobdellidae, family Hirudinea, and genus Hirudo. They are distributed all over the world due to the presence of diversified agro ecology and habitats such as in bodies of fresh water, rivers, seas, lakes, deserts, and oceans.9–11
More than 650 leech species have been identified and studied in different parts of the world. Leeches vary in shape, color,and length.10 They may be categorized into two classes, namely aquatic leeches (water-borne leeches) and terrestrial leeches (land leeches).10,12 Aquatic leeches are good swimmers and endo-parasites where the degree of infestation depends on the feeding site of different body parts. They are distributed worldwide and mainly found in different bodies of water. Land leeches are ecto-parasites and are usually found in tropical rain forest regions, where they usually live on shrubs, stones, and different plant leaves.10–14 The most prevalent pathogenic water-borne leech species, causing severe diseases in domestic and wild animals, include Limnatis nilotica, Hirudo medicinalis, Dinobdella ferox, Theromyzon tessulatum, Phytobdella catenifera, and Myxobdella Africana, while Haemadipsa sylvestris, Haemadipsa zeylanica, and Haemadipsa picta are the most common pathogenic terrestrial leeches.7,10,12,15
Anatomically leeches have two suckers, one at each end. These are called the anterior and posterior suckers. Because of jaw and teeth, anterior suckers are responsible for sucking blood, while the posterior sucker is mainly used for locomotion purposes. When feeding on host animals, leeches secrete a powerful bioactive anticoagulant called hirudin. They produce this chemical to prevent their host from forming a clot so that they can feast on blood more easily.8,16 It has been reported that domestic and wild animals infestations with leeches mostly occur through the mouth while drinking water. Then leeches attach themselves to the throat of the host animal below the nasal cavity or tongue. They are also present in structures like the pharynx, larynx, trachea, and esophagus of animals. Then leeches attach to the inner lining of these structures and start to ingest blood.17,18 All domestic animals can be infested with leeches, but it is not common in equine animals. The occurrence of livestock Hirudiniasis is higher in bovine, followed by caprine and ovine species.10
Different researchers from different parts of the world have shown coughing, bleeding through the mouth and nasal cavity, reddish discoloration of the lower lip, breathing problems, emaciation, anemia, and reduced milk yield are typical clinical signs of leech infestation in animals.7,10,16,18–20 Manual removalof the parasites, application of medicinal plants and using parasitic drugs such as levamisole and ivermectin are among the main control methods against leech infestations. Medicinal plants such as garlic (Allium sativum), ginger (Zinjiber officinale), yarrow (Achillea millefolium), and nicotine (Nicotiana tabacum) are used to treat Hirudiniasis. Also the provision of clean water for drinking is the best preventive strategy for leech infestation in humans and domestic and wild animals.10,13,21
The majority of reported research work on the prevalence of livestock Hirudiniasis is from developing countries. Many of these cases are highly endemic and commonly occurduring dry seasons where there is dwelling of different water bodies for animals to drink.6,17,21 There are very few organized and recorded research works on the epidemiological status of livestock Hirudiniasis in Ethiopia.4,8,13,22,23 These reports indicated leech infestation is a serious livestock health problem in the highlands areas of the country, where a number of small ponds and streams are used to water different livestock species.4,13,22
Many unpublished works in different parts of Ethiopia have identified leech infestation as a serious and prevalent animal health problem, especially in rural areas.4,13 The epidemiology of leech infestation has not been assessed in the Mirab Abaya district of Southern Ethiopia and has not been given due attention to sustainably address the impact on livestock productivity. Moreover, the dynamics of Hirudiniasis in different livestock species, seasons, agro-ecological zones; and also knowledge level and control practices done by livestock owners have not been studied; thus, the current study is very crucial to clarify this issue. Therefore, the main objectives of this epidemiological study was to assess farmers’ knowledge, awareness, and control practices toward leech infestation, and to estimate the prevalence and identify associated risk factors of livestock Hirudiniasis in the Mirab Abaya district of Southern Ethiopia. Furthermore, this study could complement the paucity of information and also be useful in developing new preventive and control strategies for livestock leech infestation in the study area.
Methods and Materials
Study Area Description
This study was carried out from February to September 2022 in the Mirab Abaya district of Gamo Zone, Southern part of Ethiopia (Figure 1). The district is located as part of well-known East Africa rift valley and bordered on the South by Arba Minch Zuria district, on the North by Wolayita Zone, on the East by Lake Abaya and on the West by Chencha district of Southern Ethiopia. The administrative center of Mirab Abaya district is Biribir town. The area of the district is about 121,150 hectares, of which only 38,342 hectares are used for livestock grazing. The district has lowland, midland, and highland agro-ecological zones accounting for 62%, 27%, and 11% of the total area coverage, respectively. The district receives an average annual rainfall and temperature of 500–580 mm and 27.5°C, respectively. The area lies between 1,100–2,900 m above sea level. A bimodal rainfall pattern is the characteristic feature of the district, in which the long rainy season occurs between June to mid-October and a short rainy season occurs between mid-January to April. The district has an estimated total livestock population of 64,417. A mixed crop and livestock production system is the main livelihood of the district.23,24
 |
Figure 1 Map of the study area. |
Study Population
Cattle, goats, sheep, and equines are the study animals which are kept under extensive and semi-extensive production systems. All body condition scores and age groups of domestic animals were represented in the study. The age of the study animals was determined based on the erupted stages of permanent incisor teeth.25–27 According to this, cattle below 3 years were classified as young and above 3 years as adult age groups. Goats and sheep below 2 years of age were categorized as young and above 2 years as adult. Equines less than 3 years were categorized as young and those above 3 years were categorized as adult age groups. Based on the appearance of the ribs and dorsal spines, the body condition of the study animals was categorized as poor, medium, or good.27,28 Livestock owners who participated in this study were considered the study population and their socio-demographic characteristics, knowledge status, awareness level, and leech control practices were collected using the questionnaire.
Study Design
A cross-sectional study design was carried out from February to September 2022 to estimate the prevalence of livestock Hirudiniasis and to identify potential risk factors associated with the occurrence of disease in the study area. Moreover, a questionnaire survey was also employed to assess the livestock owners’ knowledge, awareness, and control practices toward leech infestation in the study area.
Sampling and Sample Size Determination
A purposive sampling technique was implemented to select study kebeles based on the complaints raised by livestock owners in Mirab Abaya district of Southern Ethiopia. In Ethiopia, a kebele is defined as the lowest administrative sub-division of a district larger than a village.5 Three kebeles, namely Birbir 1, Algie, and Dalbo, were selected for this study. A simple random sampling technique was employed to select study animals from each kebele in which all animals have an equal chance of being selected. The total sample size of domestic animals required for this study was computed using the statistical formula given by Thrusfield et al29 and taking expected prevalencean of 50% at 95% confidence interval and absolute precision of 5%. According to this, the computed sample size was 384. Then, based on the livestock population size of each kebele, proportionate numbers of each species of animals were sampled. Consequently, a total of 112, 120, and 152 animals were sampled from Birbir 1, Algie, and Dalbo kebeles, respectively.
The questionnaire was implemented to assess the farmers’ knowledge, awareness, and control practices toward livestock Hirudiniasis in the study area in conjunction with specimen collection from each study animal. The sample size for questionnaire survey was computed using the formula (n=0.25/SE2) as per Arsham30 at the standard error (SE) of 0.04 with a 96% confidence interval. Based on this, a total of 150 livestock owners were randomly selected from study kebeles. Accordingly, 50 individuals were selected and interviewed from each of the three kebeles of the study district.
Study Methodology
Questionnaire Survey
A questionnaire survey was employed to assess the farmers’ awareness, knowledge, and control practices toward leech infestation in domestic animals. Before conducting the final interviews the questionnaire was pre-tested and modified.
Specimen Collection and Transportation
Before sample collection, each study animal was restrained and physically examined using close observation and inspection technique. Then clinical signs of livestock Hirudiniasis, such as coughing, bleeding through the mouth and nasal cavity, reddish discoloration of the lower lip, breathing problems, emaciation, and anemia were examined.7 Leeches were then severed and removed with tweezers, stored in a plastic universal bottle of well water and labeled with all the necessary information. Then collected leeches from each study animal were transported using an icebox to the Veterinary Parasitology Laboratory of Arba Minch University, Kulfo campus, where they were morphologically identified.
Morphological Identification of Leech Species
In the laboratory, a stereomicroscope was used to examine leech samples and a smartphone camera mounted directly on the stereomicroscope was used to photograph them. Then, using the morphological identification keys described in Negm-Eldin et al31 and Arfuso et al,32 the morphological structures of leeches were characterized and evaluated. Shape, color, and size, number of segments, jaw shape, and external openings of leeches were some of typical the morphological structures that were observed and assessed. All parasitological data were recorded on a data collection sheet. The leech was removed from the bottle with tweezers and placed on a petri dish, where 95% alcohol drops were added to reduce the activity and movement of the leech. Finally, the required body parts of the leech were examined.13,17,33
Data Analysis
All data generated from this study were arranged, coded, and recorded in a Microsoft Excel spreadsheet. STATA version 16.0 computer software (Stata Corp. College Station, TX, USA) was applied for the statistical analysis at 95% confidence interval. Data obtained from the questionnaire survey were computed by descriptive statistics. The prevalence of livestock Hirudiniasis was calculated by dividing the total number of leech infested animals by the total number of examined animals. In addition, both univariate and multivariate logistic regression analyses were employed to identify potential risk factors for the occurrence livestock Hirudiniasis in the study area. After checking the data for collinearity those variables with a P-value less than 0.25 in the univariable logistic analysis were included in the final multivariable logistic model. In all cases, the association was considered significant when the P-value at 95% confidence interval was less than 0.05 (p<0.05).
Results
Socio-Demographic Characteristics of the Respondents
A survey of 150 participants in the study area was conducted to determine their knowledge, awareness, and control practices regarding livestock Hirudiniasis (Table 1). Based on the responses of participants, 48% of the respondents were over the age of 46, while 16.67% were between the ages of 18 and 30. A total of 128 (85.33%) respondents were male and the remaining 22 (14.67%) were female. The largest proportion of participants had no formal education (42.6%). Moreover, the survey found that 48% of respondents had lived in the study area for more than 20 years. In contrast, only 8% had lived there for less than 5 years. Table 1 indicates the socio-demographic characteristics of the participants who were questioned in the study area.
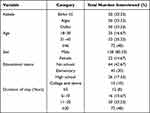 |
Table 1 Socio-Demographic Characteristics of the Respondents (n=150) |
Livestock Owners’ Knowledge, Awareness, and Control Practices of Hirudiniasis
Table 2 reveals the knowledge, awareness, and control practices of livestock owners concerning Hirudiniasis in our study area. Only 53 (35.33%) of the participants were aware of Hirudiniasis’ zoonotic impacts, while 92 (61.33%) participants knew about leech infestations in domestic animals. Among the study participants, 56.67% did not know the clinical signs of Hirudiniasis in domestic animals. However, 43.3% were aware of the disease’s clinical signs, which include drooling saliva with blood, abnormal coughing, loss of appetite, emaciation, anemia, and reduced milk production. They also indicated that cattle were the most susceptible species to leech infestation in the study area, regardless of sex or age.
 |
Table 2 Knowledge, Awareness and Control Practices of Livestock Owners on Hirudiniasis |
Based on respondents’ knowledge of Hirudiniasis predilection sites, leech infestation was seen under the tongue (45.33%), attached to the gums (43%), and hanging in the nasal cavity(22.67%) of live animals. However, 3.33% of respondents observed leech parasites in the throat and sinuses of slaughtered animals. As reported by respondents, livestock in the study area become infected with leech parasites when they drink water from leech-infested bodies of water and graze in leech-infested deforested areas. They also stated that Hirudiniasis occurs more commonly during the dry season.
Participants stated that Hirudiniasis has both direct and indirect effects on economic aspects of livestock owners in the current study area. According to the respondents, a decrease in production (60%), loss of diseased animals (10%), veterinary costs (17.33%), and combination effects (4.67%) are some of the major economic impacts of livestock Hirudiniasis in the study area. Furthermore, livestock owners in the study areas had applied various treatment, control, and preventive strategies to overcome the problem of leech infestation in domestic animals. Manual removal (40.6%), traditional medicine using different plants (30%), transport to veterinary clinic (15.33%), and salt water drenching (9.33%) are some of the measurement options taken by livestock owners in the study area.
Overall Prevalence of Livestock Hirudiniasis in the Study Area
Of a total of 384 domestic animals examined for leech infestation, 52 were found to be infested, resulting in an overall prevalence of 13.54% (52/384) in the current study area (Table 3). The highest proportion of Hirudiniasis was observed in cattle (22.14%), followed by sheep (10%), goats (8.65%), and equines (5%). In addition, leeches collected from infested animals and water bodies were examined using a stereomicroscope. Thus, Limnatis nilotica was identified as the only leech species causing livestock Hirudiniasis in the study area by morphological identification (Figure 2).
 |
Table 3 Overall Prevalence of Livestock Hirudiniasis in the Study Area |
 |
Figure 2 Leech attached on the tongue of cattle (see the arrow). |
Association Between Putative Factors and Livestock Hirudiniasis
Species, sex, age, body condition score (BCS), season, grazing area, production type, and kebeles of the study district were considered as possible risk factors for the presence of livestock Hirudiniasis in the study area, by univariable logistic regression analysis (Table 4). Among these factors, species, age, body condition score, season, grazing, and production system were found to be significantly associated (p<0.05) with livestock Hirudiniasis. In contrast, sex of animals and kebeles of the study district did not show a significant association with the prevalence of leech infestation in the current study area (p>0.05). After checking for collinearity, all risk factors with a p-value less than 0.25 in the univariable analysis were further subjected to stepwise backward multivariable logistic regression analysis. Based on the final multivariable logistic regression model for potential risk factor analysis, it was found that age, body condition score, season, grazing area, and production types were associated with Hirudiniasis prevalence in the study area and were, therefore, potential risk factors (Table 5).
 |
Table 4 Univariable Logistic Regression Analysis for Risk Factors of Livestock Hirudiniasis |
 |
Table 5 Multivariable Logistic Regression Analysis for Risk Factors of Livestock Hirudiniasis |
The multivariable logistic regression analysis for the occurrences of livestock Hirudiniasis and its potential risk factors in the study area are summarized in Table 5. According to the results, a significantly higher prevalence of livestock Hirudiniasis was observed in the dry season (OR=3.16, p<0.05), around water bodies grazing areas (OR=10.88, p<0.01), with extensive production systems (OR=3.81, p<0.01), in adult age groups (OR=2.58, p<0.01) and in poor body condition animals (OR=9, p<0.01). The odds of getting Hirudiniasis in an adult age group of study animals was 2.58-times higher than in young age group animals. Poor body-conditioned animals are 9-times moresusceptible for Hirudiniasis than good body conditioned study animals. Based on the season, the odds of getting leech infestation were 3.16-times higher in the dry study season than in the wet study season. Leech infestation around water bodies was 10.88-times higher than in dry land grazing areas. Furthermore, domestic animals reared under an extensive production system are 3.81-times more susceptible for leech infestation than animals reared in a semi-intensive production system in the study area.
Discussion
This study shows that Hirudiniasis is an indispensable disease and a potential problem to the health and productivity of livestock in the Mirab Abaya district of Southern Ethiopia. It had an overall prevalence of 13.54%. The present result is in agreement with previous reports of 11.41% by Negm-Eldin et al,31 from livestock farms in the Green Mountain area of Libya. The current result was higher than the findings of Amsalu et al4 and Nyamsingwa,34 who reported an overall prevalence of 3.4% and 0%, respectively, from livestock farms of selected districts of Northern Ethiopia and cattle in the Ngorongoro district of Tanzania. The ecological condition, seasonal difference during the study period, leech control operations used, livestock management systems of the respective study area, animal owners awareness status about the disease, variation in leech density and quantity of water in infested springs where animals drink are all factors that may be responsible for the variation in the overall prevalence of livestock Hirudiniasis across the study sites.10,13,18
During the study period, the highest proportion of Hirudiniasis was observed in cattle (22.14%), followed by sheep (10%), goats (8.65%), and equines (5%). Similarly, Amsalu et al4 and Negm-Eldin et al31 reported larger leech burdens on cattle examined in the Western Gojam Zone of Northern Ethiopia and the Green Mountain area of Libya, respectively, compared with other species of animals studied. This was supported by Bahmani et al,19 who reported a higher proportion of Hirudiniasis in cattle followed by goats, sheep, and equines. This might be due to the consumption of larger quantities of water by cattle that may expose them to greater probabilities of leech infestation than other domestic animals.
Among the total examined animals from each kebele in the study district, male prevalence was higher (15%) than female prevalence (12.04%). However, a statistically significant difference (p>0.05) in leech prevalence was not observed between the sexes. This finding coincides with previous research conducted by Amsalu et al4 in selected districts of the Amhara region, Northern Ethiopia. This is likely because both sexes would have an equal chance of getting infected if they were exposed to the parasite.
The study was conducted to see the existence of leech infestation based on the age of animals and revealed that there was a higher prevalence in adults and a lower prevalence in animals of younger age. The odds of getting Hirudiniasis in adult age group of animals was 2.58-times higher than those in young age group animals. Similar reports have been reported previously.4,31,34 This might be due to increased exposure of animals to leech infestation as age increases. This might be due to the sample size of study animals and sampling strategies. The strategies resulted in a considerably higher number of adult animals than young animals because age stratification was not performed during sampling.
When comparing animals with poor body condition scores with those with medium and good body condition scores, a significantly higher prevalence was observed. Poor body condition animals were 7.13-times more likely to develop infestations than good body condition animals in the study area. Medium body condition scores were 2.79-times more than good body condition animals. There is a strong agreement with Amsalu et al,4 who reported a higher prevalence of leech infestations in domestic animals of poor body condition in Mecha and South Achefer districts of Northern Ethiopia’s Amahra region. Leech infestation in domestic animals is clinically manifested by coughing, loss of appetite, blood loss in the mouth and nose, and anemia; and another consequence of these conditions is a loss of body condition.27,35,36 While poor body condition can arise from other factors, such as concurrent nutritional and other parasitic diseases, Hirudiniasis also results in loss of body condition.
This study found that livestock leech infestation was significantly different in wet and dry seasons of the study period. Livestock Hirudiniasis was occurred higher in the dry season than in the wet season in this study area. The odds of getting leech infestation were 3.16-times higher in the dry study season than in the wet study season. Previously, Nyamsingwa34 and Negm-Eldin et al31 had reported similar findings in Tanzania’s Ngorongoro district and Libya’s Green Mountain, respectively. A leech infestation is very common during the dry (hot) season, when there are fewer water pools for cattle to drink at. It is because during the dry study season the volume of water bodies where animals drink is decreased. In addition, the temperature of the environment is suitable for leeches to thrive in different water bodies. Also, as a body of water's volume increases, so does it’s speed, making it more difficult for leeches to settle undisturbed and as a result allowing them to be easily captured by run-off water.10,21,31,34,37
Based on the types of grazing area in the study area, a significantly higher proportion of Hirudiniasis was observed in those animals grazing around bodies of water than in those animals grazing on dry land. Domestic animals grazing near water are 10.88-times more susceptible to leech infestation than those that graze on pasture on dry land. Similar reports have been done previously.4,18,31,34 This is because leeches are more abundantly found around water (aquatic leeches) than dry land (terrestrial leeches) and hence animals grazing near different bodies of water are more susceptible to leech infestation than animals grazing on dry land.12,14,31,37
With regard to livestock production type, the incidence of leech infestation was significant in the study kebeles of Mirab Abaya district. The chance of getting Hirudiniasis in extensively managed domestic animals was 3.81-times higher than those domestic animals kept in a semi-intensive production system. Similarly, a study conducted by Amsalu et al,4 in the Mecha and South Achefer districts of the Amahra region, Northern Ethiopia, found a statistically significant difference between livestock housed in extensive and semi-intensive production methods. It may be because livestock in extensive production systems graze repeatedly on pasture and are exposed to running water bodies, which increases the chances of contracting leech disease. In addition, animals kept in semi-intensive systems have access to palatable and nourishing ad-lib feed, which, in contrast to animals kept in extensively managed systems, boosts their resistance to diseases.4,21,31
Questionnaire survey results revealed that 61.33% of respondents had knowledge of leech infestations in domestic animals and 35.33% of respondents became aware of the zoonotic impacts of Hirudiniasis. They also elaborated that leeches infected human being and animals when they drank them with water. Similarly reports by Amsalu et al4 from Ethiopia and Nyamsingwa34 from Tanzania found that livestock owners had knowledge of leech infestations in both animals and humans. Responses from interviewees with knowledge of Hirudiniasis in domestic animals reported finding leech infestations under the tongue, attached to the gums, hanging in the nose of live animals, and hanging in the sinuses and throat of slaughtered animals. This finding coincides with previous reports.31,34,36,38
Interviewees also reported high livestock Hirudiniasis in the dry season with the reason being similar with reports of Grisi et al39 and Negm-Eldin et al,31 who reported that the total population (particularly the immature) increased during the summer season when the water temperature increased. According to these authors, high temperatures during summer months favor the prevalence and intensity of infestation of farm animals by leeches.
According to the respondents, a decrease in production (60%), loss of diseased animals (10%), veterinary costs (17.33%), and combination effects (4.67%) are some of the major direct and indirect economic impacts of Hirudiniasis in the current study area. This finding is similar with the findings of Amsalu et al4 in South Achefer districts of Northern Ethiopia and Bahmani et al38 in the Ilam province of Iran.
The local population in the research areas, according to the respondents, employs a variety of treatment, control, and preventive techniques to address the issue of leech infestation in domestic animals. Respondents used traditional medicine, salt water drenching, and veterinary clinic treatment for leech-infested animals that were sick. This was similar to the reports of Amsalu et al4 in Mecha and South Achefer districts of Amahra region, Northern Ethiopia, Eguale et al13 in the Gurage Zone of Southern Ethiopia, Ogello et al40 in the Kegati Aquaculture Research Station of Kenya, and Nyamsingwa34 in the Ngorongoro district of Tanzania.
Conclusions and Recommendations
This study reveals that Hirudiniasis is an infectious disease and a potential threat to the health and productivity of livestock in the Mirab Abaya district of Southern Ethiopia. The overall prevalence of livestock Hirudiniasis in the study area was 13.54%. The highest prevalence was observed in cattle, followed by sheep, goats, and equines, respectively. Seasons, types of grazing regions, production system, age, and animal body condition scores all significant for the occurrence of Livestock Hirudiniasis. Limnatis nilotica was the only leech species found in the study area during the study period based on parasitological results. Among the participants in the study, 61.33% knew that leech infestation can occur in domestic animals, and 35.33% knew that it can also occur in humans. Additionally, the survey revealed that most farmers were unaware of the clinical signs and disease epidemiology. Decrease in production, loss of diseased animals, veterinary costs, and combination effects are the major economic impacts of livestock Hirudiniasis identified in the study area. Furthermore, manual removal, traditional medicine, salt water drenching, and treatment at veterinary clinics are among the control measures the respondents in the study area used to combat livestock Hirudiniasis. Therefore, appropriate leech control measures should be implemented to address the setback. It is pertinent to conduct awareness-raising training on disease transmission, epidemiology, and its control in the study area for smallholder livestock farmers.
Abbreviations
CSA, Central Statistical Agency; CI, Confidence interval; GZLFO, Gamo Zone Livestock and Fishery Office; GDP, Gross Domestic Product (GDP); OR: Odds ratio.
Data Sharing Statement
All raw data generated, analyzed, and presented in this article are available by the contacting the corresponding author.
Ethical Consideration
Ethical approval for this study was obtained from the Animal Research Review Committee of Arba Minch University, Ethiopia (Ref. No.: AMU/AREC/10/2015). Before starting the research in the study area, the objectives of the study were presented to all participants. Then written and signed consent was obtained from the livestock owners in the study area. Animal owner permission was also taken while taking leech samples from study animals. When livestock owners were illiterate, only informed verbal consent was obtained. Verbal informed consent was acceptable and approved by the ethics committee of Arba Minch University. All best animal health practices, proper guidelines, and regulations were applied.
Acknowledgments
The authors would like to express sincere gratitude to Arba Minch University and staff members of Animal Science Department for their unreserved technical and laboratory material support during the study period. Also the livestock owners and animal health workers of Mirab Abaya district are highly acknowledged for their cooperation and participation during sample collection and interview time.
Author Contributions
All authors made a significant contribution in the conception, study design, execution, acquisition of data, analysis, and interpretation; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was not funded by any sources or institutions.
Disclosure
All authors confirmed that they have no conflict of interest in relation to this research work.
References
1. Central Statistical Agency (CSA). Central Statistical Agency: agricultural sample survey. Report on livestock and livestock characteristics, federal democratic republic of Ethiopia, Addis Ababa; 2018:12–20.
2. Leta S, Mesele F. Spatial analysis of cattle and shoat population in Ethiopia: growth trend, distribution and market access. Springer Plus. 2014;3(1):310. doi:10.1186/2193-1801-3-310
3. Belay D, Yisehak K, Janssens GPJ. Analysis of constraints facing urban dairy farmers and gender responsibility in animal management in Jimma town. Afr J Basic Appl Sci. 2011;3(6):313–318.
4. Amsalu T, Teshager N, Alemu B. Assessment of Leeches’ impact on livestock productivity in the selected districts. Appl J Hygiene. 2019;8(1):1–11. doi:10.5829/idosi.ajh.2019.01.11
5. Seyoum W, Tora E, Kore K, Lejebo F. Seasonal patterns: bovine Trypanosomosis, Glossina pallidipes density, and infection in Rift Valleys of Gamo Zone, Southern Ethiopia. Front Vet Sci. 2022;9:1–13. doi:10.3389/fvets.2022.805564
6. Nzalawahi J, Kassuku AA, Stothard JR, Coles G. Trematode infections in cattle in Arumeru District, Tanzania are associated with infection. Parasit Vectors. 2014;7(107):1–5. doi:10.1186/1756-3305-7-107
7. Misheck AM, Joyce S, Philbert BM, Richard SS. Hirudiniasis in cattle in Mpwapwa District, Dodoma Region of Tanzania. Case Rep Vet Med. 2020;1–6. doi:10.1155/2020/3028345
8. Shitaye N, Shibabaw S. Severe anemia due to pharyngeal leech infestation; a case report from Ethiopia. BMC Surg. 2017;17:102. doi:10.1186/s12893-017-0298-7
9. Gouda HA. The effect of peritrich ciliates on some freshwater leeches from Assiut, Egypt. J Invertebr Pathol. 2006;93(3):
10. Abdisa T. Therapeutic importance of leech and impact of leech in domestic animals. MOJ Drug Des Develop Ther. 2018;2(6):
11. Getahun AM, Endayehu Y, Berhanu GD, Case A. Report on leech infestation as a cause of severe anemia in a 10-month-old infant in Ethiopia. Int Med Case Rep J. 2021;14:111–114. doi:10.2147/IMCRJ.S292226
12. Zaidi SM, Jameel S, Zaman F, Jilani S, Sultana A, Khan SA. A systematic overview of the medicinal importance of sanguivorous leeches. Altern Med Rev. 2011;16(1):
13. Eguale T, Abie G, Sahile M, Gizaw D. Control of aquatic Leeches (Limnatis nilotia) causing Phytolacca dodecandra (Endod) in Sodo district, Gurage zone, South Nations, Nationalities and People region, Ethiopia. Ethiop Vet J. 2010;14(2):125–135. doi:10.4314/evj.v14i2.63889
14. Phillips AJ, Brown RA, Figueroa AO, et al. Tyrannobdella rex N. Gen. N. Sp. and the evolutionary origins of mucosal leech infestations. PLoS One. 2010;5(4):1–8. doi:10.1371/journal.pone.0010057
15. Lai YT, Nakano T, Chen JH. Three species of land leeches from Taiwan, Haemadipsa rjukjuanacomb. n., a new record for Haemadipsa picta Moore, and an updated description of Tritetrabdella Taiwan (Oka). ZooKeys. 2011;139:1–22. doi:10.3897/zookeys.139.1711
16. Mekonnen D. Leech infestation: the unusual cause of upper airway obstruction. Ethiop J Health Sci. 2013;23(1):65–68.
17. Garca MF, Yelken MK, Okur MH, Yuca SA. Leech infestation on the nasophrynx: a rare cause of epistaxis and hemorrhage. Eur J Gen Med. 2011;8(2):141–143. doi:10.29333/ejgm/82715
18. Bahmani M, Rasouli M, Parsaei P, Banihabib E, Saki K, Ghotbian F. Case report: limnatis nilotica infestation in a ram and kid in Dehlonllam province in West Iran. Asian Pac J Trop Dis. 2013;3(2):155–157. doi:10.1016/S2222-1808(13)60061-4
19. Bahmani M, Golshahi H, Ghotbian F, Bahmani F. Internal hirudiniasis in a hen (Gallus domesticus). The first report in literature. Asian Pac J Trop Dis. 2013;3(3):
20. Sarathi K. Nasal leech infestation causing persistent epistaxis. J Emerg Trauma Shock. 2011;4(3):413–414. doi:10.4103/0974-2700.83875
21. Behçet AL, Yenen ME, Aldemir M. Rectal bleeding due to leech bite: a case report. Ulus Travma Acil Cerrahi Derg. 2011;17(1):83–86. doi:10.5505/tjtes.2011.75318
22. Amsalu T, Teshager N, Alemu B. Evaluating the Efficacy of Selected Plants on the Control Leeches. Appl J Hygiene. 2019;8(1):12–18. doi:10.5829/idosi.ajh.2019.12.18
23. Mohammed N, Taye M, Asha A, Sheferaw D. Epizootological study of small ruminant gastrointestinal strongyles in Gamo-Gofa Zone, Southern Ethiopia. J Parasit Dis. 2016;40(2):1–6. doi:10.1007/s12639-014-0528-1
24. GZLFRD. Gamo Zone Livestock and Fishery Office: Annual Report on Livestock and Livestock Characteristics. Arbaminch, Gamo, Ethiopia: Gamo Zone Livestock and Fishery Office; 2021:1–5.
25. Muylle S, Simoens P, Lauwers H, Van Loon G. Age determination in mini‐Shetland ponies and donkeys. Zentralbl Veterinarmed A. 1999;46(7):421–429. doi:10.1046/j.1439-0442.1999.00229.x
26. Ullman-Culleré MH, Foltz CJ. Body condition scoring: a rapid and accurate method for assessing health status in mice. Comparative study. Lab Anim Sci. 1999;49(3):319–323.
27. Radostits OM, Gay CC, Hinchcliff KW, Constable PD. Veterinary medicine. In: A Text Book of Diseases of Cattle, Sheep, Pigs, Goats and Horses.
28. Nicholson MJ, Butterworth MH. A Guide to Condition Scoring of Zebu Cattle. Addis Ababa: International Livestock Centre for Africa; 1986:1–29.
29. Thrusfield M, Christley R, Brown H, et al. Veterinary Epidemiology.
30. Arsham H. Descriptive sampling data analysis. Statistical thinking for managerial decision making; 2002. Available from: http://home.ubalt.edu/ntsbarsh/Business-stat/opre504.htm.
31. Negm-Eldin MM, Abdraba AM, Benamer HE. The first reported leech infestation by Limnatisnilotica (Savigny 1822) of farm animals in Libya. Travaux de l’InstitutScientifique Rabat SérieZoologie. 2013;49:33–36.
32. Arfuso F, Gaglio G, Ferrara MC, Abbate F, Giannetto S, Brianti E. First record of infestation by nasal leeches, Limnatisnilotica (Hirudinida, Praobdellidae), from cattle in Italy. J Vet Med Sci. 2019;81(10):1419–1423. doi:10.1292/jvms.19-0247
33. Forouzan S, Bahmani M, Parsaei P, et al. Anti – parasitic activities of Zinjiber Officinale Methonolic extract on Limnatisnilotica. Global Vet. 2012;9(2):144–148. doi:10.5829/idosi.gv.2012.9.2.63134
34. Nyamsingwa ZI. Studies on Prevalence and the Importance of Cattle Leech Infestation in Ngorongoro District, Tanzania [master thesis]. Sokoine University of Agriculture; 2016.
35. Urquhart GM, Armour J, Duncan JL, Dunn AM, Jennings FW. Veterinary Parasitology.
36. Ahmad R, Baharuddin KA, Zaidin H, Mohidin MA, Kheng CP, Sidek N. An unusual case of urethral hiriduniasis. Southeast Asian J Trop Med Public Health. 2008;39(2):319–323.
37. Sket B, Trontelj P. Global diversity of Leeche (Hirudinea) in freshwater. Hydrobiologia. 2008;595(1):129–137. doi:10.1007/s10750-007-9010-8
38. Bahmani M, Eftekhari Z, Mohsezadeghan A, Ghotbian F, Alighazi N. Leech (Limnatis nilotica) causing respiratory distress in a pregnant cow in Ilam province in Iran. Comp Clin Pathol. 2012;21(4):501–503. doi:10.1007/s00580-011-1236-1
39. Grisi L, Leite RC, Martins JRS, et al. Reassessment of the potential economic impact of cattle parasites in Brazil. Rev Bras Parasitol Vet. 2014;23(2):150–156. doi:10.1590/S1984-29612014042
40. Ogello EO, Kembenya EM, Obiero KO, Munguti JM. Effect of nicotina tobacum (Linnaeus) on the survival and behavioral response of the fresh water Leeches, Hirudinaria sp. Int J Aquat Sci. 2016;1(1):19–24.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
