Back to Journals » Research and Reports in Tropical Medicine » Volume 6
Prevalence of vulvovaginal candidiasis among nonpregnant women attending a tertiary health care facility in Abuja, Nigeria
Authors Emeribe A , Abdullahi Nasir I , Onyia J, Ifunanya AL
Received 17 February 2015
Accepted for publication 27 April 2015
Published 29 June 2015 Volume 2015:6 Pages 37—42
DOI https://doi.org/10.2147/RRTM.S82984
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Thomas Unnasch
Anthony Uchenna Emeribe,1 Idris Abdullahi Nasir,2 Justus Onyia,2 Alinwachukwu Loveth Ifunanya3
1Department of Medical Laboratory Science, University of Calabar, Calabar, Cross River State, Nigeria; 2Department of Medical Microbiology, University of Abuja Teaching Hospital, Gwagwalada, Federal Capital Territory, Nigeria; 3Department of Medical Laboratory, School of Health Technology, Tsafe, Zamfara State, Nigeria
Background: Candida spp. are normal flora of the vagina that eventually become pathogenic under some prevailing conditions, and thus present as a common etiology of vulvovaginitis. When prompt recognition and laboratory confirmation is not achieved, this could lead to devastating genital discomfort and a major reason for frequent hospital visits.
Aims: This was a cross-sectional prospective study that aimed to determine the prevalence and some associated risk factors of vulvovaginal candidiasis (VVC) among nonpregnant women attending University of Abuja Teaching Hospital, Gwagwalada.
Subjects and methods: A pair of high vaginal swab and endocervical swab samples was collected from each of 200 individual participating subjects. They were separately inoculated on Sabouraud's dextrose agar and incubated aerobically at 33°C for 48 hours. Ten percent KOH wet mount and Gram staining was done on swabs and colonies, respectively. Structured questionnaires were used to obtain sociodemographic and clinical data.
Results: Of the 200 participating subjects, the prevalence of Candida albicans was 6.5% and that of non-albicans candidiasis 7.5%. Candidiasis was observed mostly among the 20- to 30-year age-group. All subjects with Candida-positive culture had been on antibacterial therapy prior to participating in this study – 28 (100%). There was a statistical relationship between the prevalence of VVC with previous antibacterial therapy (P<0.05), but not with age or other prevailing health conditions studied (P>0.05).
Conclusion: The outcome of this study indicated involvement of Candida spp. in vulvovaginitis among nonpregnant women, especially those on empirical antibacterial therapy. Moreover, it is worthwhile to consider culture-test results as adjunctive in combination with clinical symptoms in the definitive diagnosis of VVC. Due to the importance of our findings, sex-education workshops should be conducted to educate women on the clinical significance of Candida in vulvovaginitis.
Keywords: vulvovaginal candidiasis, antibacterial therapy, nonpregnant, Abuja
Introduction
Vulvovaginal candidiasis (VVC) is a fungal or yeast infection of the female lower genital tract, the vulva, and the vagina caused by Candida spp.1–3 It can be referred to as candidiasis or moniliasis. VVC can be recurrent or relapsing.4,5 Recurrent or relapsing VVC occurs when a woman presents with four or more episodes per year. This condition affects less than 5% of healthy women.6
Candida spp. are part of the lower genital tract flora in 20%–50% of healthy asymptomatic women.7 C. albicans is the most frequent colonizer, and is incriminated in most cases of VVC.8 Nevertheless, over the last 10 years, research evidence has demonstrated an increment in the frequency of cases caused by non-albicans species, with C. glabrata consistently being the leading species.9,10 About 75% of women will experience at least one episode of VVC during their lifetime. In fact, 70%–75% of healthy adult women have at least one episode of VVC during their reproductive life, and half of college women will by the age of 25 years have had one episode of VVC diagnosed by a physician.11
VVC is not considered a sexually transmitted disease, because it does affect children and celibate women, and also Candida spp. are seen as normal vagina flora in healthy women. However, this does not mean that Candida cannot be sexually transmitted.12–14
Diagnosis of VVC based solely on patient history and genital examination is not possible because of the low specificity of symptoms and signs, since other causes mimic VVC, like leukorrhea and pruritus vulvae;15 therefore, to have a definitive diagnosis of VVC, cultural isolation and identification of Candida spp. are crucial.
Previous findings have provided data on the incidence of VVC. These suggested that approximately two-thirds of women experience at least one episode during their lifetime, and nearly 50% of women had multiple episodes.16,17 It is interesting to note that most previous studies focused on immunocompromised subjects, especially pregnant women, diabetics, subjects on broad-spectrum antibiotic therapy, women on oral contraception with high estrogen content, and HIV-positive subjects, with few studies on otherwise immunocompetent women.18
The normal vagina is characterized by dynamic interrelationships between Lactobacillus acidophilus and other endogenous flora, estrogen, glycogen, vaginal pH, and metabolic by-products of these microbiomes. L. acidophilus produces hydrogen peroxide (as a by-product of metabolism), which is toxic to pathogens and keeps the healthy vaginal pH acidic. Vaginitis occurs when the vaginal microflora have been altered by invading pathogens or biochemical changes in the environment.18 Changes in the vaginal environment encourage the Candida population, enhance their adherence to vaginal epithelial cells, and facilitate germination of daughter yeast cells.19 These changes may transform asymptomatic colonization into symptomatic Candida infection. VVC, like many vulvar diseases, has the potential to cause great psychological distress and negatively impact a patient’s quality of life.
Subjects and methods
Study area
This study was conducted at the University of Abuja Teaching Hospital, Gwagwalada, Abuja. Gwagwalada is one of the five local government area councils of the Federal Capital Territory of Nigeria, together with Abaji, Kuje, Bwari, and Kwali; the territory includes the city of Abuja, the capital city of Nigeria, which is located in the center (9°4′0″N, 7°29′0″E) of Nigeria. Gwagwalada has an area of 1,043 km2, and had an estimated population of 157,770 at the 2006 census.
Sample-size calculation
The sample size was determined using data from a prevalence study conducted in Nigeria with a prevalence of vaginal candidiasis of 14%, as demonstrated by Okonkwo and Umeanaeto.20 Therefore, the minimum sample size required for this study with a 5% margin of error and 95% confidence level was 185.
Study population
A pair of high vaginal swab (HVS) and endocervical swab (ECS) samples was collected from each of 200 individual participating subjects. Subjects were recruited from the General Out-patient and Gynecology departments at the University of Abuja Teaching Hospital, Gwagwalada. The selection of patients was done with the support of attending physicians and nurses of these departments.
Criteria
Inclusion criteria were age between 15 and 45 years, with or without signs and symptoms of vulvovaginal discomfort, and not pregnant. Exclusion criteria were age under 15 or over 45 years, pregnant, diabetics, and menstruating. The procedure employed consisted of a questionnaire interview and the taking of patients’ clinical history.
Ethical clearance and informed consent
This study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Human Research Ethical Committee of the University of Abuja Teaching Hospital. All subjects gave their written informed consent for inclusion before they participated in the study. All data were analyzed anonymously throughout the study.
Questionnaire
Structured questionnaires were used by attending physicians to obtain such data as age, marital status, prior antibacterial therapy, clinical signs and symptoms, and provisional diagnosis.
Sample collection
HVS and ECS specimens were carefully and appropriately collected with sterile cotton swabs from the vagina and cervix, respectively. While contamination was minimized, a pair of swabs was collected from each subject. Both were separately inoculated onto Sabouraud’s dextrose agar and incubated at 25°C and 37°C respectively, aerobically. Thereafter, 10% KOH wet mounts were made from the swabs and examined microscopically using a 40× objective lens for the presence of pseudohyphae and/or budding yeast cells suggestive of Candida.21
Identification
Yeast growths were identified by characteristic colonial morphology of Candida on Sabouraud’s dextrose agar. Growths were then confirmed to be Gram-positive budding fungal cells by Gram staining. Germ-tube tests were also performed to identify C. albicans.
Statistical methods
Data generated from questionnaires and results of the laboratory analysis were entered into Microsoft Excel and analyzed using SPSS software (version 20; IBM Corporation, Armonk, NY, USA). Results obtained were reduced to percentages, tables, and a figure. The Pearson χ2 test at a 95% confidence interval and 0.05 level of significance was used to determine the relationships between some sociodemographic/clinical data and prevalence rates.
Results
This was a 6-month study on VVC among nonpregnant women aged 15–45 years with and without clinical signs and symptoms of vulvovaginal discomfort attending the University of Abuja Teaching Hospital, Gwagwalada for medical assistance and voluntary participation. Paired HVS and ECS samples were collected from each of the 200 participating subjects and analyzed for isolation and identification of both C. albicans and non-albicans Candida spp.
Of the 200 subjects recruited, 28 had Candida-positive cultures from both HVS and ECS samples, making the prevalence of VVC 14.0%. Candida-positive cultures were observed mostly among the age-group 20–30 years (17 [8.5%]) and least among those less than 20 years and greater than 40 years (Table 1). There was no significant statistical relationship between the prevalence of VVC and age (P>0.05).
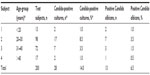  | Table 1 Age distribution of candidiasis among women attending University of Abuja Teaching Hospital |
There were equal Candida-positive cultures among women who presented with clinical symptoms of ill health and those who were apparently healthy and voluntarily came for the study (with no clinical presentations of ill health). Of 83 test subjects who presented with one or more clinical symptoms of ill health, 14 (16.9%) had candidiasis. However, of 117 women who voluntarily participated (those with no clinical symptoms of ill health), 14 (11.9%) had candidiasis. There was no statistically significant relationship between candidiasis and prevailing clinical symptoms of ill health (P=0.34, Table 2). All subjects with Candida-positive culture had been on antibacterial therapy prior to participating in this study, 28 (100%), (Table 3).
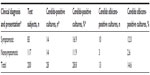  | Table 2 Distribution of Candida-positive cultures across clinical presentation |
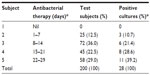  | Table 3 Distribution of vulvovaginal candidiasis and frequency of antibacterial therapy |
Of the 200 participating subjects, only 24 had notable clinical symptoms of vulvovaginal discomfort and seven candidiasis, putting the prevalence at 29.2% (Figure 1). However, the prevalence of C. albicans among the general test subjects was 6.5% (13 of 200), and that of non-albicans candidiasis was 7.5% (15 of 200) (Table 1).
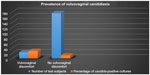  | Figure 1 Bar-chart representation of Candida-positive cultures among those with vulvovaginal discomfort and those with no vulvovaginal discomfort. |
Discussion
Vaginal candidiasis is a common mucosal membrane infection caused predominantly by C. albicans, which can affect significant numbers of otherwise healthy women of childbearing age. Vaginal candidiasis is one of the common infections of general medical practice, second only to anaerobic bacterial vaginosis. About three-quarters of all women suffer at least one episode of this condition during their lifetime.22
The prevalence of vaginal candidiasis reported by different studies was 16.5%, 21.31%, and 19%.22–24 In our study, the prevalence was found to be 14%. This relatively low prevalence of vaginal candidiasis among women attending the University of Abuja Teaching Hospital may be attributed to adequate knowledge, good personal hygiene, and normal levels of estrogens and corticoids.25
Candida-positive cultures were observed mostly among the age-group 20–30 years (17 [8.5%]) and least among those less than 20 years and greater than 40 years. These findings are not in consonance with the findings of Alo et al,26 who reported a higher prevalence of C. albicans (33.33%) within the age bracket of 36–40 years, while those between 20 and 25 years had the lowest prevalence (20.42%). This outcome agreed with Akortha et al27 and Willacy and Jackson,28 who reported peak vaginal infections between ages 20 and 40 years. This may be due to high sexual activity, poor personal hygiene, the use of contraceptives, and drug abuse among this age-group. Those 26–30 years old represent the peak of childbearing in Nigerian societies, and it was among this group that significantly high prevalence of vaginal candidiasis occurred. Advancement in age, on the other hand, reduces the effect of estrogen hormone in women, which could lead to lower infection rates as women advance in age. Most women aged over 46 years have reached menopause and are less or not sexually active.29 They rarely use contraceptives to prevent pregnancy, and they also seldom misuse drugs.29 They also have a possible increase in vaginal immunity, as they have decreased levels of estrogen and corticoids, and thus are resistant to Candida infections.30
These factors probably contributed to the lowest occurrence rate of vaginal Candida species in this age-group (>40 years). This finding is in line with a previous report by Okungbowa et al, who reported prevalence of 10% and 2% within the age-groups of 36–45 and over 46 years, respectively. They reported that this was probably due to the possible increase in vaginal immunity with age;31 however, no age-group was absolutely free of vaginal candidiasis.
There was no statistically significant relationship between the prevalence of VVC with age (P>0.05) or prevailing clinical symptoms of ill health (P>0.05). This could have been a result of recurrent infections that might have contributed to resistance of the vagina to candidiasis. This can reduce the chances of isolating the yeast organism.
Subjects with vulvovaginal discomfort had a higher percentage of Candida-positive cultures (29.1%) than those with no vulvovaginal discomfort (11.9%). This report is in agreement with the findings of Jombo et al.32 It is reasonable to believe that young women with genital discomfort consult health care centers more often than women without such symptoms.33
This report revealed the prevalence of C. albicans among general test subjects to be 6.5% (13 of 200), which was slightly lower than that of non-albicans candidiasis (7.5% [15 of 200]). This finding is in agreement with the findings of Aring et al,24 who also observed a concomitant increase in the prevalence of non-albicans species in their study group. Their study reported that among the non-albicans species, C. glabrata was the most common type (10.52%) and C. krusei the least common type (3.51%). Studies have shown that C. glabrata is one of the major causes for recurrent VVC. More than 10% of women in their study were infected with C. glabrata, which further agrees with Corsello et al.33 Vaginitis induced by non-albicans species is clinically indistinguishable from that caused by C. albicans.34 The reason for the increase in incidence of VVC caused by non-albicans species is thought to be single-dose antifungal treatment, low-dosage azole-maintenance regimens, and the use of over-the-counter antimycotics.37 Therefore, for effective control of candidiasis, it is advisable to identify the Candida spp. alongside clinical symptoms before planning for treatment.
All subjects with positive Candida-culture results had already been on antibacterial therapy prior to their hospital visit – 28 (100%). This finding is in conformity with the fact that prolonged antibacterial use usually affects vaginal bacteria microflora population and biochemical activity (mainly L. acidophilus), which thus increases vaginal pH as a result of reduced CO2 production. This feature, alongside other factors (such as hormonal factors), encourages Candida overgrowth, consequently leading to vulvovaginitis.34,35 Although the widespread use of antibiotics has been suggested as one of the major factors contributing to the rising incidence of VVC,36,37 some case-control studies38,39 found no evidence of an association between antibiotic agents and symptomatic VCC, whereas others reached the opposite conclusion.40–42
The low prevalence of candidiasis reported from these nonpregnant women could not be extensively discussed with studies from our locality due to paucity of data on similar findings. Most previous studies focused on pregnant women and other immunocompromised subjects. Therefore, there is a need to create awareness of the involvement of Candida spp. in genital discomfort, especially vulvovaginitis, among nonpregnant women with or without notable signs and symptoms, in order to avoid unnecessary and empirical antibacterial therapy. More so, it is worthwhile to consider culture-test results as adjunctive in combination with clinical symptoms in the definitive diagnosis of VVC. There is a need to build on findings generated from this study in order to identify/characterize the non-albicans Candida spp. isolated and possibly conduct antifungal susceptibility testing.
Acknowledgment
We would like to acknowledge the staff of the Medical Laboratory Services, Gynaecology and General Out-patient departments, University of Abuja Teaching Hospital for their technical support and taking part in generating clinical data from subjects.
Disclosure
The authors report no conflicts of interest in this work.
References
Sobel JD. Vulvovaginal candidosis. Lancet. 2007;369(9577):1961–1971. | |
Nyirjesy P, Sobel JD. Vulvovaginal candidiasis. Obstet Gynecol Clin North Am. 2003;30(4):671–684. | |
Marrazzo J. Vulvovaginal candidiasis. BMJ. 2002;325(7364):586–587. | |
Ferris DG, Nyirjesy P, Sobel JD, Soper D, Pavletic A, Litaker MS. Over-the-counter antifungal drug misuse associated with patient diagnosed vulvovaginal candidiasis. Obstet Gynecol. 2002;99(3):419–425. | |
Nyirjesy P. Chronic vulvovaginal candidiasis. Am Fam Physician. 2001;63(4):697–702. | |
Rex JH, Walsh TJ, Sobel JD, et al. Practice guidelines for the treatment of candidiasis. Infectious Diseases Society of America. Clin Infect Dis. 2000;30(4):662–678. | |
McClelland RS, Richardson BA, Hassan WM, et al. Prospective study of vaginal bacterial flora and other risk factors for vulvovaginal candidiasis. J Infect Dis. 2009;15;199(12):1883–1890. | |
Singh SI. Treatment of vulvovaginal candidiasis. Clin Rev. 2003;136(9):26–30. | |
Ray D, Goswami R, Banerjee U, et al. Prevalence of Candida glabrata and its response to boric acid vaginal suppositories in comparison with oral fluconazole in patients with diabetes and vulvovaginal candidiasis. Diabetes Care. 2007;30(2):312–317. | |
Ringdahl E. Treatment of recurrent vulvovaginal candidiasis. Am Fam Physician. 2000;61(11):3306–3312, 3317. | |
Sobel TD. Vaginitis. N Engl J Med. 1997;337(26):1896–1903. | |
de Leon EM, Jacober SJ, Sobel JD, Foxman B. Prevalence and risk factors for vaginal Candida colonization in women with type 1 and type 2 diabetes. BMC Infect Dis. 2002;2:1. | |
[No authors listed]. Sexually transmitted diseases treatment guidelines 2002. Centers for Disease Control and Prevention. MMWR Recomm Rep. 2002;51(RR-6):1–78. | |
Mårdh PA, Rodrigues AG, Genç M, Novikova N, Martinez-de-Oliveira J, Guaschino S. Facts and myths on recurrent vulvovaginal candidosis – a review on epidemiology, clinical manifestations, diagnosis, pathogenesis and therapy. Int J STD AIDS. 2002;13(8):522–539. | |
Geiger AM, Foxman B, Sobel JD. Chronic vulvovaginal candidiasis: characteristics of women with Candida albicans, C. glabrata and no Candida. Genitourin Med. 1995;71(5):304–307. | |
Ferrer J. Vaginal candidosis: epidemiological and etiological factors. Int J Gynaecol Obstet. 2000;71 Suppl 1:S21–S27. | |
Eschenbach DA. Chronic vulvovaginal candidiasis. N Engl J Med. 2004;351(9):851–852. | |
Odds FC. Candida and Candidiasis: A Review and Bibliography. 2nd ed. London: Bailliere Tindall; 1988. | |
Sobel JD, Faro S, Force RW, et al. Vulvovaginal candidiasis: epidemiologic, diagnostic, and therapeutic considerations. Am J Obstet Gynecol. 1998;178(2):203–211. | |
Okonkwo NJ, Umeanaeto PU. Prevalence of vaginal candidiasis among pregnant women in Nnewi Town of Anambra State, Nigeria. Afr Res Rev. 2010;4(4):539–548. | |
Cheesbrough M. Fungal pathogens. In: District Laboratory Practice in Tropical Countries. Cambridge, UK: Cambridge University Press; 2000:235–248. | |
Mahadani JW, Dekate RR, Shrikhande AV. Diagnosis of discharge per vaginum. Indian J Pathol Microbiol. 1998;41(4):403–411. | |
Nandan D, Gupta YP, Krishnan V, Sharma A, Misra SK. Reproductive tract infection in women of reproductive age group in Sitapur/Shahjahanpur district of Uttar Pradesh. Indian J Public Health. 2011; 45(1):8–13. | |
Aring BJ, Mankodi PJ, Jasani JH. Incidence of vaginal candidiasis in leucorrhoea in women attending in OPD of gynecology and obstetrics department. Int J Biomed Adv Res. 2012;3(12):867–869. | |
Fernández Limia O, Lantero MI, Betancourt A, de Armas E, Villoch A. Prevalence of Candida albicans and Trichomonas vaginalis in pregnant women in Havana City by an immunologic latex agglutination test. MedGenMed. 2004;6(4):50. | |
Alo MN, Anyim C, Onyebuchi AK, Okonkwo EC. Prevalence of asymptomatic co-Infection of candidiasis and vaginal trichomoniasis among pregnant women in Abakaliki, South-Eastern Nigeria. J Nat Sci Res. 2012;2(7):87–91. | |
Akortha E, Chikwe O, Nwaugo O. Antifungal resistance among Candida species from patients with genitourinary tract infection isolated in Benin City, Edo estate, Nigeria. Afr J Microbiol Res. 2009;3(11):694–699. | |
Willacy H, Jackson C. Vaginal and vulval candidiasis. 2011. Available from: http://www.patient.co.uk/doctor/vaginal-and-vulval-candidiasis. Accessed May 17, 2015. | |
Nelson M, Wanjiru W, Margaret MW. Prevalence of vaginal candidiasis and determination of the occurrence of Candida species in pregnant women attending the antenatal clinic of Thika District Hospital, Kenya. Open J Med Microbiol. 2013;(3):264–272. | |
Paul LF, Jessica C, Chad S. Effects of reproductive hormones experimental vaginal candidiasis. Infect Immun. 2000;68(2):651–657. | |
Okungbowa F, Isuehuemhen O, Dede A. The distribution frequency of Candida species in the genitourinary tract among symptomatic individuals in Nigerian cities. Rev Iberoam Micol. 2003;20(2):60–63. | |
Jombo GT, Opajobi SO, Egah DZ, Banwat EB, Denen Akaa P. Symptomatic vulvovaginal candidiasis and genital colonization by Candida species in Nigeria. J Public Health Epidemiol. 2010;2(6):147–151. | |
Corsello S, Spinillo A, Osnengo G, et al. An epidemiological surgery of vulvovaginal candidiasis in Italy. Eur J Obstet Gynecol Reprod Biol. 2003;110(1):66–72. | |
Bauters TG, Dhont MA, Temmerman MI, Nelis HJ. Prevalence of vulvovaginal candidiasis and susceptibility to fluconazole in women. Am J Obstet Gynecol. 2002;187(3):569–574. | |
Cauwenbergh G. Vaginal candidiasis: evolving trends in the incidence and treatment of non-Candida albicans infection. Curr Probl Obstet Gynecol Fertil. 1990;8:241. | |
Foxman B, Marsh JV, Gillespie B, Sobel JD. Frequency and response to vaginal symptoms among white and African American women: results of a random digit dialing survey. J Womens Health. 1998;7(9):1167–1174. | |
Reed BD. Risk factors for Candida vulvovaginitis. Obstet Gynecol Surv. 1992;47(8):551–560. | |
Geiger AM, Foxman B. Risk factors for vulvovaginal candidiasis: a case-control study among university students. Epidemiology. 1996; 7(2):182–187. | |
Reed BD, Huck W, Zazove P. Differentiation of Gardnerella vaginalis, Candida albicans, and Trichomonas vaginalis infections of the vagina. J Fam Pract. 1989;28(6):673–680. | |
Spinillo A, Capuzzo E, Acciano S, De Santolo A, Zara F. Effect of antibiotic use on the prevalence of symptomatic vulvovaginal candidiasis. Am J Obstet Gynecol. 1999;180(1 Pt 1):14–17. | |
Leegaard M. The incidence of Candida albicans in the vagina of “healthy young women”. How often do they have symptoms? Possible etiological factors. Acta Obstet Gynecol Scand. 1984;63(1):85–89. | |
Spinillo A, Capuzzo E, Nicola S, Baltaro F, Ferrari A, Monaco A. The impact of oral contraception on vulvovaginal candidiasis. Contraception. 1995;51(5):293–297. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
