Back to Journals » Adolescent Health, Medicine and Therapeutics » Volume 9
Prevalence of vancomycin resistance among isolates of enterococci in Iran: a systematic review and meta-analysis
Authors Moghimbeigi A, Moghimbeygi M , Dousti M, Kiani F , Sayehmiri F, Sadeghifard N, Nazari A
Received 17 July 2018
Accepted for publication 2 October 2018
Published 15 November 2018 Volume 2018:9 Pages 177—188
DOI https://doi.org/10.2147/AHMT.S180489
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Alastair Sutcliffe
Abbas Moghimbeigi,1,2 Meisam Moghimbeygi,3 Majid Dousti,4 Faezeh Kiani,5 Fatemeh Sayehmiri,6 Nourkhoda Sadeghifard,7 Ali Nazari8
1Modeling of Noncomunicable Disease Research Center, Department of Biostatistics, Faculty of Public Health, Hamadan University of Medical Sciences, Hamadan, Iran; 2Department of Biostatistics, Faculty of Public Health, Hamadan University of Medical Sciences, Hamadan, Iran; 3Department of Statistics, Faculty of Mathematical Sciences, Tarbiat Modares University, Tehran, Iran; 4Department of Parasitology and mycology, Faculty of Medicine, Shiraz University of Medical Sciences, Fars, Iran; 5Student Research Committee, Ilam University of Medical Sciences, Ilam, Iran; 6Proteomics Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran; 7Microbiology Research Center, School of Medicine, Ilam University of Medical Sciences, Ilam, Iran; 8School of Medicine, Ilam University of Medical Sciences, Ilam, Iran
Introduction: Enterococcus is responsible for 10% of hospital-acquired infections. The purpose of this review was to evaluate the prevalence of vancomycin-resistant Enterococcus (VRE) isolates in Iran using a meta-analysis method.
Materials and methods: Iranian databases, including Magiran and IranDoc, and international databases, including PubMed and MedLib, were examined carefully, and a total of 20 articles published between 2000 and 2011 were extracted. The data were subjected to meta-analysis and random-effects models. In addition, heterogeneous studies were assessed using the I2 index. Finally, the data were analyzed using R and STATA software.
Results: The results showed that the strain of Enterococcus faecalis had been more common than Enterococcus faecium in clinical infection (69% vs 28%). However, resistance to vancomycin was higher among strains of E. faecium compared with strains of E. faecalis (33% vs 3%). The complete resistance, intermediate resistance, and sensitivity to vancomycin among Enterococcus isolates were 14% (95% CI: 11, 18), 14% (95% CI: 5, 23), and 74% (95% CI: 65, 83), respectively. The resistance patterns, depending on the sample type, did not show a significant difference. In addition, the resistance of isolated strains to vancomycin in outpatients was significantly higher than that in inpatients (16% vs 1%). Moreover, 80%–86% of resistant strains were genotype van A and 14%–20% of resistant strains were genotype van B.
Conclusion: The findings of the present review show that there is a high frequency of resistant Enterococcus in Iran. Therefore, consideration of the prevalence and frequency of subjected resistant strains can be helpful for decision makers to implement proper health policies in this direction.
Keywords: clinical infections, gram-positive bacteria, enterococci, antibiotic resistance, glycopeptide antibiotics
Introduction
Enterococcus is a catalase-negative, gram-positive coccus. It is one of the important members of normal flora of the gastrointestinal tract of most warm-blooded organisms, including human beings. However, it has been suggested that different strains of gram-positive cocci could be opportunistic pathogens causing various infectious diseases.1,2 The most common human infectious strains of Enterococcus are Enterococcus faecalis (85%–90%) and Enterococcus faecium (10.5%), and they cause cause urinary tract infections, endocarditis, intra-abdominal abscesses, wound infections, bacteremia, sepsis in babies, etc.2–4 In addition, it has been proven that Enterococcus is the second leading cause of urinary tract and wound infections and the third leading cause of bacteremia in hospitals.2,3
The main reasons for the stability and development of Enterococcus in a hospital environment are as follows: inherent resistance to a wide range of antibiotics used in the treatment of infections caused by gram-positive bacteria; the ability of these bacteria to acquire resistance genes to antibiotics through mutation or acquisition of external genetic materials (plasmids, transposons, and mobile genetic indicators); and resistance gene transfer by conjugation or other transmission methods.3–5 In addition, the evidence suggests that regardless of its virulence factors the pathogenic strength of Enterococcus is because of inherent or acquired resistance to various antibiotics.3
Antibiotics have been used to treat bacterial infections for almost 70 years.6–8 Vancomycin with an antibiotic from the aminoglycoside family is prescribed instead of penicillin in the treatment of enterococcal infections. Due to the bactericidal activity of these antibiotics against Staphylococcus and other gram-positive bacteria which are resistant to methicillin, these drugs are widely used to treat and prevent against infections caused by these organisms.4 However, Enterococcus easily acquires antibiotic resistance and is able to transfer resistance genes to other strains.5 In most cases, vancomycin is prescribed as a last resort to treat infections of gram-positive bacteria, especially Enterococcus. However, in recent years, increased prescription of vancomycin in clinics plays a major role in vancomycin resistance of subjected pathogens.9 Because of its resistance against various antibiotics, vancomycin-resistant Enterococcus (VRE) has created a major problem in the treatment of patients.3
It should be mentioned that antimicrobial resistance to antibiotics can be different worldwide depending upon genetic variations of subjected strains, differences in access to broad spectrum of antibiotics, etc.10 The acquisition of antibiotic resistance genes over time in different geographical areas and the resultant changed susceptibility pattern of bacteria to the antibiotics have led to an important issue. In this circumstance, the selection of an appropriate antibiotic for better treatment is a challenge.11,12 It is of high importance to determine the prevalence of antibiotic resistance to effectively treat and control enterococcal infections.10 Therefore, further studies with the aim of gaining knowledge about antibiotic resistance patterns are necessary to guide empirical and specific treatments against this pathogen.13,14
One of the most important goals of meta-analyses is to provide an accurate and reliable result by increasing the sample size and reducing the width of the 95% CI from the range of the various applicable studies.15 So far, several studies in the field of antibiotic-resistant enterococci have been done. Since antibiotic treatment of infectious diseases caused by this organism is different based on epidemiology and antimicrobial resistance, it seems to be necessary to perform a meta-analysis study in this field to validate the results of studies and provide an accurate and reliable measure. This review was carried out to determine the prevalence of vancomycin resistance in Enterococcus isolates using a systematic literature review and meta-analysis method in Iran.
Materials and methods
Literature review
A systematic review and meta-analysis was performed by searching Iranian databases including SID, Magiran, IranDoc, and IranMedex, and international databases MedLib, PubMed, ISI, Web of Science, Scopus, and Google Scholar to find published studies about the prevalence of resistance to vancomycin in Enterococcus isolates. The search was performed using Persian keywords and their English equivalent (clinical infections, gram-positive bacteria, enterococci, antibiotic resistance, glycopeptide antibiotics, vancomycin) with all possible combinations. In addition, the titles and references from selected articles were an additional search tool. To reduce the bias, the search process was conducted independently by two researchers.
Inclusion and exclusion criteria for studies
We considered all cross-sectional or cohort studies that reported the prevalence of vancomycin resistance in Enterococcus isolates in patients suspected of having clinical infection. The published studies were examined in three steps: title, abstract, and full text. Exclusion criteria for the analysis were as follows: studies with insufficient information; studies that were not cross-sectional or cohort; studies that were done in other organisms except enterococci; review studies; abstracts of congresses; articles published in languages other than Persian and English; and systematic review, meta-analysis, and repetitive studies. In addition, to check the quality control of the data, the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE)1 checklist was used. This checklist has 22 parts that cover different sections of reports. In addition, each section was scored between 0 and 2, and the total score for each article was calculated.16 If necessary, the authors were contacted for further information.
Data extraction
After determining the quality of studies, the following data were extracted: first author; year of publication; year of study; place of study; sample size; sample type; prevalence of all kinds of Enterococcus and their resistance to vancomycin; prevalence of complete resistance, prevalence of mean resistance, and prevalence of sensitivity to vancomycin in Enterococcus isolates; and antimicrobial susceptibility determination methods and the criteria of antibiotic susceptibility test (Table 1). Data extraction was carried out independently by two researchers, and if the results did not match, study investigators resolved the differences together. Afterward, the extracted data were entered into an Excel spreadsheet to perform statistical analyses.
Statistical analyses
Since the main index of the review was the value of prevalence, its variance and 95% CI were calculated by considering the binomial distribution. To combine the prevalence values of various studies, the variance of the weighted mean was used to calculate the 95% CIs. Each study was given weight proportional to its inverse variance. The heterogeneity was investigated using the Q-test and I2 index at a significance level of <10%. In addition, due to the heterogeneity of studies, the random-effects model was used in this meta-analysis. The results were plotted in forest plots (point estimates and 95% CI). Finally, to analyze the data, R and STATA (version 11.2; StataCorp LP, College Station, TX, USA) software were used.
Antibiotic resistance definition
In most studies, the criteria of antibiotic sensitivity and resistance were as follows: minimum inhibitory concentration (MIC) <8 mg/dL as sensitivity, MIC 8–16 mg/dL as intermediate, and MIC >18 mg/dL was considered resistant. In some studies, MIC >32 mg/dL was defined as complete resistance and MIC >256 mg/dL or MIC >500 mg/dL was defined as high-level resistance.
Results
Fifty-three articles were found by searching Iranian databases including SID, Magiran, IranDoc, and IranMedex, and international databases MedLib, PubMed, ISI, Web of Science, Scopus, and Google Scholar. After primary evaluation, 12 articles were excluded from the study based on the titles and abstracts. In addition, another three articles were removed because of the unavailability of the full text. Therefore, 38 articles remained for studying the full text. In the next step, and after evaluating the full-text articles, 18 articles were excluded (three review articles, five duplicate articles, three low-quality articles, and seven articles due to insufficient information) and finally 20 articles published between 2000 and 20111,3,17–34 were entered into the meta-analysis (Figure 1). General information and data about these articles are summarized in Table 1.
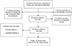  | Figure 1 Flowchart of the studies identified in the systematic review and meta-analysis. |
As mentioned previously, due to the heterogeneity of studies, the random-effects model was used in all next steps. According to this model, it is assumed that the observed differences derive from different samplings and differences in measured parameters (prevalence of enterococcal resistance in Iran) in studies.
In this review, a total of 6,829 Enterococcus isolates from inpatients and outpatients were analyzed. The samples were obtained from urine, stool, rectal swab, wound, blood, sterile liquid, lung secretion, abscess, catheter, etc. Although isolates were different based on sampling locations, most of the isolates were obtained from urine (>70%).
In most studies, Enterococcus-type identification was performed through biochemical tests. As summarized in Table 2, E. faecalis and E. faecium are the most common Enterococcus strains that cause clinical infections with a frequency of 69% (95% CI: 74, 64) and 28% (95% CI: 24, 32), respectively. In addition, the frequency of other types of Enterococcus is ~3% (95% CI: 1, 4).
Figure 2 shows the frequency of full-resistant, intermediate-resistant, and sensitive isolates of Enterococcus to vancomycin. Enterococcus isolates were sensitive to vancomycin antibiotic at a rate of 74% (95% CI: 65, 83). The frequency of intermediate- and full-resistant isolates to vancomycin at rates of 14% (95% CI: 5, 23) and 14% (95% CI: 11, 18), respectively. Figure 3 shows the prevalence of resistance to vancomycin in Enterococcus isolates in Iran and 95% CI in the reviewed studies.
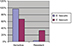  | Figure 2 Frequency of resistance and sensitivity to vancomycin in Enterococcus isolates. Abbreviations: E. faecalis, Enterococcus faecalis; E. faecium, Enterococcus faecium. |
In addition, the prevalence of vancomycin resistance among isolates of E. faecalis and E. faecium was 3% (95% CI: 2, 5) and 33% (95% CI: 21, 45), respectively. These findings show that vancomycin resistance among E. faecium isolates is significantly higher than that among E. faecalis isolates (Table 2).
In this review, the amount of resistance was also evaluated based on sample type. As mentioned earlier, >70% of isolates were obtained from urine samples. The remaining <30% of isolates were mostly extracted from stool samples, and other few isolates were extracted from different clinical samples. Therefore, the analysis in subgroups of the samples was limited to only three groups: urine, stool, and other clinical samples. Accordingly, vancomycin resistance in Enterococcus isolates obtained from urine samples was 15% (95% CI: 10, 19), stool samples was 16% (95% CI: 9, 23) and other samples was 12% (95% CI: 10, 14; Table 3). The results do not show significant differences in this area (Figure 4).
  | Table 3 The prevalence of vancomycin resistance among Enterococcus isolates according to patients’ status |
Another result of this review was to study the prevalence of vancomycin resistance among isolates of Enterococcus based on patients’ status (inpatients, outpatients). The results showed that vancomycin resistance among Enterococcus isolates obtained from inpatients was significantly higher than that from outpatients (16% vs 1%; Table 3). Based on the results of this review, genotype van A had the highest frequency of resistance to vancomycin (Table 2). In addition, we found that among total strains with resistance to vancomycin, 86% (95% CI: 73, 98) were genotype van A and 20% (95% CI: 16, 24) were genotype van B.
Discussion
Enterococcus is the second leading cause of urinary tract infections and the third leading cause of bacteremia. In addition, in the past two decades, Enterococcus was introduced as the third leading cause of hospital-acquired infections after Escherichia coli and Staphylococcus.35 It has been evidenced that Enterococcus is responsible for 10%–20% of all hospital infections, 10%–12% urinary tract infections in hospitals, and 5%–10% of hospital bacteremia.36 Diagnosis and treatment of clinical infections are vital, and a delay in treatment may result in irreversible harm to patients. Because of improper antibiotic consumption due to self-medication in our society, urine and stool cultures of patients were often reported as negative. Therefore, in many cases, the treatment was based on the most common urinary infection strains and their antibiotic susceptibility.37 In this review, we aimed to determine the prevalence of vancomycin resistance among the most common cause of clinical infections (Enterococcus) in Iran by using systematic reviews and meta-analyses.
The results of this review indicate that two species, namely E. faecalis and E. faecium, are the most common Enterococcus strains that cause human infections. We found that 69% of the isolated species belong to E. faecalis and 28% belong to E. faecium. In addition, we observed that this distribution is different among various geographical places; in several studies in countries such as Iran, USA, UK, and many European countries, E. faecalis is the dominant isolated species. However, a few reports showed that in some countries, such as India and Japan, E. faecium formed a higher percentage of Enterococcus.38 In Yeh et al’s study, >90% of Enterococcus isolates were E. faecalis and the remaining 10% were E. faecium.39 Enterococcus faecalis has a higher role in enterococcal infections due to high connectivity and proliferation in the intestine. But, high potential of E. faecium in the acquisition of resistance materials (genes, mutations, plasmids, etc.) makes this strain highly resistant to various antibiotics.40,41 As the results of this review showed that the prevalence of E. faecalis was higher than that of E. faecium (69% vs 28%), the prevalence of resistance to vancomycin among E. faecium isolates was considerably higher than that of E. faecalis isolates (33% vs 3%). In addition, the prevalence of vancomycin-resistant genes among E. faecium isolates was higher than that of E. faecalis (85% vs15%). Many studies showed that E. faecium has high resistance, and it is the dominant species among VRE.19,42 This property is indicative of the important role of E. faecium in the spread of resistance to vancomycin.
The results of this review showed that 16% of colonized Enterococcus isolates in inpatients were resistant to vancomycin. In addition, the amount of vancomycin resistance in Enterococcus isolates obtained from inpatients was significantly higher than that from outpatients (16% vs 1%). A study in France reported that the frequency of resistance to vancomycin was 37% for inpatients and 11.8% for outpatients.43 VRE is of high risk for inpatients. A study in the USA showed that the percentage of Enterococcus isolates resistant to vancomycin in hospital ICUs is on the rise.44 VRE is an important factor in hospital-acquired infections and can lead to increased rate of diseases, mortality, and costs.45–47 It is possible that excessive consumption of vancomycin and other antibiotics, such as cephalosporins, plays a key role in the colonization of VRE.47,48 Benenson et al’s49 study of 1,215 inpatients showed that 9.8% of patients were fecal carriers of VRE and previous hospitalization and antibiotic treatment are important risk factors. The results of Cohen et al’s50 study of 1,039 patients in different phases showed that VRE carriers are 3.8% of patients at reception, 15% of discharged patients, and 32% in inpatients.
In this review, the rate of vancomycin resistance in Enterococcus isolates based on the place of the sampling did not show a significant difference. In addition, most samples were obtained from urine (>70%) and this shows the importance of Enterococcus colonization in the urinary tract after hospitalization of patients. Different studies have shown that Enterococcus is the leading cause of urinary tract infection among gram-positive cocci and the third leading cause of bacterial infection in women’s urinary tracts in Iran after E. coli and Klebsiella pneumonia.19
The results of this review showed that vancomycin resistance in Enterococcus isolates in Iran is 14%. The prevalence of vancomycin resistance in South Korea, Belgium, and England was reported as 16%,51 12.8%,52 and 12.2%,53 respectively. These results are close to our results in this subject. Some studies have reported a lower prevalence, eg, in Spain only three cases were resistant to vancomycin from 437 Enterococcus samples.54 A prevalence of 6.7% was reported in a study in Canada,55 9% in a study in New York,56 and 2%–9% in a separate study in the USA.57–60 In soEurope, a prevalence of 1% in France and 59% in Portugal was reported.61 This may be a reflection of drug and antibiotic utilization patterns in a region.
Drug resistance to antibiotics is different due to genetic changes in strains, difference in antibiotic utilization, and differences in access to broad-spectrum and new antibiotics in different regions of Iran and the world. Some predisposing factors should be considered in Enterococcus colonization or infection with these microorganisms in patients. These factors can be listed as a long stay in hospital, inappropriate use of third-generation antibiotics, such as cephalosporins and vancomycin, organ transplants, taking metronidazole, surgery, diabetes, leukemia, weakened immune system for any reason, and kidney failure.23
Based on our findings, van A has a higher frequency of resistance to vancomycin. From all vancomycin-resistant strains, 80% had genotype van A and 20% had genotype van B. The most important vancomycin-resistant genes are van A, van B, van C1, and van C2/C3. Van A and B (as the most important genes for resistance) are on transposons, such as Tn1546 and Tn1547, respectively, and they can be found in plasmids or chromosomes.1 Increased resistance to glycopeptides, such as vancomycin, results in limited therapeutic and drug choices because an alternative treatment in Iran has not improved. In addition, it increases the risk of transferring resistance genes to other bacteria, such as Staphylococcus.1–3 On the other hand, the infections caused by Enterococcus that are resistant to several antibiotics are also increasing simultaneously. Vancomycin is the optional drug for infections caused by multi-resistance strains. Reports showed that multidrug resistance is usually observed in patients who have been recently treated with antibiotics. Resistant strains, especially multidrug resistant strains are colonized in these patients’ gastrointestinal tracts because the sensitive strains have been eliminated with antibiotic treating. In this way, the direct and indirect transfer rates of resistant strains increase.31 Multidrug-resistant Enterococcus strains are causing a series of problems, including the emergence of resistance to aminoglycosides and beta-lactams. If resistance to vancomycin is found, the situation will become more critical. Therefore, using newer compounds, such as oxazolidinedione and streptogramin, in the treatment of patients can somewhat reduce this problem.19
One of the main limitations of this review was in the checking of the prevalence of resistance to vancomycin separately for males and females because the resistance was calculated separately for the two sexes in only a very small number of studies. Patients’ age is an important factor which may contribute in antibiotic resistance. Since in most studies the details of age were not mentioned and because of nonexistent studies of similar age groups, we could not calculate resistance values according to age. Another limitation in our review was the lack of unit definition of resistance in the analysis of the literature that was used. In addition, the lack of access to the full text of some articles was another limitation of this review.
Conclusion
Drug-resistant Enterococcus is an important epidemic cause of nosocomial infections and can increase disease, mortality, and costs. Vancomycin is an antibiotic that because of its activity against methicillin-resistant Staphylococcus aureus and other gram-positive bacteria can be used widely for the treatment and prevention of infections caused by these organisms. According to the results, there was a high resistance to this drug in Enterococcus strains in many regions of Iran, whereas in many developed countries there is a low resistance. Therefore, there is a difference in the pattern of bacterial sensitivity and resistance in different geographic regions. In addition, the use of methods that are able to detect resistant strains and application of them in prevention strategy design to control the spread of resistant strains is important. To limit the drug-resistant Enterococcus prevalence, it is necessary to be cautious in using vancomycin. Also, permanent control of the prevalence of glycopeptide-resistant Enterococcus strains is essential in a hospital environment.
Disclosure
The authors report no conflicts of interest in this work.
References
Mohammadi F, Tabaraie B, Sadeghifard N. Evaluation of drug resistance frequency among Entrococci faecium and E. faecalis strains and detection of vanA/B genes in vancomycin resistance isolated by PCR method in Ilam And Kermanshah hospitals. J Ilam Univ Med Sci. 2011;19(2):1–8. | ||
Arbabi l, Vndyvsfy C, Bouzari M, Rahimi F, Rahimi F. Vancomycin-resistant Enterococcus antibiotic resistant strains isolated from stool samples of meat and dairy farms in Tehran. Infect Dis Trop Med. 2010;15(49):11–16. | ||
Dadfarma N, Oskoei M, Imani Fouladi A, Farokh P. Study of aac(6′) Ie-aph(2″)Ia gene in clinical strain of Enterococci and identification of high-level gentamicin resistant Enterococci. Sci J Hamadan Univ Med Sci. 2010;17(3)25-32. | ||
Arbabi l, Vndyvsfy C, Bouzari M, Rahimi F, Rahimi F. Vancomycin-resistant Enterococcus strains in poultry Tehran. Infect Dis Trop Med. 2010;15(48):33–38. | ||
Shaghaghi B, Nakhost Lotfi M, Mahmoudi N, Poorshafi M. Antibiotic resistance in Enterococcus isolates from dairy wastewater in Tehran. Infect Dis Trop Med. 2007;12(37):61–66. | ||
Farrell DJ, Morrissey I, De Rubeis D, Robbins M, Felmingham D. A UK multicentre study of the antimicrobial susceptibility of bacterial pathogens causing urinary tract infection. J Infect. 2003;46(2):94–100. | ||
Malinverni R. Urinary tract infections and antibiotic resistance. Ther Umsch. 2002;59(1):31–35. | ||
Paterson DL. Serious infections caused by enteric gram-negative bacilli–mechanisms of antibiotic resistance and implications for therapy of gram-negative sepsis in the transplanted patient. Semin Respir Infect. 2002;17(4):260–264. | ||
Molazade A, Shahi A, Najafipour S, et al. Antibiotic Resistance Pattern of Bacteria Causing Urinary Tract Infections in Children of Fasa During the Years 2012 and 2014. J Fasa Univ Med Sci. 2015;4:493–499. | ||
Asadi Manesh F, Sharifi A, Mohammad Hosini Z, et al. Antibiotic Resistance of Urinary Tract Infection of Children Under 14 Years Admitted To The Pediatric Clinic of Imam Sajjad Hospital, 2012. Armaghane Danesh. 2014;19(5):411–420. | ||
Molazade A, Shahi A, Gholami MS, et al. The antibiotic resistance pattern of gram-negative bacilli isolated from urine cultures of adult outpatients admitted to Vali Asr Hospital of Fasa Clinical Laboratory in 2012–13. Par J Med Sci. 2014;12(3):15–22. | ||
Jafari S, Najafipour S, Kargar M, et al. Phenotypical evaluation of multi-drug resistant Acinetobacter Baumannii. J Fasa Univ Med Sci. 2013;2(4):254–258. | ||
Moghadas AJ, Irajian G. Asymptomatic urinary tract infection in pregnant women. Iran J Pathol. 2009;4(3):105–108. | ||
Forbes BA, Sahm DF, Weissfeld AS. Study Guide for Bailey & Scott’s Diagnostic Microbiology. 12th ed. Missouri: Mosby Elsevier; 2007:842–55. | ||
Steiner M. Postnatal depression: a few simple questions. Fam Pract. 2002;19(5):469–470. | ||
von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology [STROBE] statement: guidelines for reporting observational studies. Gac Sanit. 2008;22(2):144–150. | ||
Ghasemi A. Mniri A, Mousavi S. Multidrug resistance in Enterococcus faecalis strains isolated from clinical specimens in Shahid Beheshti hospital and maternity Shabikhani in 2008. J Med Microbiol. 2008;3(3.2):21–26. | ||
Vahhabi A, Hasani A, Nahaei M, Farajnia S. The prevalence of ampicillin-resistant enterococci, gentamicin and vancomycin in stool samples from hospitalized patients and outpatients in the hospital of Tabriz University of Medical Sciences. J Tabri Univ Med Sci. 2011;33(3):78–85. | ||
Feizabadi M. Asadi M, Khatibi Q, Etemadi G, Parvin M, Oskouei M. The pattern of drug resistant strains of Enterococcus faecalis and Enterococcus faecium in hospitals and martyr Chamran Labbafinejad years 1382–1379. Pajouhandeh. 2004;9(42):333–339. | ||
Sharifi Y, Hasani A, Ghotaslou R, et al. Vancomycin-resistant enterococci among clinical isolates from north-west Iran: identification of therapeutic surrogates. J Med Microbiol. 2012;61(4):600–602. | ||
Shokoohizadeh L, Mobarez AM, Zali MR, et al. High frequency distribution of heterogeneous vancomycin resistant Enterococcus faecium (VREfm) in Iranian hospitals. Diagn Pathol. 2013;8:163. | ||
Ghaffarpasand I, Moniri R, Kheradi I. The prevalence of fecal carriage of antibiotic resistant enterococci among hospitalized patients in Shahid Beheshti hospital, Kashan, Iran at 2007. Feyz. 2010;14(1):70–75. | ||
Hosseinizadeh A, Abtahi H, Shojapour M, Akbari M, Nazari R, Sofian M. Prevalence and antimicrobial susceptibility pattern of vancomycin resistant enterococci isolated from clinical sample of educational hospitals in Arak. J Arak Univ Med Sci. 2012;15(64):11–16. | ||
Askarian M, Afkhamzadeh R, Monabbati A, Daxboeck F, Assadian O. Risk factors for rectal colonization with vancomycin-resistant enterococci in Shiraz, Iran. Int J Infect Dis. 2008;12(2):171–175. | ||
Assadian O, Askarian M, Stadler M, Shaghaghian S. Prevalence of vancomycin-resistant enterococci colonization and its risk factors in chronic hemodialysis patients in Shiraz, Iran. BMC Infect Dis. 2007;7:52. | ||
Fatholahzadeh B, Hashemi FB, Emaneini M, Aligholi M, Nakhjavani FA, Kazemi B. Detection of vancomycin resistant enterococci (VRE) isolated from urinary tract infections (UTI) in Tehran. DARU. 2006;14(3):141–145. | ||
Haghi-Ashteiani M, Sadeghifard N, Abedini M, Soroush S, Taheri-Kalani M. Etiology and antibacterial resistance of bacterial urinary tract infections in Children’s Medical Center, Tehran, Iran. Acta Medica Iranica. 2007;45(2):153–157. | ||
Feizabadi MM, Sayadi S, Shokrzadeh L, Parvin M, Yadegarynia D. Increase in prevalence of vancomycin resistant isolates of Enterococcus faecium at Labbafinejad hospital. Iran J Clinic Infect Dis. 2008;3(2):73–77. | ||
Javadi A, Ataei B, Khorvash F, Toghyani S, Mobasherzadeh S, Soghrati M. Prevalence of vancomycin resistant Enterococci colonization in gastrointestinal tract of hospitalized patients. Iran J Clinic Infect Dis. 2008;3(3):137–141. | ||
Aligholi M, Emaneini M, Jabalameli F. Antibiotic Susceptibility Pattern of Gram-positive Cocci Cultured from Patients in Three University Hospitals in Tehran, Iran during 2001–2005. Acta Medica Iranica. 2009;47(4):329–334. | ||
Saifi M, Pourshafie MR, Eshraghian MR, Soltan Dallal MM. Anti-microbial resistance of Enterococci isolated from urinary tract infections in Iran. Iran Biomed J. 2008;12(3):185–190. | ||
Pourshafie MR, Talebi M, Saifi M, et al. Clonal heterogeneity of clinical isolates of vancomycin-resistant Enterococcus faecium with unique vanS. Trop Med Int Health. 2008;13(5):722–727. | ||
Rahbar M, Hajia M, Farzanehkhah M. Activity of Nitrofurantoin Against Urinary Tract Infection (UTI) Isolates of Vancomycin-Resistant Enterococci (VRE): A Three-Year Survey in an Iranian Hospital. Iran J Pathol. 2007;2(4):171–174. | ||
Emaneini M, Aligholi M, Aminshahi M. Characterization of glycopeptides, aminoglycosides and macrolide resistance among Enterococcus faecalis and Enterococcus faecium isolates from hospitals in Tehran. Pol J Microbiol. 2008;57(2):173–178. | ||
Fisher K, Phillips C. The ecology, epidemiology and virulence of Enterococcus. Microbiology. 2009;155(Pt 6):1749–1757. | ||
Gikas A, Christidou A, Scoulica E, et al. Epidemiology and molecular analysis of intestinal colonization by vancomycin-resistant enterococci in Greek hospitals. J Clin Microbiol. 2005;43(11):5796–5799. | ||
Esmaeili R, Hashemi H, Moghadam Shakib M, Alikhani MY. Bacterial Etiology of Urinary Tract Infections and Determining their Antibiotic Resistance in Adults Hospitalized in or Referred to the Farshchian Hospital in Hamadan. Ilam Univ Med Sci. 2013;21(7):281–287. | ||
Galimand M, Sabtcheva S, Courvalin P, Lambert T. Worldwide disseminated armA aminoglycoside resistance methylase gene is borne by composite transposon Tn1548. Antimicrob Agents Chemother. 2005;49(7):2949–2953. | ||
Yeh KM, Lu JJ, Siu LK, Peng MY, Chang FY. Phenotypes and genotypes of vancomycin-resistant enterococci isolated during long-term follow-up in a patient with recurrent bacteremia and colonization. J Microbiol Immunol Infect. 2002;35:243–248. | ||
Zarrilli R, Tripodi MF, Di Popolo A, et al. Molecular epidemiology of high-level aminoglycoside-resistant enterococci isolated from patients in a university hospital in southern Italy. J Antimicrob Chemother. 2005;56(5):827–835. | ||
Alekshun MN, Levy SB. Molecular mechanisms of antibacterial multidrug resistance. Cell. 2007;128(6):1037–1050. | ||
Billström H, Lund B, Sullivan A, Nord CE. Virulence and antimicrobial resistance in clinical Enterococcus faecium. Int J Antimicrob Agents. 2008;32(5):374–377. | ||
Gambarotto K, Ploy MC, Turlure P, et al. Prevalence of vancomycin-resistant enterococci in fecal samples from hospitalized patients and nonhospitalized controls in a cattle-rearing area of France. J Clin Microbiol. 2000;38(2):620–624. | ||
Endtz HP, van den Braak N, van Belkum A, et al. Fecal carriage of vancomycin-resistant enterococci in hospitalized patients and those living in the community in The Netherlands. J Clin Microbiol. 1997;35(12):3026–3031. | ||
Boisivon A, Thibault M, Leclercq R, Collége de Bactériologie-Virologie-Hygiène des Hôpitaux Généraux Français. Colonization by vancomycin-resistant enterococci of the intestinal tract of patients in intensive care units from French general hospitals. Clin Microbiol Infect. 1997;3(2):175–179. | ||
Gordts B, Van Landuyt H, Ieven M, Vandamme P, Goossens H. Vancomycin-resistant enterococci colonizing the intestinal tracts of hospitalized patients. J Clin Microbiol. 1995;33(11):2842–2846. | ||
Boyle JF, Soumakis SA, Rendo A, et al. Epidemiologic analysis and genotypic characterization of a nosocomial outbreak of vancomycin-resistant enterococci. J Clin Microbiol. 1993;31(5):1280–1285. | ||
Moaddab SR, Rafi A. Prevalence of vancomycin and high level aminoglycoside resistant enterococci among high-risk patients. Southeast Asian J Trop Med Public Health. 2003;34(4):849–854. | ||
Benenson S, Cohen MJ, Block C, et al. Vancomycin-resistant enterococci in long-term care facilities. Infect Control Hosp Epidemiol. 2009;30(8):786–789. | ||
Cohen MJ, Adler A, Block C, et al. Acquisition of vancomycin-resistant enterococci in internal medicine wards. Am J Infect Control. 2009;37(2):111–116. | ||
Yang J, Lee D, Kim Y, Kang B, Kim K, Ha N. Occurrence of the van genes in Enterococcus faecalis and Enterococcus faecium from clinical isolates in Korea. Arch Pharm Res. 2007;30(3):329–336. | ||
Ieven M, Vercauteren E, Descheemaeker P, van Laer F, Goossens H. Comparison of direct plating and broth enrichment culture for the detection of intestinal colonization by glycopeptide-resistant enterococci among hospitalized patients. J Clin Microbiol. 1999;37(5):1436–1440. | ||
Taylor ME, Oppenheim BA, Chadwick PR, et al. Detection of glycopeptide-resistant enterococci in routine diagnostic faeces specimens. J Hosp Infect. 1999;43(1):25–32. | ||
Pérez-Hernández X, Méndez-Alvarez S, Delgado T, et al. Low prevalence of vancomycin-resistant enterococci in clinical samples from hospitalized patients of the Canary Islands, Spain. Int Microbiol. 2002;5(3):117–120. | ||
Zhanel GG, Decorby M, Laing N. Canadian Antimicrobial Resistance Alliance (CARA), Hoban DJ Antimicrobial-resistant pathogens in intensive care units in Canada: results of the Canadian National Intensive Care Unit (CAN-ICU) study, 2005–2006. Antimicrob Agents Chemother. 2008;52(4):1430–1437. | ||
Fishbane S, Cunha BA, Mittal SK, Ruggian J, Shea K, Schoch PE. Vancomycin-resistant enterococci in hemodialysis patients is related to intravenous vancomycin use. Infect Control Hosp Epidemiol. 1999;20(7):461–462. | ||
Roghmann MC, Fink JC, Polish L, et al. Colonization with vancomycin-resistant enterococci in chronic hemodialysis patients. Am J Kidney Dis. 1998;32(2):254–257. | ||
D’Agata EM, Green WK, Schulman G, Li H, Tang YW, Schaffner W. Vancomycin-resistant enterococci among chronic hemodialysis patients: a prospective study of acquisition. Clin Infect Dis. 2001;32(1):23–29. | ||
Atta MG, Eustace JA, Song X, Perl TM, Scheel PJ. Outpatient vancomycin use and vancomycin-resistant enterococcal colonization in maintenance dialysis patients. Kidney Int. 2001;59(2):718–724. | ||
Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004;39(3):309–317. | ||
Bouchillon SK, Johnson BM, Hoban DJ, et al. Determining incidence of extended spectrum beta-lactamase producing Enterobacteriaceae, vancomycin-resistant Enterococcus faecium and methicillin-resistant Staphylococcus aureus in 38 centres from 17 countries: the PEARLS study 2001–2002. Int J Antimicrob Agents. 2004;24(2):119–124. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




