Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 11 » Issue 1
Prevalence of undiagnosed airflow obstruction among people with a history of smoking in a primary care setting
Authors Fu SN , Yu WC, Wong C, Lam M
Received 12 February 2016
Accepted for publication 5 May 2016
Published 27 September 2016 Volume 2016:11(1) Pages 2391—2399
DOI https://doi.org/10.2147/COPD.S106306
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Sau Nga Fu,1 Wai Cho Yu,2 Carlos King-Ho Wong,3 Margaret Choi-Hing Lam1
1Department of Family Medicine and Primary Health Care, Kowloon West Cluster, Hospital Authority, 2Department of Medicine and Geriatrics, Princess Margaret Hospital, 3Department of Family Medicine and Primary Care, Li Ka Shing Faculty of Medicine, University of Hong Kong, Hong Kong SAR
Purpose: The purpose of this study was to define the prevalence of undiagnosed airflow obstruction (AO) among subjects with a history of smoking but no previous diagnosis of chronic lung disease. The finding of AO likely represents diagnosis of chronic obstructive pulmonary disease.
Patients: People aged ≥30 years with a history of smoking who attended public outpatient clinics for primary care services were included in this study.
Methods: A cross-sectional survey in five clinics in Hong Kong using the Breathlessness, Cough, and Sputum Scale, the Lung Function Questionnaire, and office spirometry was conducted.
Results: In total, 731 subjects (response rate =97.9%) completed the questionnaires and spirometry tests. Most of the subjects were men (92.5%) in the older age group (mean age =62.2 years; standard deviation =11.7). Of the 731 subjects, 107 had AO, giving a prevalence of 14.6% (95% confidence interval =12.1–17.2); 45 subjects with AO underwent a postbronchodilator test. By classifying the severity of chronic obstructive pulmonary disease using the Global Initiative for Chronic Obstructive Lung Disease, 27 (60%) were considered to be in mild category and 18 (40%) in moderate category. None of them belonged to the severe or very severe category. The total score of Lung Function Questionnaire showed that majority of the subjects with AO also had chronic cough, wheezing attack, or breathlessness, although most did not show any acute respiratory symptoms in accordance with the Breathlessness, Cough, and Sputum Scale. Diagnosis of AO was positively associated with the number of years of smoking (odds ratio =1.044, P=0.035) and being normal or underweight (odds ratio =1.605, P=0.046). It was negatively associated with a history of hypertension (odds ratio =0.491, P=0.003).
Conclusion: One-seventh of smokers have undiagnosed AO. Spirometry screening of smokers should be considered in order to diagnose AO at an early stage, with an emphasis on smoking cessation.
Keywords: chronic obstructive pulmonary disease, COPD, airflow obstruction, smokers, early diagnosis, general practice
Introduction
Chronic obstructive pulmonary disease (COPD) causes suffering and adds substantial burden to national health care budget because of repeated hospital admissions.1 However, the condition often remains undiagnosed until the patients are sent to the emergency room with acute exacerbation or complications such as pneumonia or pneumothorax, by which time the disease is often already in the advanced stage.2 In 2002, COPD was the fifth leading cause of mortality worldwide. Total deaths from COPD are projected to increase by >30% in the next 10 years.3 The estimated overall prevalence of COPD among the general population varies among different regions, with 6.3% in the Asia-Pacific region,4 8.2% in the People’s Republic of China,5 4% in the UK, and 6% in the USA.6 In Hong Kong, the COPD prevalence was 3.5% among those aged >30 years estimated using epidemiology relationship of smoking and other risk factors.4 In the community, 25.9% of those aged >60 years attending elderly centers were found to have airflow obstruction (AO) in a survey using office spirometry.7
Early COPD diagnosis may motivate smoking cessation, which is the only measure known to radically improve future prospects for the patients.8–10 One feasible method for early COPD diagnosis is to screen for AO. AO is spirometrically defined as a reduced ratio of forced expiratory volume in the first second (FEV1) to forced vital capacity (FVC), such that FEV1/FVC is <0.70. The yield of undiagnosed AO patients is highly dependent on the inclusion criteria of the high-risk group. For instance, history of smoking is known to be a strong risk factor for AO.11
Although the most common diagnosis in smokers with AO is COPD,12 doctors still need to consider other differential diagnoses such as asthma, bronchiectasis,13 and pulmonary tuberculosis.14 Patients with AO are often relatively asymptomatic in the early stages. Smokers, men, and older people have a higher risk of being affected.15 The prevalence of undiagnosed AO among people with smoking history varies worldwide,12 and it ranges from 10.1% in the UK,16 13.1% in Japan,17 and 34.8% in Denmark among smokers with chronic respiratory symptoms.18
It is generally agreed that primary health care professionals play a significant role in accurately diagnosing COPD at the early stage.19 They have ample opportunities to make a diagnosis during primary health care encounters.20 The active case finding of high-risk group can potentially identify 70% more undiagnosed AO than opportunistic identification alone. Providing treatment for these new cases could potentially reduce hospitalizations and deaths.21 The most commonly used strategy is to invite patients in the high-risk group to complete questionnaires and perform spirometry during their primary care visits,16–18,20 although it is questionable whether such strategy is applicable and acceptable in older population from the lower socioeconomic class. In view of the large variability of response and yield in COPD screening programs worldwide, this study was conducted to examine the situation in Hong Kong.
Hypothesis to be tested
This study investigates the prevalence of AO among persons aged ≥30 years who have a history of smoking but had no previous diagnosis of obstructive lung diseases. In addition, it investigates whether there is any significant association of AO with demographic and clinical parameters.
Materials and methods
This is a multicenter cross-sectional prevalence study performed from April to July 2014. The study was approved by the Hong Kong Hospital Authority, Kowloon West Cluster Research Ethics Committee. Written informed consent was obtained before the commencement of spirometry and data collection. Subjects with acute respiratory symptoms underwent a spirometry test 2–6 weeks later to ensure the reliability of the result.
Setting
This study was conducted in five public primary care community clinics in the Kwai Chung and Tsing Yi districts of Hong Kong. The study clinics mainly provide primary health care services to the older population from lower socioeconomic class, and ~10% of the patients attending the study clinics were active smokers. A number comparable to the prevalence of daily smokers in the Hong Kong adult population.22 All Hong Kong residents have access to the clinics’ smoking cessation counseling service with or without referral. Doctors can arrange for any patient to do an office spirometry. In the Hong Kong primary care setting, only a small proportion of physician-diagnosed COPD patients have documented spirometry findings.23 In the absence of any structured COPD screening program, and with the uncommon use of spirometry for the evaluation of patients with chronic respiratory symptoms, the prevalence of undiagnosed AO is anticipated to be high, especially among high-risk patients such as chronic smokers.
Study subjects
All potential subjects were identified by using computerized patient records with a history of smoking, regardless of their reason for consultation. They were then recruited by trained research nurses during their next visit to the clinics for health care services.
Inclusion and exclusion criteria
All subjects must be aged ≥30 years with a history of smoking (ie, persons who reported smoking at least 100 cigarettes during their lifetime, according to the 2011 Centre of Disease Control and Prevention Criteria).24 Subjects who were unable to perform spirometry and who had previous diagnosis of chronic lung diseases including asthma, COPD, pulmonary fibrosis, bronchiectasis, and restrictive lung disease were excluded from this study. Pregnant women were also excluded from the study.
Basic data collection
The research nurses interviewed participants using standard questionnaires in Cantonese (a variety of the Chinese languages) to collect the following data:
- Demographics: sex, age, and occupation.
- Smoking status, including intensity and duration by using the World Health Organization Global Adult Tobacco Survey.24
- Known chronic illnesses according to subjects’ medical record.
- Assessment of acute respiratory symptoms by using the validated Chinese version of Breathlessness, Cough, and Sputum Scale (BCSS)© 2003,25 used with permission from the AstraZeneca Company (London, UK).
- Assessment of chronic respiratory symptoms by using the Chinese version of Lung Function Questionnaire (LFQ),26 used with permission from GlaxoSmithKline plc (Middlesex, UK).
The BCSS is a three-item, patient-reported questionnaire regarding the presence of three common COPD symptoms during the interview: breathlessness, cough, and sputum. Subjects were asked to evaluate each symptom on a 5-point Likert-type scale, ranging from 0 to 4, with higher scores indicating more severe manifestation of symptoms. The total score is expressed in the range of 0–12. The tool has been validated to assess daily respiratory symptoms in COPD patients.27
The LFQ is a five-item tool asking how often the subject experiences productive cough, wheezing attack, and shortness of breath by a 5-point Likert-type scale. The fourth item is the number of years of smoking, and the fifth item is the age of the subject. The total score ranges from 5 (very frequent symptoms) to 25 (never had any symptoms). A score of ≤18 indicates the presence of chronic respiratory symptoms and high risk of AO. The tool has been used to estimate the prevalence of COPD among smokers in primary care settings. It provides additional information to identify high-risk patients with AO.28 The Chinese version of LFQ was translated by bilingual authors Margaret Choi-Hing Lam and Sau Nga Fu and subsequently back-translated by Wai Cho Yu; all authors agreed on the final version. The translated LFQ was tested on five subjects to confirm content validity.
Spirometry
Spirometry was done according to the American Thoracic Society criteria29 by trained research nurses using the Spirolab III® spirometer (MIR Medical International Research, Roma, Italy) with subjects in seated position. The spirometric reference values for Hong Kong adults according to Ip et al were adopted.30 For those with FEV1/FVC <0.70, spirometry was repeated 15–20 minutes after inhalation of 400 μg Ventolin® metered dose inhaler (salbutamol; GlaxoSmithKline plc) administered using a spacer. Each spirogram was reviewed using the American Thoracic Society guidelines.29 Positive bronchodilator (BD) reversibility is defined as an increase in FEV1 of 200 mL and 12%.29
Outcome measurement
The primary outcome is the proportion of subjects with AO, defined by preBD FEV1/FVC ratio of <0.70.
Secondary outcomes are:
- proportion of subjects with AO and chronic respiratory symptoms, assumed by LFQ score ≤18, indicating a high possibility of COPD;
- proportion of subjects with different Global Initiative for Chronic Obstructive Lung Disease (GOLD) stages according to the postBD FEV1 % predicted value of Hong Kong adults;30 and
- factors associated with AO.
Statistical analysis
Descriptive statistics were presented as mean and standard deviation (SD) for continuous variables and as a proportion for categorical variables. The prevalence rate of undiagnosed AO and its 95% confidence interval were calculated. Sociodemographic and clinical characteristics between subjects with and without undiagnosed AO were compared and tested using a χ2 test for categorical variables and independent t-test and Fisher’s exact test for continuous variables. Among those who underwent postBD spirometry, the proportions of subjects with mild, moderate, severe, and very severe conditions according to the GOLD stage standard were reported.
Since undiagnosed AO is the primary outcome, logistic regression model was used to determine sociodemographic and clinical factors associated with the presence of undiagnosed AO. Odds ratios (ORs) of sociodemographic and clinical factors associated with undiagnosed AO were estimated. All significance tests were two-tailed, and P<0.05 was considered significant. Statistical analyses were conducted by using SPSS Version 23.0 (IBM® SPSS Inc., Armonk, NY, USA).
Results
Subject characteristics
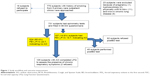
Figure 1 presents the research workflow. Of the 770 eligible subjects approached, 16 refused to participate (response rate =97.9%), and 23 subjects were excluded due to the following conditions: pregnancy (n=1), having kyphoscoliosis (n=2), being physically unfit to blow (n=11), and having known chronic lung disease (n=9). The remaining 731 subjects completed the questionnaires and spirometry tests. Table 1 shows the sociodemographic and clinical characteristics of the subjects. Most of the subjects were men (92.5%) in the older age group (mean age =62.2 years; SD =11.7). The majority of them (73.1%) only attended middle school or below. One-third (32.4%) of them were actively employed. Approximately half of them (48.3%) were current smokers, with all being cigarette smokers except for one bamboo pipe smoker. The mean years since first smoking was 42.9 (SD =12.9). The mean pack-year was 34.2 (SD =25.1). Many subjects were either overweight (24.1%) or obese (47.1%) according to the World Health Organization Asian body mass index cutoff level.31 Two-third of them had hypertension, and one-third of them had diabetes mellitus. The mean BCSS score was 0.95 (SD =1.12). In total, 335 subjects (45.8%) had no acute respiratory symptoms (BCSS score =0), while 381 (52.1%) had only mild acute symptoms (BCSS score =1–3). Of the 105 subjects with AO who responded to LFQ, 100 (95.2%) scored ≤18 in LFQ, which indicated the presence of significant chronic respiratory symptoms.
Spirometry results
Table 2 shows the spirometry results of the 731 subjects. 107 were found to have AO determined by a preBD FEV1/FVC ratio of <0.70. The prevalence was 14.6% (95% confidence interval =12.1–17.2). In other words, one in seven subjects were screened positive for AO. The Pearson’s correlation between FEV1 % predicted and LFQ was 0.282 (P=0.004). The higher LFQ score (less chronic symptoms) indicated a higher FEV1 % predicted in spirometry test.
A significant number of subjects refused to undergo the postBD test. Of the 45 subjects (42.1%) who completed postBD spirometry, 12 (20.7%) showed significant BD reversibility. Assuming that the subjects with AO were suffering from COPD, the GOLD classification of severity of COPD showed that conditions of 60% of the subjects were mild, while that of 40% were moderate. None of the subjects were classified as suffering from severe or very severe COPD.
Factors associated with airflow obstruction
Table 3 shows the sociodemographic and clinical factors associated with AO and AO with chronic respiratory symptoms (LFQ score ≤18) by logistic regression. Sex, educational level, and occupation of the subjects were not significantly associated with the presence of AO. The longer the subjects have smoked, the more likely they would have AO (total number of years of smoking, OR =1.04; P=0.035). However, the number of pack-years did not demonstrate a significant association.
Some clinical parameters were also found to show positive association with AO and AO with chronic respiratory symptoms. Subjects with body mass index <23 kg/m2 (normal or underweight) were more likely to have AO (OR =1.61; P=0.046). There were 537 (73.5%) subjects with predefined chronic illnesses such as cardiovascular diseases, hypertension, diabetes mellitus, and ischemic heart disease for at least 6 months. Subjects with hypertension were found less likely to have AO (OR =0.49; P=0.003). Having other chronic illnesses did not show any significant association with AO.
Discussion
The prevalence of undiagnosed AO among subjects with smoking history who participated in this study was 14.6%, which is similar to that reported in the study of Sekine et al conducted in Japan (13.1% prevalence among people with smoking history).17 The response rate of this cross-sectional survey (97.9%) was high. Most subjects were in the older age group with a relatively low educational level, who were likely to be representative of the public primary care clinics encounters in Hong Kong.32 On top of the overall prevalence of diagnosed COPD (3.5%) in Hong Kong,4 doctors were likely to diagnose more COPD patients with smoking history at the early stage of disease by the use questionnaire and spirometry test. If diagnosed at an early stage, COPD patients could potentially preserve their lung function by smoking cessation, thus preventing the subsequent development of complications and disabilities.8–10 This finding calls for a more active and effective screening of AO among patients with a smoking history.
In contrast to the findings from recent studies showing the association of pack-years33 and age34 with risk of COPD, this study found no significant association of both factors with AO. The negative association with age could probably be explained by the relatively homogenous characteristics of the dominant older male population, which was a representative of the smoker population in Hong Kong. According to the statistics in Hong Kong, the majority of cigarette smokers (82.8%) are male.22 Nevertheless, the number of years of smoking, calculated by subtracting the age at last smoking from the age at first smoking, showed significant association with AO (OR =1.044; P=0.035). This observation might be attributed to the fact that more than half of the subjects were past smokers (51.7%). There might have been recall bias or underreporting of the number of cigarettes smoked per day. In view of this, the conventional adoption of pack-years of smoking but not of duration of smoking to assess risk of AO should be reconsidered, especially when a significant proportion of screened subjects are ex-smokers. Both factors (ie, duration of smoking and pack-years) should be assessed in daily clinical practice. Further research is required in this area.
The majority of subjects had no or mild respiratory symptoms (eg, breathlessness, cough, or sputum) on the day of assessment as determined by the BCSS. However, it was found that the BCSS had no association with the diagnosis of AO. In other words, the presence of acute respiratory symptoms in patients with a history of smoking is not a helpful indicator of predicting AO. On the other hand, the presence of chronic symptoms by LFQ (eg, productive cough, wheezing, and shortness of breath) is a more accurate predictor of possible undiagnosed AO. Therefore, doctors should assess the duration of chronic respiratory symptoms in the patients with a history of smoking and order spirometry accordingly.
Apart from clinical symptoms and duration of smoking, another factor that is significantly associated with AO is whether a subject was of normal weight or underweight. It has been found that there is depletion of body weight, fat-free mass, and muscle mass in patients with moderate and severe COPD.31 In the present study, most subjects with AO suffered from mild lung function impairment, and the data would suggest that loss of body mass occurs early in the course of COPD. Further studies with more detailed nutritional assessment are required.
Yet another observation was that subjects with hypertension had a significantly lower risk of AO. It is well known that hypertension and COPD commonly co-occur in the same individual, particularly in the elderly. Mills et al have in fact shown that patients with COPD have increased arterial stiffness and blood pressure in comparison with controls matched for age and smoking status.35 Our findings seem to be contradictory to what is known about the relationship between COPD and systemic arterial hypertension. However, patients in the study by Mills et al had more severe conditions compared to those in the present study (Stage I, 2%; Stage II, 36%; Stage III, 39%; and Stage IV, 20%), which again raises the possibility that different stages of COPD may have different manifestations and associations. Further studies are thus required to examine the relationship between systemic arterial hypertension and different GOLD stages of COPD and thus to provide more definitive answers.
The response rate for spirometry test among the participants in the present study is very encouraging. It enhances the confidence of health care professionals that the test is well accepted by most of the primary care patients who are smokers. However, more than half (57.1%) of the subjects with AO who underwent preBD spirometry refused to undergo postBD spirometry. The research nurses in this project reported that most of the subjects who refused did not want to wait for the 15 minutes that was required to complete the test. Others simply did not want to blow again because of the physical discomfort associated with the test. However, although BD reversibility may provide useful clinical information, it is not required for the diagnosis of AO; therefore, the primary objective of this study was not adversely affected. LFQ in this study correlated well with the prevalence of AO, which was also demonstrated in a previous study.28 Possible future strategies to improve patient acceptance include a more widespread use of LFQ as a screening tool of AO, providing patients with clear explanation of procedures involved and educating them on the importance of completing both pre- and postBD spirometry.
Limitations
The majority of the study population (92.5%) were men from the older age group (mean age =62.2 years; SD =11.7) with a relatively low educational level and were unemployed. Therefore, the prevalence of AO could just be a representative of the male population of the lower socioeconomic class. The prevalence of AO among men of younger age group, women, and those from higher socioeconomic class may be different when using the same screening method.
Although this method received a high response rate from the study population, the diagnosis of AO may not necessarily lead to the diagnosis of COPD. Other differential diagnoses such as asthma and bronchiectasis should also be considered in clinical practice. The relatively low response rate for postBD test may lead to underdetection of asthmatic element in subjects with AO. Even though screened subjects are clinically compatible to COPD diagnosis, the severity cannot be graded due to the absence of postBD spirometry results. This limitation warrants a better designed screening program for AO with enhanced acceptance of postBD spirometry.
Conclusion
One in seven smokers who seek primary care services for various ailments in this study had undiagnosed AO. Although smokers who exhibited AO mostly suffered from COPD, all subjects in this study showed mild to moderate COPD condition. Most subjects with AO had chronic respiratory symptoms, although they might not show any acute respiratory symptoms. The presence of AO was associated with number of years of smoking, being normal or underweight, and having no hypertension. Spirometry screening of subjects with smoking history, particularly those with chronic respiratory symptoms, should be considered to allow early diagnosis of AO, so that appropriate intervention measures including smoking cessation might be instituted.
Acknowledgments
The authors would like to thank all the supporting staff, nurses, and doctors from Ha Kwai Chung General Outpatient Clinic (GOPC), South Kwai Chung GOPC, North Kwai Chung GOPC, Tsing Yi Town GOPC, and Tsing Yi Cheung Hong GOPC for the recruitment of research subjects and assisting in data collection. The authors would also like to thank Dr Yuk-Kwan Yiu, Chief of Service of the Department of Family Medicine and Primary Health Care, Kowloon West Cluster, Hospital Authority, for authorizing them to perform this study. This study was financially supported by the Princess Margaret Hospital Respiratory Research Fund.
Disclosure
The authors report no conflicts of interest in this work.
References
Jansson SA, Backman H, Stenling A, Lindberg A, Ronmark E, Lundberg B. Health economic costs of COPD in Sweden by disease severity–has it changed during a ten years period? Respir Med. 2013;107(12):1931–1938. | ||
Bastin AJ, Starling L, Ahmed R, et al. High prevalence of undiagnosed and severe chronic obstructive pulmonary disease at first hospital admission with acute exacerbation. Chron Respir Dis. 2010;7(2):91–97. | ||
Lopez AD, Shibuya K, Rao C, et al. Chronic obstructive pulmonary disease: current burden and future projections. Eur Respir J. 2006;27(2):397–412. | ||
Regional COPD Working Group. COPD prevalence in 12 Asia-Pacific countries and regions: projections based on the COPD prevalence estimation model. Respirology. 2003;8(2):192–198. | ||
Zhong N, Wang C, Yao W, et al. Prevalence of chronic obstructive pulmonary disease in China: a large, population-based survey. Am J Respir Crit Care Med. 2007;176(8):753–760. | ||
Halbert RJ, Natoli JL, Gano A, Badamgarav E, Buist AS, Mannino DM. Global burden of COPD: systematic review and meta-analysis. Eur Respir J. 2006;28(3):523–532. | ||
Ko FW, Woo J, Tam W, et al. Prevalence and risk factors of airflow obstruction in an elderly Chinese population. Eur Respir J. 2008;32(6):1472–1478. | ||
Anthonisen NR, Connett JE, Kiley JP, et al. Effects of smoking intervention and the use of an inhaled anticholinergic bronchodilator on the rate of decline of FEV1. The Lung Health Study. JAMA. 1994;272(19):1497–1505. | ||
Scanlon PD, Connett JE, Waller LA, et al. Smoking cessation and lung function in mild-to-moderate chronic obstructive pulmonary disease. The Lung Health Study. Am J Respir Crit Care Med. 2000;161(2 Pt 1):381–390. | ||
Kanner RE, Connett JE, Williams DE, Buist AS. Effects of randomized assignment to a smoking cessation intervention and changes in smoking habits on respiratory symptoms in smokers with early chronic obstructive pulmonary disease: the Lung Health Study. Am J Med. 1999;106(4):410–416. | ||
Fletcher C, Peto R. The natural history of chronic airflow obstruction. Br Med J. 1977;1(6077):1645–1648. | ||
Coultas DB, Mapel DW. Undiagnosed airflow obstruction: prevalence and implications. Curr Opin Pulm Med. 2003;9(2):96–103. | ||
Roberts HR, Wells AU, Milne DG, et al. Airflow obstruction in bronchiectasis: correlation between computed tomography features and pulmonary function tests. Thorax. 2000;55(3):198–204. | ||
Allwood BW, Myer L, Bateman ED. A systematic review of the association between pulmonary tuberculosis and the development of chronic airflow obstruction in adults. Respiration. 2013;86(1):76–85. | ||
Vestbo J, Hurd SS, Agusti AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4):347–365. | ||
Al Omari M, Khassawneh BY, Khader Y, Dauod AS, Bergus G. Prevalence of chronic obstructive pulmonary disease among adult male cigarettes smokers: a community-based study in Jordan. Int J Chron Obstruct Pulmon Dis. 2014;9:753–758. | ||
Sekine Y, Yanagibori R, Suzuki K, et al. Surveillance of chronic obstructive pulmonary disease in high-risk individuals by using regional lung cancer mass screening. Int J Chron Obstruct Pulmon Dis. 2014;9:647–656. | ||
Ulrik CS, Lokke A, Dahl R, et al. Early detection of COPD in general practice. Int J Chron Obstruct Pulmon Dis. 2011;6:123–127. | ||
Price DB, Yawn BP, Jones RC. Improving the differential diagnosis of chronic obstructive pulmonary disease in primary care. Mayo Clin Proc. 2010;85(12):1122–1129. | ||
Jones RC, Price D, Ryan D, et al. Opportunities to diagnose chronic obstructive pulmonary disease in routine care in the UK: a retrospective study of a clinical cohort. Lancet Respir Med. 2014;2(4):267–276. | ||
Jordan RE, Lam KB, Cheng KK, et al. Case finding for chronic obstructive pulmonary disease: a model for optimising a targeted approach. Thorax. 2010;65(6):492–498. | ||
Centre for Health Protection, Department of Health, HKSAR. Statistics on behavioural risk factors, cigarette smoking; April 2014. Available from: http://www.chp.gov.hk/en/data/1/10/280/4015.html. Accessed May, 2015. | ||
Yu WC, Fu SN, Tai EL, et al. Spirometry is underused in the diagnosis and monitoring of patients with chronic obstructive pulmonary disease (COPD). Int J Chron Obstruct Pulmon Dis. 2013;8:389–395. | ||
Warren CW, Lee J, Lea V, et al. Evolution of the Global Tobacco Surveillance System (GTSS) 1998–2008. Global Health Promot. 2009;16(2 Suppl):4–37. | ||
Leidy NK, Schmier JK, Jones MK, Lloyd J, Rocchiccioli K. Evaluating symptoms in chronic obstructive pulmonary disease: validation of the Breathlessness, Cough and Sputum Scale. Respir Med. 2003;97(Suppl A):S59–S70. | ||
Yawn BP, Mapel DW, Mannino DM, et al. Development of the Lung Function Questionnaire (LFQ) to identify airflow obstruction. Int J Chron Obstruct Pulmon Dis. 2010;5:1–10. | ||
Desai H, Eschberger K, Wrona C, et al. Bacterial colonization increases daily symptoms in patients with chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2014;11(3):303–309. | ||
Mintz ML, Yawn BP, Mannino DM, et al. Prevalence of airway obstruction assessed by lung function questionnaire. Mayo Clin Proc. 2011;86(5):375–381. | ||
Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26(5):948–968. | ||
Ip MS, Ko FW, Lau AC, et al. Updated spirometric reference values for adult Chinese in Hong Kong and implications on clinical utilization. Chest. 2006;129(2):384–392. | ||
Schols AM, Soeters PB, Dingemans AM, Mostert R, Frantzen PJ, Wouters EF. Prevalence and characteristics of nutritional depletion in patients with stable COPD eligible for pulmonary rehabilitation. Am Rev Respir Dis. 1993;147(5):1151–1156. | ||
Yam H, Mercer SW, Wong L, Chan WK, Yeoh EK. Public and private healthcare services utilization by non-institutional elderly in Hong Kong: is the inverse care law operating? Health Policy. 2009;91(3):229–238. | ||
Forey BA, Thornton AJ, Lee PN. Systematic review with meta-analysis of the epidemiological evidence relating smoking to COPD, chronic bronchitis and emphysema. BMC Pulm Med. 2011;11:36. | ||
Sandelowsky H, Stallberg B, Nager A, Hasselstr AJ. The prevalence of undiagnosed chronic obstructive pulmonary disease in a primary care population with respiratory tract infections – a case finding study. BMC Fam Prac. 2011;12:122. | ||
Mills NL, Miller JJ, Anand A, et al. Increased arterial stiffness in patients with chronic obstructive pulmonary disease: a mechanism for increased cardiovascular risk. Thorax. 2008;63(4):306–311. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.