Back to Journals » Journal of Multidisciplinary Healthcare » Volume 13
Prevalence of the SARS-CoV-2 Infection Among Post-Quarantine Healthcare Workers
Authors Alshahrani MS , Alnimr A , Alnassri S , Alfarag S, Aljehani Y, Alabdali M
Received 2 September 2020
Accepted for publication 29 October 2020
Published 15 December 2020 Volume 2020:13 Pages 1927—1936
DOI https://doi.org/10.2147/JMDH.S279469
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Mohammed S Alshahrani,1 Amani Alnimr,2,3 Samia Alnassri,3 Sukyana Alfarag,4 Yasser Aljehani,5 Majed Alabdali6
1Emergency and Critical Care Department, King Fahad Hospital of the University – Imam Abdulrahman Bin Faisal University, Dammam, Kingdom of Saudi Arabia; 2Department of Microbiology, College of Medicine, Imam Abdulrahman Bin Faisal University, Dammam, Kingdom of Saudi Arabia; 3Department of Infection Control, King Fahad Hospital of the University, Dammam, Kingdom of Saudi Arabia; 4Emergency Department, King Fahad Hospital of the University – Imam Abdulrahman Bin Faisal University, Dammam, Kingdom of Saudi Arabia; 5Department of Surgery, King Fahad Hospital of the University – Imam Abdulrahman Bin Faisal University, Dammam, Kingdom of Saudi Arabia; 6Department of Neurology, King Fahad Hospital of the University – Imam Abdulrahman Bin Faisal University, Dammam, Kingdom of Saudi Arabia
Correspondence: Mohammed S Alshahrani
Emergency and Critical Care Departments, King Fahad Hospital of the University – Imam Abdulrahman Bin Faisal University, Dammam 31952, Kingdom of Saudi Arabia
Tel +966-556-966663
Email [email protected]
Background: Coronavirus disease 2019 is an emerging highly communicable disease. Nosocomial transmission needs to be prevented through the implementation of stringent screening and infection control measures.
Objective: The objective of the study is to estimate the prevalence of severe acute respiratory syndrome– coronavirus 2 (SARS-CoV-2) infection among health care workers (HCWs) post quarantine period.
Methods: This is a prospective, observational study conducted at a teaching University hospital in Alkhobar, Saudi Arabia, during the period between May 1 and June 15, 2020. All (HCWs) joining work back from the quarantine areas had a real-time polymerase chain reaction (qRT-PCR) test for SARS-CoV-2. The demographic and clinical data from the staff were collected.
Results: Of the 301 HCWs screened, 18 (6%) had positive PCR. The age means of the positive cases was 32.9 Y ± 8.7 compared to 33.8 Y ± 7.0 in the negatively tested group (p value = 0.90). Of the 18 PCR-positive HCWs, 7 (38.9%) were male. Majority of those who tested positive were trainees (8.2%) followed by nurses (5.1%). In PCR-positive group, a clear epidemiological exposure was found in 4/18 cases (22.2%). Male gender and residency in specific districts were observed more in the positive cases (p value = 0.01 and 0.0001, respectively). In regards to symptoms, most of the positive PCR tested HCWs (n=12, 66.7%) remained asymptomatic. Most prevalent initial symptoms were gastrointestinal symptoms (diarrhea, abdominal pain) in six HCWs representing 33.3%. No significant difference was noted in co-morbidities reported by both groups.
Conclusion: Health care workers tested post-quarantine period were found to be at risk of SARS-CoV-2 infection despite very minimal or no known risks of exposure, where most of them were asymptomatic. This potentially carries risk of nosocomial transmission inside healthcare facilities. Implanting policies for routine post-quarantine screening for HCWs is recommended.
Keywords: COVID‐19, asymptomatic HCW, quarantine, PCR, SARS-CoV-2, screening
Introduction
The novel coronavirus disease (COVID-19) was declared as a pandemic by the World Health Organization (WHO) on 11 March 2020, in response to the increasing numbers of cases across the world.1 More than 34 million confirmed COVID-19 cases were reported globally including an excess of one million deaths (https://coronavirus.jhu.edu/map.html, PMID: 32670917). The reasons for the rapid spread of the disease caused by the severe acute respiratory syndrome–coronavirus 2 (SARS-CoV-2) can be explained by many factors. Of these, the presence of asymptomatic carriers is an important yet unidentified reservoir of the infection.2–5 Since then, countries have variably implemented stringent measures to contain the local and international spread of the infection. Such public health measures that are employed worldwide include stay-at-home ordinances, massive screenings, social distancing, travel restriction, curfews and quarantines. Citywide quarantines have been imposed in areas whereas potential exposure to the contagious disease has likely happened in a large population, to ascertain if they become unwell. This will help to reduce the risk of them infecting others by the implementation of separation and restriction of movement.6–8 An epidemiological study has shown that the combined interventions of travel restrictions around Wuhan and other public measures followed by compulsory quarantine were associated with progressive reductions in the incidence of confirmed cases in Wuhan and a decrease in the effective reproduction number from >3 before the interventions to 0.3 after them.9
Multiple governmental actors have enforced quarantines to curb the rapid spread of the virus, leading to half the world’s population being in total lockdown in April 2020.10 The Kingdom of Saudi Arabia, amongst other countries, adopted a cautious approach, utilising official directives. The city-wise breakdown of the new cases of SARS-CoV-2 during the early surge of the pandemic in March 2020 was identified in the Qatif district in Eastern Saudi Arabia. Accordingly, the Kingdom of Saudi Arabia imposed a mandatory quarantine on the area for 7 weeks between 8 March and 29 April 2020. This temporary suspension of entry and exit across the whole district along with the implementation of strict precautionary measures resulted in a drastic decline in the number of cases in the Qatif area, as reported by the Ministry of Health officials.11 Published studies on COVID-19 have mainly focused on the clinical, diagnostic and management aspects of the disease. Little attention has been given to following up on post-quarantine individuals. A recently published study conducted in a homeless shelter in the United States found that 36% of the screened population tested positive, while the majority of them (87.8%) remained asymptomatic.12 Another study highlighted the importance of multi-timepoint surveillance by identifying up to 7.1% positive cases amongst asymptomatic healthcare workers (HCWs) in an academic medical centre in the United Kingdom.13 However, there are no massive screening studies available on HCWs concerning geographical quarantines.
In this study, we aimed to assess the prevalence of the COVID-19 infection among HCWs that came from a predefined quarantine area and the role of universal real-time reverse transcriptase PCR (qRT-PCR) screening in the early detection of asymptomatic cases. Besides, we evaluated the association of the risk of exposure during the quarantine period with the qRT-PCR results.
Methods
Design, Setting and Participants
In this prospective cohort study, all HCWs at King Fahad Hospital of the University (KFHU) that were residents of the Qatif region, a quarantined area announced by the government, were included upon re-joining work. The qPCR testing was done for all joining HCWs. Demographic data of the hospital staff (that is, age, gender, nationality and the town of residency) were collected, as well as information on their working areas and epidemiological exposure risks in other healthcare facilities or the community. Further, a screening for COVID-19 symptoms and comorbidities was carried out via an electronic, detailed questionnaire sent by email to all the negative PCR HCWs. For PCR-positive cases, phone interviews were performed to collect the data using the same questions set >14 days after testing.
A contact investigation was conducted on each positive case whenever there were potential exposure risks. All HCWs with positive COVID-19 PCRs were followed up for clearance following the local protocol. This project was run as a part of the mandatory institutional infection control plan for re-joining work after a quarantine period. The project utilised the hospital policy for HCW testing for COVID-19, wherein a test-based strategy was followed for all symptomatic and asymptomatic HCWs to join work (Figure 1). The asymptomatic HCWs diagnosed with COVID-19 were tested periodically every 48–72 hours until the infection cleared. A single negative PCR test was needed for clearance ≥5 days, along with physical assessments in the employee health clinic for all joining HCWs. All personal information was kept confidential throughout the study; no extra testing was required for those cases and no further specimens were collected. The study was been approved by the Institutional Review Board (IRB-2020-475-Med).
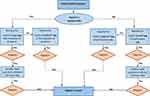 |
Figure 1 The follow-up algorithm for a confirmed case of an HCW with COVID-19. |
Laboratory Testing
One nasopharyngeal swab sample was collected from each individual during the first week of joining and inoculated in a tube that contained a viral transport medium (VTM) (Vircell, Spain). The viral nucleic acid was extracted by the QIAamp Viral RNA Mini Kit (Qiagen, Germany), followed by a qRT-PCR assay using the RealStar SARS-CoV-2 RT-PCR Kit (Altona Diagnostics, Germany). The tests detected the E gene sequence of the lineage B-betacoronavirus and the S gene sequence specific to SARS-CoV-2. All samples were initially screened by the detection of the E gene, followed by a confirmatory step to detect the S gene sequence, with RNA positive and negative controls corresponding to the target genes. When only the E gene was detected, the result was considered as inconclusive and another sample was requested for submission on the same day, according to the WHO for interpreting SARS-CoV-2 PCR tests.14
Main Outcomes and Measures
Primary Outcome
The primary outcome was to assess the prevalence of laboratory-confirmed COVID-19 infections (defined as a positive qRT-PCR test) among HCWs re-joining from within the quarantine area.
Secondary Outcome
There were two secondary outcomes. The first was to determine the risks of exposure and predictors of positivity before re-joining work. The second was to report any associated clinical manifestations.
Statistical Analysis
The data were tabulated in excel sheets and expressed in mean and SD forms. Comparative studies were performed between positive and negative cases using one-way ANOVA. A p-value of <0.05 was considered statistically significant. All statistical analyses were performed using the GraphPad Prism software for Mac (version 7).
Results
Of the 301 HCWs evaluated, the qRT-PCR results indicated that 18 individuals (6%) tested positive in the PCR. All positive testing HCWs were interviewed by phone calls and while an electronic questionnaire sent to all negatively testing individuals. Of the latter, only 119 responded and provided the requested data. We found no significant differences between the age mean ± SD in the negative cases (33.8 Y ± 7.0) or positive cases (32.9 Y ± 8.7) (p-value = 0.90). Of the 18 PCR-positive testing HCWs, 7 (38.9%) were male compared to only 16 (13.4%) in the negative PCR group, which was statistically significant (Table 1). Most HCWs in this cohort belonged to the age group of 20–29 Y, which was also evident in the age distribution in both positive and negative cases.
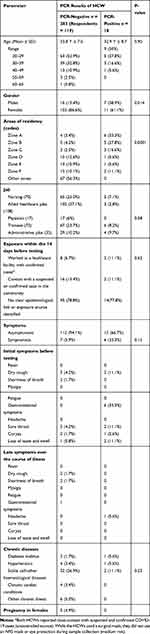 |
Table 1 Demographic and Clinical Characteristics for the Quarantined Individuals |
Considering the large quarantine area, we stratified the zones and found that residents in some zones were at higher risks of having positive PCR results compared to other zones, as shown in Table 1 (Figure 1).
The HCWs came from different job categories. These categories included nurses (70), allied healthcare jobs (108), physicians (17), trainees (73) and administrative jobs (33). It was found that a majority of those that tested positive were trainees (6, 8.2%) followed closely by 5 nurses (5.1%), 4 administrative HCWs (97%) and 3 allied healthcare jobs (2.8%), while no physicians had positive results (Table 1). However, no statistical difference was found between both groups regarding the nature of the job’s p-value = 058.
Exposure Risks Within the 14 Days Before Testing
The study evaluated the potential source of the infection for all the 18 patients that had positive PCR results. The identification of the source of infection could have established the pathway of the spread of the disease across the population and determined the risk to healthcare workers, making it possible to rely on a risk-based testing algorithm. Nevertheless, only 4 of the 18 cases (22.2%) had an identified risk of exposure (Table 2). These were 2 HCWs (11.1%) who reported working in a healthcare facility within the quarantine area and had handled confirmed cases, compared to 8 (6.7%) of the HCWs who had PCR-negative results with the same environmental risks. Community exposure through contact with a suspected or confirmed case in the community was reported in 16 (13.4%) of PCR-negative results and 2 (11.1%) of the PCR-positive cases. No clear epidemiological link or exposure source was identified in most (n = 14, 77.8%) PCR-positive cases. This risk of exposure results did not show any significant differences between the p-value of both groups (= 0.62).
 |
Table 2 N = 301, Positive Cases = 18. Description of the 18 Screened Cases That Tested Positive Through the PCR Screening for COVID-19 Among KFHU’s Staff Living in Qatif |
Pre-Testing and Post-Testing COVID-19 Symptoms, Comorbidities and Viral Clearance
The study evaluated the distribution of the symptoms among screened HCWs. Among the PCR-positive patients, most remained asymptomatic carriers of the virus (66.7%). The most prevalent initial symptoms identified in the PCR-positive HCWs were gastrointestinal symptoms (diarrhoea and abdominal pain) in six HCWs, representing 33.3% of the total symptoms. Additionally, other symptoms identified included the loss of taste and smell and sore throat in 2 out of 18 HCWs (11.2%) for each symptom. Coryza was only present in one patient (5.6%) from among those who had PCR-positive results. Remarkably, only one patient out of 18 PCR-positive HCWs indicated a headache as a symptom during the late symptoms throughout the illness. The rest of the patients had no symptoms associated with COVID-19 during the late course of illness (Table 1). Concerning comorbidities, among the PCR-positive HCWs, two were found to have a pre-existing sickle cell and other haematological diseases (11.2%), 1 had diabetes mellitus and another HCW had hypertension representing, 5.6% for each of existing comorbidity (Table 1). The viral shedding and clearance are illustrated in Figure 2 where the median duration of shedding is 9 days ± 1.307, indicating that 50% of the HCWs cleared the infection by day 9, while all were cleared by day 12. This means that a time-based clearance strategy based on 10 days would have missed two persistently PCR-positive cases (11.1%).
 |
Figure 2 Viral clearance in days (Kaplan–Meier curve), where the X-axis represents the time in days while the Y-axis shows the percentage of cases still shedding the virus (median = 9 days ± 1.307). |
Discussion
The results of this study indicated that implementing PCR screening for HCWs that reside in quarantined areas at the end of the quarantine period through the universal testing strategy can help detect infected HCWs. These HCWs may be nosocomial carriers and potential sources of COVID-19 outbreaks (6% in our study). Relying on symptoms and identifiable epidemiological links triaged only 33.3% of the positive testing HCWs coming from quarantine sites; these HCWs were mostly asymptomatic, increasing the risk of transmission within the healthcare facility. Thus, this suggests the need of carrying out larger-scale studies that consider implementing a policy for universal SARS-Cov2 screening tests for HCWs post quarantine (or lockdown) periods. Healthcare systems must address the potential of HCWs as sources of COVID-19 nosocomial infections and limit their possible unprotected exposure to the infection within the community and at work. Further, this must be supplemented by determining the need for work restrictions, self-quarantine and testing upon indication. A follow-up study identified high a transmission of COVID-19 in HCWs in the early stages of the outbreak due to inadequate responses and preventive measures,15 highlighting the importance of policy implementation by the governments and healthcare systems to minimise these risks. Despite the predominance of asymptomatic HCW cases, a conservative approach is suggested when allowing the HCWs to resume duties because of the rarity of false-positive PCR results, except in a few conditions where carry-over contamination is suspected or specific diagnostic platforms are used.16 Continuous viral shedding supports the true positivity of those cases. The present study found that 88.9% of the HCWs cleared the infection in 10 days (Figure 2). This finding is consistent with a study by Liu et al, stating that 90% of 76 patients in China were clear of the virus by day 10, with a longer duration of viral shedding in more severe cases.17 In contrast, a longer duration of shedding (>3 weeks) has been reported in 56 mild cases of SARS-CoV-2 infections by Xiao et al.18 There are no studies that have specifically evaluated the shedding of SARS-CoV-2 in infected HCWs and its potential to increase patients’ risks. Of note, the analytical sensitivity of the assays and lower limit of detection may influence the reported duration of viral shedding, which must be considered. Assays with modest analytical sensitivity, such as the 1.00E-01 assay used in this study, are likely to result in a shorter duration of viral shedding being reported. Importantly, viral cell cultures were not performed in most of the studies examining the duration of SARS-CoV-2 shedding. The currently available evidence suggests the non-viability of the virus detected by nucleic acid assays during the late shedding of the virus.19 However, this was concluded from small studies of symptomatic cases and it is still unknown whether it applies to asymptomatic cases, particularly in HCWs that may transmit the infection to highly vulnerable patient populations. Viable SARS-CoV-2 samples have also been cultured for up to 4 weeks from non-respiratory samples such as stools from diarrheal cases, a symptom encountered in HCWs screened in the presented study.20 The prevalence of COVID-19 infection is higher in HCW than in the general population which indicates lower threshold for laboratory testing in front-line HCW who can be a source of nosocomial infections (32,575,505, 32,369,541).
A detailed description of the cohort indicated that the highest rate of infection of COVID-19 was among the trainees (8.2%) followed by nurses (7.1%) and those in administrative jobs (9.7%). As described in the results, a higher percentage of positive-testing HCWs had the risk of exposure as they worked in other healthcare facilities with COVID-19 patients inside the quarantine area compared to negative-testing patients. According to the Center for Disease Control and Prevention (CDC) report, HCWs at the highest risk of infection are those providing direct patient care for COVID-19 patients, such as conducting physical examinations, giving nursing care, generating procedures, performing aerosols, carrying out a specific collection and radiologic testing.15 The report by CDC confirms the current study findings that trainees, nurses and HCWs with administrative jobs are at a high risk of infection. Lai et al conducted a single-centre case series that involved a sample of 9,684 HCWs, of which 110 were identified to have contracted the infection. In the study, the highest proportion of HCWs infected with COVID-19 were those who were in close contact with patients, especially the nurses who were younger than 45 years of age.21 Similarly, a letter to the editor published by Wang et al identified that HWC workers with direct contact with infected patients were at the highest risk of infection.22 However, our findings suggest that although a large proportion of positive-testing HCWs have no clear exposure identified, they can be potential sources of infection in hospital settings in which direct exposure to patients is encountered. This highlights the importance of compliance with the fundamental preventive measures of infection for various categories of healthcare jobs through education and auditing. Although not statistically significant, the proportion of HCW with diabetes mellitus was more in infected group (5.6 vs. % 1.7%). Because COVID-19 infection in diabetic patients is more likely to have serious complications, intensive care admissions, longer length of stay, and more mortality, HCW with diabetes need to be given special attention (32,233,013, 32,890,227). (32,445,595). On the other hand, the proportion of the sickle cell disease SCD was notably high in both groups and more in the negative arm (26.9% vs 11.1%), as this study was conducted on HCW quarantined in an area with high endemicity of inherited hematological disorders (16,868,367). This suggests that SCD may not be a risk factor for acquisition of COVID-19 infection although there are no population-based studies to date addressing this association, but low morbidity and mortality with COVID‐19 in SCD patients have been reported (https://onlinelibrary.wiley.com/doi/10.1002/jha2.87). Nevertheless, COVID-19 infection can mimick acute chest syndrome in case of SCD which can delay the diagnosis (32,267,016). The possible complications of COVID-19 infection and adverse reactions of therapeutics in this patient group should not be ignored. Further studies are needed to address the optimal care in those cases based on the pathological and pharmacological challenges (Br J Haematol. 2020;190(2):e86–e89. doi:10.1111/bjh.16880).
Furthermore, this study found that the majority (66.7%) of the PCR-positive cases were asymptomatic, with only 33.3% of the confirmed cases showing symptoms linked to COVID-19. The findings of asymptomatic cases are higher than those in previously published reports; Kim et al reported that of the 213 participants that tested positive for SARS-Cov2, 41 (19.2%) were asymptomatic.23 This can be attributed to the universal testing strategy adopted in this study, which is likely to detect more asymptomatic infections than risk-based algorithms. The proportion of asymptomatic infections has not been systematically evaluated yet. An estimated proportion of 40% was suggested by a narrative review of three large-scale population-based studies, while variable proportions (30–90%) were reported in other groups.24 Furthermore, no longitudinal follow-up studies were performed on asymptomatic cases to investigate for any symptom development. Our cohort highlighted that the majority (66.7%) of those HCWs that initially tested asymptomatically positive did not develop any symptoms until they cleared the infection.
For symptomatic cases, the most common symptoms identified included shortness of breath (11.2%), gastrointestinal symptoms (33.3%), sore throat (5.6%), loss of taste and smell (5.6%) and coryza during the pre-testing phase. Headache was identified in one patient during the post-testing phase. These symptoms are also identified in previous studies where the most prevalent SARS-CoV-2 symptoms included fever, dry cough, tiredness, sore throat, diarrhoea, headache and loss of taste and smell. The results of the current study are almost identical to the previous studies conducted on SARS-CoV-2.25–28 Most of the symptoms encountered in the current study are similar to the previously published studies on symptoms of SARS-CoV-2.25–28 Of note, gastrointestinal symptoms were the most frequent manifestation of symptomatic Covid-19 cases in this small cohort, where 4/18 (22.2%) presented only with diarrhea. This was not reported in other reports where gastrointestinal symptoms were infrequently seen (31,986,264, 32,302,078). A systematic review of studies reporting the gastrointestinal symptoms in COVID-19 patients has elucidated a pooled prevalence of 17.6%, of which diarrhea was the most common (12.5%) (32,251,668). There was no clear explanation for the frequent reporting of diarrhea in our study but it suggests that new-onset gastrointestinal symptoms alone in HCW should not be ignored as they can be significant and may indicate SARS-CoV-2 testing irrespective of absence of other symptoms. To the best of our knowledge, this is the first study to address the role of universal PCR testing within a geographical quarantine setting and longitudinally assess asymptomatic, infected HCWs for the subsequent development of symptoms.
Limitations
The study had several limitations. First, the study had a single-centre focus and did not present data from a wide sample to create outcomes that were generalisable to HCW populations. Future research should consider the multi-centre evaluation of a larger sample of HCWs. The large female to male ratio in this cohort could have underestimated the post-quarantine prevalence of Covid-19 since male gender is a recognized independent risk factor of acquiring the SARS-CoV-2 infection as shown in a multivariate analysis study (32,422,197). Only less than 50% of the negative-testing HCWs responded to the questionnaire related to the comparative data needed. However, all positively tested groups were interviewed, and their data were obtained. Another possible limitation may be the recall bias since the questionnaire for non-infected HCWs was sent after the end of the incubation period.
Conclusions
HCWs re-joining their work and residing in quarantined areas are still at risk of acquiring the SARS-CoV-2 infection, despite reporting very minimal chances of exposure. Based on the presented data, we suggest adopting routine COVID-19 testing for post-quarantine HCWs upon resuming clinical duties whenever resources permit, as the majority of the HCWs were found to be asymptomatic, carrying the risk of silent transmission. Emphasis on compliance to appropriate infection preventive measures, including universal masks and hand hygiene, must be continuously addressed within healthcare facilities to minimise patients’ risks until further evidence is available to clarify the clinical significance of asymptomatic shedding in HCWs.
Data Sharing Statement
The raw data of this study are available from the corresponding author upon justifiable request.
Ethical Considerations
The study followed the ethical standards of the institutional and national research committees and the Helsinki Declaration. Ethical approval for this study was obtained from the institutional review board of Imam Abdulrahman Bin Faisal University. Due to the pandemic restrictions, interviewed for data collection was done over the phone where participants were consented and approved participation in the study. Verbal informed consent was obtained and that’s was approved by the local IRB. This study is presented following the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE). This research was conducted under Biosafety Level 3 conditions.
Acknowledgments
The authors thank the routine microbiology laboratory staff at KFHU and the Ministry of Health for supporting this study.
Author Contributions
All authors contributed significantly to the conception, conduction, analysis and writing of this article. The corresponding author attests that all listed authors meet the authorship criteria and that no others meeting the criteria have been omitted. All authors approved the final revised version of the manuscript and agreed on the journal to which it was submitted and agreed to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this study.
References
1. Coronavirus disease (COVID-19) pandemic. World Health Organization (WHO). Available from: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200316-sitrep-56-covid-19.pdf?sfvrsn=9fda7db2_6.
2. Nishiura H, Kobayashi T, Miyama T, et al. Estimation of the asymptomatic ratio of novel coronavirus infections (COVID-19). Int J Infect Dis. 2020;94:154–155. doi:10.1016/j.ijid.2020.03.020
3. Chan JF‐W, Yuan S, Kok K‐H, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395(10223):514–523.
4. Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382(13):1199–1207. doi:10.1056/NEJMoa2001316
5. Rothe C, Schunk M, Sothmann P, et al. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N Engl J Med. 2020;382(10):970. doi:10.1056/NEJMc2001468
6. Manuell ME, Cukor J. Mother nature versus human nature: public compliance with evacuation and quarantine. Disasters. 2011;35:417–442.
7. Barbisch D, Koenig KL, Shih FY. Is there a case for quarantine? Perspectives from SARS to Ebola. Disaster Med Public Health Prep. 2015;9(5):547–553. doi:10.1017/dmp.2015.38
8. Centers for Disease Control and Prevention. Quarantine and isolation. 2017. Available from: https://www.cdc.gov/quarantine/index.html.
9. Pan A, Liu L, Wang C, et al. Association of public health interventions with the epidemiology of the COVID-19 outbreak in Wuhan, China. JAMA. 2020;10. doi:10.1001/jama.2020.6130
10. Sandford A. Euronewswith AP. AFP. 2020. Available from: https://www.euronews.com/2020/04/02/coronavirus-in-europe-spain-s-death-toll-hits-10-000-after-record-950-new-deaths-in-24-hou.
11. Ministry of Health of Saudia Arabia. Ministry of health dashboard for COVID-19. 2020. Available from: https://covid19.moh.gov.sa.
12. Baggett TP, Keyes H, Sporn N, et al. Prevalence of SARS-CoV-2 infection in residents of a large homeless shelter in Boston. JAMA. 2020;27. doi:10.1001/jama.2020.6887
13. Treibel TA, Manisty C, Burton M, et al. COVID-19: PCR screening of asymptomatic health-care workers at London hospital. Lancet. 2020;395(10237):1608–1610. doi:10.1016/S0140-6736(20)31100-4
14. World Health Organisation (WHO). Laboratory testing for 2019 novel coronavirus (2019-nCoV) in suspected human cases. 2020. Available from: https://www.who.int/publications/i/item/laboratory-testing-for-2019-novel-coronavirus-in-suspected-human-cases-20200117.
15. Centers for Disease Control and Prevention. Coronavirus Disease 2019 (COVID-19). 2020. Available from: https://www.cdc.gov/coronavirus/2019-ncov/hcp/non-us-settings/public-health-management-hcw-exposed.html.
16. U.S. Food and Drug Administration (FDA) letter to Health care providers 07/06/2020: False positive results with BD SARS-CoV-2 reagents for the BD max system - Letter to clinical laboratory staff and health care providers. Available from: https://www.fda.gov/medical-devices/letters-health-care-providers/false-positive-results-bd-sars-cov-2-reagents-bd-max-system-letter-clinical-laboratory-staff-and.
17. Liu Y, Yan LM, Wan L, et al. Viral dynamics in mild and severe cases of COVID-19. Lancet Infect Dis. 2020;20(6):656–657. doi:10.1016/S1473-3099(20)30232-2
18. Xiao AT, Tong YX, Zhang S. Profile of RT-PCR for SARS-CoV-2: a preliminary study from 56 COVID-19 patients. Clin Infect Dis. 2020;ciaa460. doi:10.1093/cid/ciaa460
19. Wölfel R, Corman VM, Guggemos W, et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581(7809):465–469. doi:10.1038/s41586-020-2196-x
20. Xiao F, Sun J, Xu Y, et al. Infectious SARS-CoV-2 in feces of patient with severe COVID-19. Emerg Infect Dis. 2020;26(8):1920–1922. doi:10.3201/eid2608.200681
21. Lai X, Wang M, Qin C, et al. Coronavirus disease 2019 (COVID-2019) infection among health care workers and implications for prevention measures in a tertiary hospital in Wuhan, China. JAMA Netw Open. 2020;3(5):e209666. doi:10.1001/jamanetworkopen.2020.9666
22. Wang J, Zhou M, Liu F. Reasons for healthcare workers becoming infected with novel coronavirus disease 2019 (COVID-19) in China. J Hosp Infect. 2020;105(1):100–101. doi:10.1016/j.jhin.2020.03.002
23. Oran DP, Topol EJ. Prevalence of asymptomatic SARS-CoV-2 infection: a narrative review. Ann Intern Med. 2020;M20–3012. doi:10.7326/M20-3012
24. Kim G, Kim M, Ra S, et al. Clinical characteristics of asymptomatic and symptomatic patients with mild COVID-19. Clin Microbiol Infect. 2020;26(7):
25. Tabata S, Imai K, Kawano S, et al. Clinical characteristics of COVID-19 in 104 people with SARS-CoV-2 infection on the diamond princess cruise ship: a retrospective analysis. Lancet Infect Dis. 2020;20(9):1043–1050. doi:10.1016/S1473-3099(20)30482-5
26. Jin J, Bai P, He W, et al. Gender differences in patients with COVID-19: focus on severity and mortality. Front Public Health. 2020;8:152.
27. Adhikari S, Meng S, Wu Y, et al. Epidemiology, causes, clinical manifestation and diagnosis, prevention and control of coronavirus disease (COVID-19) during the early outbreak period: a scoping review. Infect Dis Poverty. 2020;9(1). doi:10.1186/s40249-020-00646-x
28. Fu L, Wang B, Yuan T, et al. Clinical characteristics of coronavirus disease 2019 (COVID-19) in China: a systematic review and meta-analysis. J Infect. 2020;80(6):656–665. doi:10.1016/j.jinf.2020.03.041
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
