Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 11
Prevalence of the metabolic syndrome according to different criteria in the male population during the Blue November Campaign in Natal, RN, Northeastern Brazil
Authors Espósito RC , Medeiros PJ , Silva FS, Oliveira AG , Soares Aragão CF , Oliveira Rocha HA , Moreira SA, Farias Sales VS
Received 22 March 2018
Accepted for publication 16 May 2018
Published 6 August 2018 Volume 2018:11 Pages 401—408
DOI https://doi.org/10.2147/DMSO.S168430
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ming-Hui Zou
Regina Carmen Espósito,1 Paulo Jose de Medeiros,2 Fernando de Souza Silva,3 Antonio Gouveia Oliveira,4 Cícero Flávio Soares Aragão,4 Hugo Alexandre Oliveira Rocha,5 Sueli Aparecida Moreira,6 Valéria Soraya de Farias Sales1
1Department of Clinical and Toxicology Analysis, Clinical Immunology Laboratory, Postgraduate Program in Development in Innovation Technogical in Medicines, Federal University of Rio Grande do Norte, Natal, Rio Grande do Norte, Brazil; 2Division of Urology, Department of Integrated Medicine, Federal University of Rio Grande do Norte, Natal, Rio Grande do Norte, Brazil; 3Urology Clinic University of Hospital Onofre Lopes, Federal University of Rio Grande do Norte, Natal, RN, Brazil; 4Department of Pharmacy, Federal University of Rio Grande of Norte, Natal, Rio Grande do Norte, Brazil; 5Department of Biochemistry, Biosciences Center, Federal University of Rio Grande do Norte, Natal, Rio Grande do Norte, Brazil; 6Hideas Feeding and Nutritional Security Research Group, Biosciences Center, Federal University of Rio Grande do Norte, Natal, RN, Brazil
Background: Metabolic syndrome (MetS) is an aggregation of risk factors associated with increased incidence of cardiovascular disease, type 2 diabetes mellitus, and all-cause mortality. Information on MetS prevalence is scarce in the northeast region, Brazil. This study aims to estimate the prevalence of MetS according to different diagnostic criteria in a community sample of men during the November Blue Campaign living in the metropolitan area of Natal, Rio Grande do Norte, Brazil.
Methods: This is a cross-sectional study on 500 men aged 40 years or older invited by the Blue November Campaign of 2015, an awareness program aimed at the prevention of male diseases. The evaluation included blood pressure, anthropometric measurements (weight, height, and waist circumference), fasting blood glucose, and blood lipid profile. The diagnosis of MetS was made according to the criteria of International Diabetes Federation (IDF)/American Heart Association (AHA)/National Heart, Lung, and Blood Institute (NHLBI), IDF, and National Cholesterol Education Program’s Adult Treatment Panel III (NCEP-ATPIII).
Results: The prevalence was high by considering the following three criteria: IDF/AHA/NHLBI (66.8%), IDF (60.0%), and NCEP-ATPIII (46.4%). Concordance between diagnostic criteria measured by the kappa statistic (k) was excellent between IDF/AHA/NHLBI and IDF (k=0.85, P<0.0001) and moderate between IDF/AHA/NHLBI and NCEP-ATPIII (k=0.59) and IDF and NCEP-ATPIII (k=0.54).
Conclusion: Prevalence of MetS in the male population was high using the three diagnostic criteria. IDF/AHA/NHLBI and IDF criteria have a high level of agreement, but NCEP-ATPIII criteria identify a lower number of MetS cases.
Keywords: metabolic syndrome, preventive medicine, male population
Introduction
The metabolic syndrome (MetS) is a complex and worldwide epidemic disorder with high socioeconomic impact due to its association with increased morbidity and mortality.1 The main causes are increasing urbanization, nutrition transition, and reduced physical activity.1 MetS consists of a cluster of several metabolic and physiological abnormalities, including abdominal obesity, impaired glucose metabolism, hypertriglyceridemia, decreased high-density lipoprotein cholesterol (HDL-C), and arterial hypertension. It is believed that this syndrome increases the risk of type 2 diabetes mellitus (T2DM) and cardiovascular diseases (CVDs).2,3 People with MetS are estimated to have twice the risk of developing CVD compared to healthy individuals and a fivefold increased risk of T2DM.4
The Framingham Offspring Study demonstrated that the attributable risk in the population for diabetes associated with MetS was 62% in men and 47% in women with the modified National Cholesterol Education Program (NCEP) criteria.5 The MetS’s pathogenesis remains unclear, as does the establishment of diagnostic criteria. It has been demonstrated clearly that the syndrome is common and that its prevalence is rising worldwide, which is largely a consequence of increasing obesity and sedentary lifestyles. As a result, the MetS is now both a public health issue and a clinical problem.3 The MetS has an estimated prevalence of 20%–25% in the general population. Its prevalence is greater for older people, reaching 42% in individuals older than 60 years.6
One in approximately every four or five adults develops MetS, depending on the environmental conditions and daily lifestyle habits of the country where he/she resides. The prevalence of this syndrome has been estimated to increase with age for individuals older than 50 years,7 the use of new eating patterns,8 changes in the work environment,9 the number of meals during the day,10 and the duration of sleep11–14 and in low-income people15 and smokers.15 Meanwhile, the prevention and treatment of MetS are based on changes in lifestyle through a multifactor approach based on education, regular physical exercise, and a healthy diet, as well as pharmacological strategies.16
In Brazil, the Ministry of Health’s Comprehensive Male Health Care Policy (PNAISH) promotes the improvement of the health conditions of the male population from 25 to 59 years, a preponderant part of the productive force, contributing to the reduction in morbidity and mortality in this population through rational coping of risk factors and facilitating access, actions, and comprehensive health care services.17 The Blue November Campaign is a part of these actions and includes a set of awareness strategies on the importance of prevention and early diagnosis of diseases that affect the male population, especially the most prevalent.18
Considering that MetS represents a higher risk for T2DM and CVD, combined with the scarcity of data in Brazil, this study aims at determining the prevalence of MetS in men living at the metropolitan area of Natal, Rio Grande do Norte, northeast region of Brazil. The community-based sample was taken on the Blue November Campaign of 2015 using the criteria defined by consensus among the International Diabetes Federation (IDF)/American Heart Association (AHA)/National Heart, Lung, and Blood Institute (NHLBI). The prevalence of MetS according to IDF/AHA/NHLBI was compared with the criteria set forth by the IDF and NCEP’s Adult Treatment Panel III (NCEP-ATPIII).
Methods
Study population
The research was carried out according to the Norms and Ethical Guidelines of the National Health Council Resolution 510/2016 of the Brazilian Ministry of Health and approved by the Research Ethics Committee of the University Hospital Onofre Lopes, Federal University of Rio Grande do Norte, in 2014. The study was a cross-sectional survey based on an urban community sample of men 40 years or older in Natal, Rio Grande do Norte, with the Certificate of Presentation for Ethical Appreciation (CAAE) 25997913.1.0000.5292. The participants were invited between November 2015 and April 2016 through television advertisements, seeking volunteers to take part in the Blue November Campaign of 2015, which is the prevention of male diseases’ awareness program, promoted by the University Hospital Onofre Lopes.
A total of 500 men who agreed to participate in the study signed the informed consent form. Then, they were included in the survey, all were submitted to anamnesis, clinical, and laboratorial evaluation, and data were collected on the individual components of the MetS (blood pressure, waist circumference [WC], fasting glucose [FG], HDL-C, triglycerides [TG], and medication history). Also, we measured total cholesterol, low-density lipoprotein cholesterol (LDL-C) and body mass index (BMI).
Measurements
The blood pressure was measured with a mercury sphygmomanometer applied to the left arm, with the subject in the sitting position. The BMI was calculated by dividing the subject’s weight in kilograms by the square of height in meters (kg/m2) using an automatic anthropometric scale (Filizola, São Paulo, Brazil) calibrated by INMETRO. WC was measured using an inelastic measuring tape at the midpoint between the iliac crest and the lowest rib, with the patient in the standing position.
Blood samples were drawn from overnight fasting and using automated methods and commercially available assays at the Laboratory of Clinical Analysis of the University Hospital Onofre Lopes. We measured FG by the enzymatic test kit AA and lipid profile (including TG by the enzymatic test kit GOP/PAP AA, total cholesterol by the enzymatic test kit AA, and HDL-C by colorimetric test kit monofase AA plus) with an automated CMD 800 iX1 equipment (Wiener Lab, Rosario, Argentina).
The diagnosis of MetS was established according to the IDF/AHA/NHLBI criteria and compared with the IDF and NCEP-ATPIII criteria. The cutoff used for WC in IDF/AHA/NHLBI and IDF criteria was that of European ethnicity, according to Table 1.
Statistical analysis
Prevalence rates are presented with binomial 95% CIs. The kappa statistic was used to analyze concordance between diagnostic criteria. The Chi-square test was used to compare proportions. Significance level was set at P<0.05. Stata 11 (Stata Corp., College Station, TX, USA) was used for statistical calculations.
Results
The present study assessed, on a community-based sample of the male population living in the metropolitan area of Natal, the prevalence of MetS and the agreement between diagnostic criteria, according to the latest criteria, IDF/AHA/NHLBI, compared with the IDF and NCEP-ATP III criteria. During a 6-month period, 500 men aged 43–83 years who participated in a voluntary health examination were assessed. The clinical and demographic characteristics of the study population are presented in Table 2, which showed a mean age of 57.3 years, the WC of 100.4 cm, the serum TG of 189.5 mg/dL, and the FG of 123.2 mg/dL.
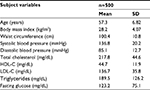  | Table 2 Characteristics of the study population in Natal, Rio Grande do Norte, at 2015 Abbreviations: HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol. |
The prevalence rates among age groups (40–49, 50–59, 60–69, and ≥70 years) according to the three diagnostic criteria did not find a statistically significant difference, as shown in figure 1. The group of 60–69 years presented the highest prevalence in all criteria, such as IDF/AHA/NHLBI, IDF, and NCEP-ATPIII.
The percentage prevalence of MetS found for the three criteria studied is shown in Table 3. In this study, it was higher using the IDF/AHA/NHLBI criteria (66.8%) followed by the IDF criteria and the NCEP-ATPIII criteria. Concordance analysis of the three diagnostic criteria used for the definition of MetS was analyzed by determining the kappa statistic. The concordance showed excellent agreement between IDF/AHA/NHLBI and IDF criteria (kappa statistic 0.85, P<0.0001) but a moderate agreement between either of those criteria and NCEP-ATPIII criteria (kappa statistic 0.59 and 0.54, respectively).
Discussion
MetS and age
The literature shows great variability in the prevalence of MetS among the elderly, which depends greatly on the criteria used for the diagnosis and also the ethnic and regional characteristics of the study population.19 Park et al,15 in the United States, showed that the prevalence of MetS increases with advancing age, peak at the sixth decade of life for men and the eighth decade of life for women. The emerging prevalence data suggest that MetS is quite prevalent especially among older people. This effect can be explained largely by age-related rises in blood pressure and glucose.20
In Brazil, Nakazone et al,21 in a follow-up of 200 gender-matched cardiology patients aged 31–50 years in Ribeirão Preto, São Paulo, Brazil, showed the prevalence of MetS as 35.5% by the NCEP-ATPIII criteria and 46% by the IDF criteria. Rigo et al,22 in a study at Novo Hamburgo, Rio Grande do Sul, Brazil, with 378 elderly individuals aged >60 years, estimated the prevalence of MetS as 56.9, 53.4, and 50.3%, by the IDF, NCEP-ATPIII reviewed by AHA/NHLBI, and NCEP-ATPIII criteria, respectively. In a study, conducted by Saad et al,19 of elderly population treated at the Outpatient Clinic of Internal Medicine and Geriatrics of Fundação Municipal de Saúde de Niterói, Rio de Janeiro, Brazil, the prevalence of MetS in males was 69.8, 60.3, 58.7, and 44.4% according to the Joint Interim Statement (JIS), IDF, World Health Organization (WHO), and NCEP-ATPIII criteria, respectively.
Kelliny et al23 surveyed a sample representative of the general population aged 25–64 years in Seychelles, Africa, and found the prevalence of MetS in men to be 33.5% by the IDF criteria, 33.4% by the WHO criteria, and 31.9% by the NCEP-ATPIII criteria.
MetS and criteria
MetS is a widely prevalent and multifactorial disorder and has been given several names such as insulin resistance (IR) syndrome, plurimetabolic syndrome, Reaven’s syndrome, syndrome X, and the deadly quartet.7 There are various MetS definitions. The first formal definition of the MetS was proposed in 1998, by a consultation group for the definition of diabetes on behalf of the WHO.3 A different criterion was proposed by the NCEP-ATPIII in 2001 establishing that, for the diagnosis of MetS, an individual must have at least three of the following five criteria: central obesity measured by WC (>102 cm), hypertriglyceridemia, low HDL-C, hypertension (≥130/85 mmHg), and fasting hyperglycemia (≥110 mg/dL).16
In 2005, the IDF produced another set of criteria for use both in epidemiology and in clinical practice worldwide, with the aims of identifying people with MetS, to clarify the nature of the syndrome and to focus on therapeutic strategies to reduce the long-term risk of CVD. The IDF established that, for the diagnosis of MetS, an individual must have central obesity, measured by the WC, with ethnicity-specific values as one required component and two other components (hypertriglyceridemia or low HDL-C or hypertension or fasting hyperglycemia) (≥100 mg/dL).24
In 2009, both the IDF and AHA/NHLBI agreed that abdominal obesity should not be a prerequisite for diagnosis but that it should be one of the five criteria, so that the presence of any three of the following five risk factors constitutes a diagnosis of MetS: increased WC with ethnicity-specific reference values (eg, WC men ≥94 cm and women ≥80 cm in Caucasians), elevated TG (≥150 mg/dL or 1.7 mmol/L) or drug treatment for elevated TG, decreased HDL-C (<40 mg/dL or 1.03 mmol/L in males; <50 mg/dL or 1.29 mmol/L in females) or drug treatment for reduced HDL-C, elevated FG (≥100 mg/dL or 5.6 mmol/L) or drug treatment for elevated glucose, and elevated blood pressure (≥130/85 mm Hg) or use of antihypertensive drugs in a patient with a history of hypertension.3 In this study, the higher prevalence by the IDF/AHA/NHLBI and IDF criteria was probably due to the lower cutoff of WC and fasting glycemia established by them.3
In the United States, Ford et al26 showed the prevalence of MetS in participants of the National Health and Nutrition Examination Survey, according to the JIS definition, to be higher with the JIS-94 definition (60.1%) than with the JIS-102 definition (59.3%) in men aged between 60 and 69 years. Alkerwi et al25 found a prevalence of MetS in Luxembourg, according to the JIS definition, estimated from the ORISCAV-LUX study, also higher with the JIS-94 definition (68.3%) than with the JIS-102 definition (60.4%) in men aged between 60 and 69 years.The use of lower cutoff points of WCs is beneficial, as it raises the risk level for cardiometabolic disease among those identified as having the MetS27 and then indicates the need for early cardiovascular risk reduction. This approach implies a large impact on preventive strategies and health care resources.
The different prevalence values for MetS found in these studies indicate the need to strongly consider the population ethnic aspects and regional habits. The findings demonstrate the difficulty in having a diagnostic criterion that is accurate, sensitive, and specific and that can be useful for assessing the general population, overcoming the limitations of regional specificities. Oliveira et al28 showed the prevalence of MetS in the population with rheumatoid arthritis treated at the Rheumatology Outpatient Clinic of Hospital Universitário Walter Cantídio of Universidade Federal do Ceará, Ceará, Brazil, to be 53.4% by the IDF criteria and 50% by the NCEP-ATPIII criteria. Xi et al29 used data from the China Health and Nutrition Surveys in 2009 to describe the prevalence of MetS in China and showed that the prevalence in men according to revised NCEP-ATPIII criteria for Asians was 20.9%, according to IDF criteria was 16.2%, and according to the Chinese Diabetes Society criteria was 12.2%.
MetS in Brazil
Similar to what has been found in Western countries, the prevalence of MetS is rapidly increasing in developing countries. In Brazil, studies estimating the prevalence of MetS for urban regions in the southeast reported 29.8% in 200730 and 25.4% in 200831 by the NCEP-ATPIII criteria, 35.5 and 46.0% in 200721 by the NCEP-ATPIII and IDF criteria respectively, 34.0% in 201132 by IDF criteria, 35.9 and 43.2% in 201133 by the NCEP-ATPIII and IDF criteria, respectively, and 45.2, 64.1, 69.1, and 51.9% in 201419 by the NCEP-ATPIII, IDF, Joint Interim Statement (JIS), and WHO criteria, respectively. According to the Brazilian region, the prevalences have been estimated as follows: in the Northeast, 50% by the NCEP-ATPIII criteria and 53.4% by the IDF criteria in 2016;28 in the South, 50.3% by the ATPIII criteria, 53.4% by ATPIII reviewed by the AHA/NHLBI criteria, and 56.9% by the IDF criteria in 2009;22 and in the Midwest region, 32.0% by the harmonized MetS criteria3 in 2012.34 In a rural population of southeastern Brazil, the MetS was found in 21.6% in 200735 and 14.9% in 201136 by the NCEP-ATPIII criteria, and in the northeastern region, the MetS was found in 24.8% by the NCEP-ATPIII criteria in 2006.37 Among the indigenous population in the north, the MetS was found 34% by the NCEP-ATPIII and 43% by the IDF criteria in 2009,38 and in the midwest region, the MetS was found 66.1% by the IDF/AHA/NHLBI criteria in 2015.39
MetS and lifestyle
Kesse-Guyot et al8 showed that the increased prevalence of MetS worldwide had been attributed to changes in lifestyle, particularly regarding new eating patterns and sedentarism. The study by Straif et al9 found the growth in MetS prevalence due to the working hours that once occurred during daytime being extended in the last decades for a large number of services and production areas. Knutson et al10 reported that meals more often breakfast, lunch, and dinner and snacks in the intervals lead to better appetite control, greater effect of postprandial thermogenesis, largest mobilization of lipids due to repeated stimulation of the sympathetic nervous system, lower elevation in plasma glucose, and less variation in insulin levels and C-peptide, therefore maintaining the control of MetS. Wu et al11 demonstrated that reduced sleep time (<6 h/day) was positively associated with MetS, while Gangwisch et al,12 Meier-Ewert et al,13 and Stamatakis and Punjabi14 observed that decreased sleep duration has been linked to several metabolic disorders, such as glucose intolerance, IR, dyslipidemia, hypertension, and systemic inflammatory processes. Park et al15 showed that the prevalence is higher in low-income people (mainly women), smokers, and sedentary men. The scientific literature supports the beneficial effects of low-calorie diet associated with physical exercise, reinforcing the importance of changing lifestyle in the management of MetS.40
MetS and concordance analysis
Similar results of this study, about concordance analysis, were also observed in Brazil, where Saad et al19 found a very good concordance between IDF and JIS (k=0.89) and moderate concordance between IDF and NCEP-ATPIII (k=0.55), NCEP-ATPIII and JIS (k=0.53), WHO and NCEP-ATPIII (k=0.51), WHO and IDF (k=0.47), and WHO and JIS (k=0.45). Alkerwi et al25 in Luxembourg showed excellent concordance between JIS-94/80 and IDF (k=0.93), between JIS-94/80 and NCEP-ATPIII (k=0.91), and between IDF and NCEP-ATPIII (k=0.84) criteria, but in the United States, the agreement was k=0.92 between the NCEP-ATPIII and IDF criteria in postmenopausal women,26 and in 2008, Kelliny et al23 in Africa found excellent agreement between NCEP-ATPIII and IDF (k=0.82) and moderate agreement between WHO and IDF (k=0.61) and between WHO and NCEP-ATPIII (k=0.59).
The difference in concordance between the MetS diagnostic criteria in different populations is probably due to ethnic characteristics, dietary habits, and lifestyle, thus making it difficult to use a single diagnostic criterion for all populations. Substantial socioeconomic and demographic changes that have occurred in the Brazilian population over the past decades have been associated with a deterioration of the metabolic profile because of adverse changes in lifestyle habits. Although the MetS has been a feature of urban westernized lifestyle, risk factors for CVD are now emerging as a major public health problem in the urban population of developing countries. The main focus of treating MetS is managing the risk factors with lifestyle changes that include losing weight, following a heart-healthy diet, reducing one’s blood pressure, improving one’s cholesterol and blood sugar levels, getting more regular physical activity, and quitting smoking. Maintaining a healthy lifestyle is a lifelong commitment. Successfully controlling MetS takes a long-term effort and teamwork with health care providers. At the same time, early diagnosis, prevention, and management of MetS are considered the key approaches to reduce the risk of progression of atherosclerosis and the development of CVD.7
Conclusion
We observed that the prevalence of MetS in the male population was high using the three diagnostic criteria and that IDF/AHA/NHLBI and IDF criteria have a high level of agreement. However, NCEP-ATPIII criteria identify a lower number of MetS cases. It is essential to evaluate the occurrence of MetS and its associated factors in order to contribute to the elaboration of health promotion public policies that have a significant impact on the prevention and treatment of T2DM and CVD.
Limitations of this study can be explained in part by the sample selection criterion, which comprised all the demand served in the Blue November Campaign. The Campaign may contribute to explain the gaps in men’s health, addressing the risk factors of men aging in urban settings, poor eating habits, and sedentary lifestyle. While different regions may require further regional studies due to the characteristics of its populations to better assess their prevalence of MetS, main risk factors associated with men aging in urban settings are known and can be identified and intervened. The positive aspect was to note the importance of the Blue November Campaign as an opportunity to propose interventions for men’s health care.
Acknowledgment
This research received funding from the Federal University of Rio Grande do Norte/PROAD/PPg UFRN.
Disclosure
The authors report no conflicts of interest in this work.
References
Misra A, Khurana L. Obesity and the metabolic syndrome in developing countries. J Clin Endocrinol Metab. 2008;93(suppl 1):S9–S30. | ||
Corona G, Rastrelli G, Morelli A, Vignozzi L, Mannucci E, Maggi M. Hypogonadism and metabolic syndrome. J Endocrinol Invest. 2011;34(7):557–567. | ||
Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120(16):1640–1645. | ||
Grundy SM. Metabolic syndrome pandemic. Arterioscler Thromb Vasc Biol. 2008;28(4):629–636. | ||
Wilson PW, D’Agostino R Jr, Parise H, Sullivan L, Meigs JB. Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation. 2005;112(20):3066–3072. | ||
Santos HCM, Orange LG, Lima CR, Azevedo MMS, Dourado KF, Andrade SP. Metabolic syndrome and other risk factors for cardiovascular disease in an obese population. Rev Bras Cardiol. 2013;26(6):442–449. | ||
Gupta A, Gupta V. Metabolic syndrome: what are the risks for humans? Biosci Trends. 2010;4(5):204–212. | ||
Kesse-Guyot E, Ahluwalia N, Lassale C, Hercberg S, Fezeu L, Lairon D. Adherence to Mediterranean diet reduces the risk of metabolic syndrome: a 6-year prospective study. Nutr Metab Cardiovasc Dis. 2013;23(7):677–683. | ||
Straif K, Baan R, Grosse Y, et al. Carcinogenicity of shift-work, painting, and fire-fighting. Lancet Oncol. 2007;8(12):1065–1066. | ||
Knutson KL, Spiegel K, Penev P, Van Cauter E. The metabolic consequences of sleep deprivation. Sleep Med Rev. 2007;11(3):163–178. | ||
Wu MC, Yang YC, Wu JS, Wang RH, Lu FH, Chang CJ. Short sleep duration associated with a higher prevalence of metabolic syndrome in an apparently healthy population. Prev Med. 2012;55(5):305–309. | ||
Gangwisch JE, Heymsfield SB, Boden-Albala B, et al. Short sleep duration as a risk factor for hypertension: analyses of the first National Health and Nutrition Examination Survey. Hypertension. 2006;47(5):833–839. | ||
Meier-Ewert HK, Ridker PM, Rifai N, et al. Effect of sleep loss on C-reactive protein, an inflammatory marker of cardiovascular risk. J Am Coll Cardiol. 2004;43(4):678–683. | ||
Stamatakis KA, Punjabi NM. Effects of sleep fragmentation on glucose metabolism in normal subjects. Chest. 2010;137(1):95–101. | ||
Park YW, Zhu S, Palaniappan L, Heshka S, Carnethon MR, Heymsfield SB. The metabolic syndrome: prevalence and associated risk factor findings in the US population from the Third National Health and Nutrition Examination Survey, 1988–1994. Arch Intern Med. 2003;163(4):427–423. | ||
Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Expert panel on detection, evaluation, and treatment of high blood cholesterol in adults. Executive summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001;285(19):2486–2497. | ||
Brasil Ministério da Saúde. Política de Atenção Integral à Saúde do Homem. Brasília: Brasil Ministério da Saúde; 2008:40. | ||
Instituto Nacional do Câncer. Coordenação-Geral de Atenção às Pessoas com Doenças Crônicas. Posicionamento do Ministério da Saúde acerca da integralidade da saúde do homem no contexto do Novembro Azul. Rio de Janeiro: INCA; 2015. Nota técnica conjunta Nº001/2015. Available from: u.saude.gov.br/images/pdf/2015/novembro/09/Integralidade-sa--de-homens.pdf. Accessed March 02, 2018. | ||
Saad MA, Cardoso GP, Martins WA, Velarde LG, Cruz Filho RA. Prevalence of metabolic syndrome in elderly and qgreement among four diagnostic criteria. Arq Bras Cardiol. 2014;102(3):263–269. | ||
Alexander CM, Landsman PB, Grundy SM. The influence of age and body mass índex on the metabolic syndrome and its components. Diabetes Obes Metab. 2008;10(3):246–250. | ||
Nakazone MA, Pinheiro A, Braile MC, et al. Prevalence of metabolic syndrome using NCEP-ATPIII and IDF definitions in Brazilian individuals. Rev Assoc Med Bras. 2007;53(5):407–413. | ||
Rigo JC, Vieira JL, Dalacorte RR, Reichert CL. Prevalência de síndrome metabólica em idosos de uma comunidade: comparação entre três métodos diagnósticos. Arq Bras Cardiol. 2009;93(2):85–91. | ||
Kelliny C, William J, Riesen W, Paccaud F, Bovet P. Metabolic syndrome according to different definitions in a rapidly developing country of the African region. Cardiovasc Diabetol. 2008;7:27–37. | ||
Alberti KG, Zimmet P, Shaw J. Metabolic syndrome – a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med. 2006;23(5):460–480. | ||
Alkerwi A, Donneau AF, Sauvageot N, et al. Prevalence of the metabolic syndrome in Luxembourg according to the Joint Interim Statement definition estimated from the ORISCAV-LUX study. BMC Public Health. 2011;11(1):4. | ||
Ford ES, Li C, Zhao G. Prevalence and correlates of metabolic syndrome based on a harmonious definition among adults in the US. J Diabetes. 2010;2(3):180–193. | ||
Katzmarzyk PT, Janssen I, Ross R, Church TS, Blair SN. The importance of waist circumference in the definition of metabolic syndrome: prospective analyses of mortality in men. Diabetes Care. 2006;29(2):404–409. | ||
Oliveira BMGB, Medeiros MMC, Cerqueira JVM, Souza Quixadá RT, Oliveira IMAX. Síndrome metabólica em pacientes com diagnóstico de artrite reumatoide acompanhados em um Hospital Universitário do Nordeste brasileiro. Rev Bras Reumatol. 2016;56(2):117–125. | ||
Xi B, He D, Hu Y, Zhou D. Prevalence of metabolic syndrome and its influencing factors among the Chinese adults: the China Health and Nutrition Survey in 2009. Prev Med. 2013;57(6):867–871. | ||
Salaroli LB, Barbosa GC, Mill JG, Molina MC. Prevalência de síndrome metabólica em estudo de base populacional, Vitória, ES–Brasil. Arq Bras Endocrinol Metabol. 2007;51(7):1143–1152. | ||
Marquezine GF, Oliveira CM, Pereira AC, Krieger JE, Mill JG. Metabolic syndrome determinants in an urban population from Brazil: social class and gender-specific interaction. Int J Cardiol. 2008;129(2):259–265. | ||
Silva EC, Martins IS, de Araújo EA. Síndrome metabólica e baixa estatura em adultos da região metropolitana de São Paulo (SP, Brasil). Cien Saude Colet. 2011;16(2):663–668. | ||
Gronner MF, Bosi PL, Carvalho AM, et al. Prevalence of metabolic syndrome and its association with educational inequalities among Brazilian adults: a population-based study. Braz J Med Biol Res. 2011;44(7):713–719. | ||
Dutra ES, de Carvalho KM, Miyazaki E, Hamann EM, Ito MK. Metabolic syndrome in central Brazil: prevalence and correlates in the adult population. Diabetol Metab Syndr. 2012;4(1):20. | ||
Velásquez-Meléndez G, Gazzinelli A, Côrrea-Oliveira R, Pimenta AM, Kac G. Prevalence of metabolic syndrome in a rural area of Brazil. Sao Paulo Med J. 2007;125(3):155–162. | ||
Pimenta AM, Gazzinelli A, Velásquez-Meléndez G. Prevalência da síndrome metabólica e seus fatores associados em área rural de Minas Gerais (MG, Brasil). Cien Saude Colet. 2011;16(7):3297–3306. | ||
Oliveira EP, Souza ML, de Lima MD. Prevalência de síndrome metabólica em uma área rural do semi-árido baiano. Arq Bras Endocrinol Metabol. 2006;50(3):456–465. | ||
Medeiros CL. Síndrome metabólica em idosos quilombolas e não quilombolas no Estado do Amapá. [dissertação]. Brasília: Universidade Católica de Brasília; 2009. | ||
Soares LP, Fabbro AL, Silva AS, et al. Prevalence of metabolic syndrome in the Brazilian Xavante indigenous population. Diabetol Metab Syndr. 2015;7:105. | ||
Leão LS, de Moraes MM, de Carvalho GX, Koifman RJ. Nutritional interventions in metabolic syndrome: a systematic review. Arq Bras Cardiol. 2011;97(3):260–265. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



