Back to Journals » Journal of Asthma and Allergy » Volume 15
Prevalence of Patients with Uncontrolled Asthma Despite NVL/GINA Step 4/5 Treatment in Germany
Authors Bergmann KC, Skowasch D , Timmermann H, Lindner R, Virchow JC, Schmidt O, Koschel D, Neurohr C, Heck S, Milger K
Received 18 March 2022
Accepted for publication 9 June 2022
Published 4 July 2022 Volume 2022:15 Pages 897—906
DOI https://doi.org/10.2147/JAA.S365967
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Amrita Dosanjh
Karl-Christian Bergmann,1 Dirk Skowasch,2 Hartmut Timmermann,3 Robert Lindner,4 Johann Christian Virchow,5 Olaf Schmidt,6 Dirk Koschel,7 Claus Neurohr,8 Sebastian Heck,9 Katrin Milger10
1Institute for Allergology, Charité – Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin, Humboldt-Universität Zu Berlin, and Berlin Institute of Health, Berlin, Germany; 2Department of Internal Medicine II – Pneumology, University Hospital Bonn, Bonn, Germany; 3Schwerpunktpraxis Colonnaden, Hamburg, Germany; 4IQVIA Commercial GmbH & Co. OHG, Frankfurt Am Main, Germany; 5Universitätsmedizin Rostock - Zentrum für Innere Medizin, Medizinische Klinik I, Abteilung Pneumologie & Interdisziplinäre Internistische Intensivmedizin, Rostock, Germany; 6Pneumologische Gemeinschaftspraxis und Studienzentrum KPPK, Koblenz, Germany; 7Fachkrankenhaus Coswig, Lung Centre, Coswig, and Division of Pulmonology, Medical Department I, University Hospital Carl Gustav Carus, Dresden, Germany; 8Abteilung für Pneumologie und Beatmungsmedizin, Robert-Bosch-Krankenhaus Lungenzentrum, Stuttgart, Germany; 9GlaxoSmithKline GmbH & Co. KG, Munich, Germany; 10Department of Medicine V, University Hospital, Ludwig-Maximilians-University (LMU) Munich, Comprehensive Pneumology Center Munich (CPC-M), Member of the German Center for Lung Research (DZL), Munich, Germany
Correspondence: Karl-Christian Bergmann, Institute for Allergology-Charité, Luisenstraße 2, Berlin, 10117, Germany, Tel +491711934508, Fax +49 30 549090609, Email [email protected]
Purpose: Asthma is one of the most prevalent chronic diseases in Germany affecting 4– 5% of all adults and 10% of children. Despite the availability of biologicals in recent years, studies show patients with inadequately controlled severe asthma in real life. The aim of the current study was to characterize and estimate the number of patients with NVL/GINA level 4 or 5 asthma and signs of poor control in Germany.
Patients and Methods: In 2021, we retrospectively analyzed data collected during 2019 using the IQVIA™ LRx and IQVIA™ Disease Analyzer databases which contain anonymized longitudinal data covering approximately 80% of statutory health insurance (GKV) prescriptions in Germany with most relevant information about prescriptions, basic patient demographics or location of the prescriber; the IQVIA™ Disease Analyzer anonymized electronic medical records from a representative sample of office-based GPs and specialists. An expert committee of pulmonologists from different hospitals and expert practices supported the study. Asthma patients treated according to NVL/GINA 4/5 who used SABAs frequently (≥ 3 on days with no ICS-containing prescriptions/year) and/or received prescriptions for oral corticosteroids (OCS) (score of ≥ 2/year, a pulmonologist prescription scored 1.0, GP 0.75) were classified as severe, uncontrolled asthma.
Results: In 2019, 3.4 million patients received at least two prescriptions of respiratory medications and 2.4 million patients on maintenance respiratory treatment have asthma. A total of 625,000 asthma patients were treated according to NVL/GINA step 4 or 5. Among these, 54,000 were uncontrolled according to the pre-defined OCS and/or SABA use, which corresponds to approximately 15% of patients in certain regions.
Conclusion: In 2019, approximately 54,000 patients in Germany treated according to NVL/GINA step 4/5 had evidence suggestive for poor asthma control, up to 15% of patients in certain regions. Yet, only 12,000 patients overall were being treated with biologicals suggesting a possible treatment gap that requires further investigation.
Keywords: prescription database, disease analyzer, uncontrolled asthma, oral corticosteroid, OCS, short-acting β 2-agonist, SABA
Introduction
One of the most prevalent chronic diseases in Germany is asthma with 4–5% of all adults and more than 10% of children being affected.1 Although effective asthma therapies are available optimal asthma management is limited by non-compliance, budget constraints or a lack of confidence in the therapy.1 Asthma recommendations have changed over the years and are currently based on the level of asthma control rather than disease severity.2,3 Asthma control can be achieved in the majority of patients, but surveys repeatedly show that this is not the case in real life.2
“Severe Asthma” encompasses a group of patients who require treatment on steps 4–5 of GINA guidelines to prevent their asthma from becoming “uncontrolled”, or who remain “uncontrolled” despite this therapy.4 Treatment options for severe uncontrolled asthma have increased recently, especially with the introduction of novel biologic therapies.3,5 Most therapies for severe asthma target type-2 (T2) high asthma and include biologics approved for use in the United States and Europe including omalizumab, mepolizumab, benralizumab, reslizumab and dupilumab.5,6 In registries of severe asthma approximately 90% of patients are T2 high.7,8 In Germany, regardless of the introduction of biologicals for the treatment of severe asthma, a high number of patients are still inadequately controlled possibly due to suboptimal use of available therapies in a routine care setting, and therefore still rely on the use of oral corticosteroid (OCS) maintenance therapy,9 but current data are not available.
The aim of the current study, therefore, was to estimate the number of patients with treatment according to NVL/GINA step 4 or 5 in Germany and to define the characteristics of uncontrolled asthma in order to estimate the extent of patients who might benefit from intensified diagnostic and treatment with the hypothesis that there are still high numbers of patients with inadequately controlled severe asthma in the different regions of Germany.
Patients and Methods
Study Design and Materials
The study was performed retrospectively based on data collected from January to December 2019 and ended before the start of the COVID-19 pandemic to exclude possible effects of the pandemic. The study period was 1 year because a 1-year epidemiological analysis ensures that seasonal effects such as allergic contributions to asthma are captured. It also provides a broader picture of the treatment options in severe asthma with new product launches and changes in guidelines.
An expert committee of pulmonologists from different hospitals and expert practices supported the study and defined the patient cohorts, the methodology and the data analyses in cooperation with GlaxoSmithKline GmbH & Co KG.
Data Sources
IQVIA™ Longitudinal Prescription Data (LRx) was used as main data source for patient quantification. This longitudinal anonymized prescription database contains approximately 80% of the statutory health insurance (SHI/GKV (Gesetzliche Krankenversicherung)), under which ~90% of the German population are insured, prescriptions claimed in retail pharmacies. IQVIA™ LRx contains most relevant information from SHI prescriptions such as prescribed product, substance and pack (identified via Pharmazentralnummer PZN), prescription date and prescriber specialty. Basic patient demographics (age and gender) are included as well as the location of the prescriber on KV (Kassenärztliche Vereinigung, Association of Statutory Health Insurance Physicians, 17 regional KVs in Germany) -district level (63 districts in Germany).
The IQVIA™ Disease Analyzer, a database of anonymized electronic medical records from a representative sample of office-based GPs and specialists in Germany10 was used to establish a machine learning model and to validate different IQVIA™ LRx results.
Human Ethics Statement
The database used includes only anonymized data in compliance with the regulations of the applicable data protection laws. German law allows the use of anonymous electronic medical records for research purposes under certain conditions. According to this legislation, it is not necessary to obtain informed consent from patients or approval from a medical ethics committee for this type of observational study that contains no directly identifiable data.
Because patients were only queried as aggregates and no protected health information was available for queries, no Institutional Review Board approval was required for the use of this database or the completion of this study.
Machine Learning Model
The databases are not linked for data privacy reasons and the prescription data collected in LRx does not contain diagnosis. Therefore a gradient boosted tree model was trained on Disease Analyzer data that does contain diagnosis to recognize demographics and prescription histories of asthma patients and predict indication in LRx data to determine which patients on respiratory therapeutics actually suffer from asthma and not from other respiratory diseases (mostly COPD or chronic bronchitis). The purpose of the model was to determine which treatment courses in LRx most likely belong to asthmatics, and to exclude treatment courses from the analysis that were related to COPD or other non-asthma conditions.
Training sample: 165,979 Patients with at least one statutory health insurance prescription of the drug classes mentioned or listed below (referred to as core market) between 1 Jan 2019 and 31 Dec 2019. Drug classes defined by EPhMRA ATC class (anatomical classification of pharmacy products that groups substances into classes of similar action or use): R03A3 (LABA), R03B2 (Xanthines), R03D1 (pure ICS), R03F1 (ICS/LABA), R03J2 (LTRA), R03M0 (asthma-only biologics), + omalizumab and dupilumab. In all patients were 101,615 from 907 general practitioner (GPs), 46,168 patients from 26 pulmonologists, 18,196 patients from 184 pediatricians. The sample was reduced to 89,978 patients who had at least two prescriptions within one year, the latter being within 2019. Patients were assigned asthma or COPD based on the full history of ICD-10 codes (J45, J46 for asthma; J44 for COPD) that a patient has been diagnosed with in the doctor´s office (earliest diagnoses date back to 1992).
A weighting scheme was used to assign a unique indication if both diagnoses were present in a patient’s history: Diagnoses explicitly linked to a prescription in the core market (this can be done by the prescriber) had the highest weight, followed by diagnosis issued on the same day as a core market prescription. Other diagnoses weighted less, with further decreasing weights for historic diagnoses.
Asthma labels were assigned to 51,663 patients and 25,468 were assigned COPD. There were 12,847 patients with ambiguous diagnoses and were subsequently removed from the training sample. There were 8,736 patients who had no documented diagnosis and were also removed from the training sample.
The model was tested on a holdout sample of 24,428 patients that did not go into model training. Predicted diagnosis was compared with the actual label assigned by ICD codes as described above. Prediction was correct for 81% of patients.
Prediction of Asthma Diagnosis
Because of this huge amount of different treatment possibilities and heterogeneous patient profiles it was important to assure the asthma diagnosis. Patients with inhaled therapy only were predicted an indication by the machine learning model. The basis of this model was the documented explicit diagnosis of 90,000 patients from > 1.100 general practitioner or specialist practices of the IMS Disease Analyzer. Patients who had received one of the five approved asthma biologics (mepolizumab, benralizumab, reslizumab, omalizumab, dupilumab) were predicted using a rule-based approach: mepolizumab, benralizumab and reslizumab patients were assigned asthma as it was the only approved indication during the study period. For omalizumab and dupilumab, prescriber specialty and co-medication were considered to distinguish between asthma patients and patients with dermatological indications such as urticaria (omalizumab) or atopic dermatitis (dupilumab).
Selection of Patients with GINA 4/5 Asthma
Treatment combinations of patients were classified into the five GINA steps.3 Daily inhaled ICS dose is relevant for GINA classification and needs to be estimated as the prescription data does not indicate how the drug is used. The selection criteria for the cohort of patients with treatment according to NVL/GINA step 4 or 5 were first: maintenance therapy for at least half of the study period as a quality criterion to the data, in order to select patients with good database observability and not only seasonal flares of high dose medication, and second: treatment primarily according to GINA3 step 4 or 5, or treatment according to GINA step 3 and noticeable step up treatment during the study period for at least 120 days OCS+ICS+LABA+ (LAMA or leukotriene-receptor antagonist [LTRA]) as criteria to select patients with asthma treated according to GINA 4 or 5.
Selection of Patients with Uncontrolled NVL4/5 Asthma
In the subset of 625,301 patients with GINA 4/5 asthma high amounts of SABA or OCS prescriptions were used as proxy for insufficient asthma control. Not all OCS prescriptions can be assumed to be specific to asthma and with SABA prescriptions stockpiling must be accounted for. Patients who had high amounts of OCS prescriptions, high amounts of SABA prescriptions or both, were selected as uncontrolled. Several cutoffs were discussed and finally, the following intermediate scenario was selected:
OCS: “Acute-type” OCS prescriptions from GPs, pulmonologists and hospital outpatient departments in 2019 were counted. We found in a database with prescription-indication linkage (IQVIA Disease Analyzer) that only ¾ of high-dose OCS prescriptions issued to asthma patients by non-pulmonologists were in fact asthma related. In order to not overestimate the population of asthma-related OCS prescriptions, we discounted non-pulmonologist prescriptions of high-dose OCS by this factor. Prescriptions from pulmonologists scored 1.0, prescriptions from other specialists or GPs scored 0.75, Patients that had a score of 2 or more were flagged as high OCS. The definition of acute OCS Rx is shown in Figure 1. This content was validated with dosing recommendations issued by pulmonologists and documented Disease Analyzer.
 |
Figure 1 Definition of acute OCS Rx. |
SABA: Patients with at least 3 SABA prescriptions during 2019 issued on days with no prescription of ICS-containing maintenance medication were flagged as high SABA.
The study has been approved by the respective ethic committees.
Results
Patients with Asthma Treatment
In Germany, 3.4 million patients received at least two prescriptions of medications licensed for respiratory diseases in 2019 and had data of at least 180 days during the study period. Characteristics of patients on asthma maintenance therapy: 50.7% female (mean age 50.0, median 52 years); 34.5% male (mean age 42.3, median 45 years); 14.8% not known (mean age 41.2, median 45 years). The asthma medication included ICS, long-acting β2-agonists (LABA), long-acting muscarinic antagonists (LAMA), ICS/LABA combinations, theophylline, montelukast or biologicals.
Around 70% of patients get prescribed maintenance respiratory treatment to control their asthma, 30% for COPD. An analysis of the IQVIA-LRx database was performed to select the cohort of patients with asthma maintenance treatment and an asthma diagnosis. In addition a Machine Learning Model was used to confirm the asthma diagnosis (ICD-10 J45) and differentiate from COPD, acute or chronic bronchitis. According to demographic and therapeutic variables the system assessed the most important predictors to classify patients. Thirty features were identified; patient age was the most important, number of prescriptions and amount of ICS was second and third respectively. The validation of this model showed that by using these features the model identified patients on maintenance therapy with a diagnosis of asthma with a precision of 86% and a recall of 86%.
Therapy courses of 1.56 million patients in the LRx database met the study inclusion criteria and were predicted to have a diagnosis of asthma. Projection of the sample to national level (statutory plus private health insurance) resulted in a population of 2.4 million patients on asthma maintenance therapy in 2019 (projections of populations are shown in Figure 2).
The number of patients with GINA 4 or 5 therapy during the majority of treatment days in 2019 was 594,257–633,662 and they were selected with at least 180 days of maintenance treatment supply during 2019. The number of patients (31,442) who did not strictly meet the GINA 4/5 criteria but had at least 180 days of maintenance therapy and at least 120 days of GINA 4/5 therapy or at least 120 days of a therapy consisting of ICS/LABA, and OCS and LAMA or LTRA were also selected. In total, 625,301 asthma patients fulfilled these inclusion criteria and were defined as patients with asthma treatment according to GINA 4/5 (Figure 2).
Patients with Uncontrolled Asthma Treated According to GINA Step 4/5
To select the patients with uncontrolled asthma the IMS LRx data was analyzed according to OCS or excessive reliever (SABA) use as markers of uncontrolled asthma or exacerbations in patients on maintenance treatment for GINA step 4/5.
GPs are absolutely and relatively (per patient) the main prescriber of SABA. In our cohort they prescribed a mean SABA amount of 260 puffs per patient. Eleven percent of all asthma patients (250 thousand patients) on maintenance therapy received at least 4×200 puffs of SABA during the study period (Figure 3). It is expected that not all SABA that is prescribed is used by the patients, so that an excessive reliever (SABA) use was defined by time to SABA refills. More than 43,000 patients with severe asthma received 4 or more SABA prescriptions during the study year with a time to SABA refill of 60 days (Figure 3). In all, 27,835 SABA patients with at least 3 SABA prescriptions during 2019 issued on days with no prescription of ICS-containing maintenance medication were flagged as high SABA.
Most of the OCS prescriptions are issued by general practitioners; 20% are not asthma specific but low dose OCS, e.g. for rheumatic diseases. After quality check with the IMS Disease Analyzer more than 60% of the high dose OCS prescriptions by general practitioner were related to asthma and more than 90% of the high dose OCS prescriptions by pneumologists. OCS can be used for maintenance therapy as well as for a short period in case of exacerbations. The dose used by the patient is not known so that OCS maintenance therapy was calculated according to the dose and package size. A validation with the IMS Disease Analyzer pulmonologists showed an accuracy of this model of 89%. Eleven thousand patients with severe asthma received 3, and 29 thousand patients with severe asthma received 2 and more OCS prescriptions during the study period (Figure 4). There were 30,167 patients that had a score of 2 or more and were flagged as high OCS.
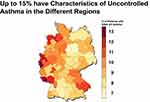
Taking into account overlaps between the SABA and OCS population, a total of 54,026 patients were flagged as uncontrolled GINA 4/5 asthma. The percentage of patients in GINA 4/5 who fulfilled the characteristics of uncontrolled asthma showed regional differences ranging from 7 to 15% (Figure 5). Twelve thousand patients with GINA 4/5 asthma were treated with biologicals and 10,000 of these asthmatics were controlled; only 2,000 were not controlled and included in the group of the 54,000 uncontrolled GINA 4/5 asthmatics.
Discussion
The present study aimed to characterize and estimate the number of patients with NVL/GINA level 4 or 5 asthma and signs of poor control in Germany and to evaluate possible areas for improvement.
The study was performed in 2019 before the start of the COVID-19 pandemic to exclude possible influences of SARS-CoV-2 related prescriptions. Experts in pneumology from hospitals and practices supported the study as an expert committee. They defined the suitable patient cohorts, the methodology and the data analysis that were performed, they reviewed, evaluated and interpreted the data, provided critical feedback and approved the final version. The IQVIA Supply Database provided the possibility to evaluate real life prescription of 80% of the statutory health insurance in Germany. This broad coverage of all available data as well as the interdisciplinary approach and the fact that longitudinal treatment data is available make this database unique. In addition, quality control could be performed using the EMR Disease Analyzer with diagnosis according to ICD 10, free text and lab data of 90,000 patients from > 1100 GPs or specialist practices.
Supplementary to performing all the analysis on the basis of this broad database of high quality we used a conservative approach for all our analyses. In the group of patients with asthma prescription we included only those that had at least two prescriptions of licensed asthma medications during the study period. This approach might have excluded patients with seasonal treatment only. Follow-up of at least 180 days during the study period ensured a continuous disease history but did not exclude death or treatment initiation during the study year. Using this procedure, we found 3.4 million patients with an asthma treatment. In 2010 Virchow J. C. reported a prevalence of asthma in Germany of 4–5% of adults corresponding to 3.2 to 4 million patients.1 In 2014/2015 Steppuhn et al11 performed a survey about the prevalence of asthma during the last 12 months in Germany, the Gesundheit in Deutschland aktuell (GEDA) study. Using a questionnaire patients ≥ 18 years of age were asked if they have had asthma or allergic asthma during the last 12 months. Of the 24,016 participating adults (13,144 women, 10,872 men) 6.2% reported asthma or asthma disorders, corresponding to around 5 million patients.11 With our conservative approach we accessed less than 70% of this number (3.4 million patients with asthma treatment) or less than 50% (2.4 million asthma patients on maintenance treatment according to GINA), respectively.
OCS as well as reliever prescriptions and the number of refills were quantifiable in our database. Excessive use of reliever or OCS could acted as an indicator of uncontrolled asthma or exacerbations in patients on GINA 4/5 maintenance treatment. Approximately 54,000 of the 625,000 patients with asthma treated according to GINA 4/5 fulfilled the definition of having uncontrolled asthma and only 12,000 of the 625,000 patients were treated with biologicals. According to Worth et al annually, 47% of GINA 4 patients treated by GPs (40% treated by respiratory physicians [RPs]) and 52% of GINA 5 patients treated by GPs [54% treated by RPs] received ≥ 3 SABA inhalers in 2017/2018 in Germany (Disease Analyzer database [IQVIA], n=15,640 patients).12 In a study based on five European countries including Germany, Janson et al reported a prevalence of SABA overuse, defined as ≥ 3 inhalers per year, of 16% in Germany (Disease Analyzer database [IQVIA], 2013–2018, n=53,866 patients).13 The main difference in the methodology between the study by Janson et al and the Worth study was the required observation time: follow-up for at least 12 months before and after study entry (Janson et al), observability criterion of having had at least two visits with their physician during the study period (Worth et al). These German retrospective studies showed that the prevalence of SABA overuse in patients treated in general and pneumologist practices in Germany was very high compared to our conservative approach of using only SABA patients with at least 3 SABA prescriptions during 2019 issued on days with no prescription of ICS-containing maintenance medication (1.2% of asthma patients on maintenance therapy). Despite new treatment options for severe asthma, Lommatzsch et al reported a prevalence of OCS >30 d/y during the years 2015–2017 of 9.2% (Y1), 9.5% (Y2) and 8.8% (Y3) for GPs and 6.3% (Y1), 6.4% (Y2) and 6.2% (Y3) for RPs irrespective of treatment step (GINA 1–5) using a German electronic medical records database (IMS®) covering 1289 GPs and 28 RPs.14 These numbers are even higher than our conservative approach and further data is needed to understand the reasons for these high number of patients not being controlled and how to overcome this treatment gap.9,12 There is existing data on SABA overuse and uncontrolled status of symptoms in asthmatics in Germany in general, but not in relation to the definition of severe uncontrolled asthma which would qualify for use of biologics. We stated more clearly that there are so far no existing data estimating the number of uncontrolled severe asthmatics in Germany and therefore our study provides important novel data. In addition German data already available have assessed asthma control using the ACT whereas we used prescription data. The ACT is standard in clinical practice to define whether a patient has uncontrolled asthma or not. However, although being a valid tool, the ACT is not available for such a large cohort of patients, but rather limited to research centers which would represent a selection bias. By use of prescription data we were independent of the existence of ACT scores and we may have covered a broader range of patients by accessing the LRx database.
Limitations of the study are: The dynamic changes in therapies available and updates to guidelines can change the relationships overtime. No diagnoses are included in the IQVIA-LRx database. Accordingly the prescription of an asthma medication had to be used as indirect indicator for a diagnosis of asthma. However the validation of the model using the EMR Disease Analyzer recorded patients on maintenance therapy having an asthma diagnose with a precision of 86% and a recall of 86%. The actual OCS dose administered by individual patient is unknown. Accordingly, the amount of OCS maintenance therapy had to be calculated according to the dose and package size, but again a validation with the EMR Disease Analyzer pulmonologists showed an accuracy of this model of 89%. The treatment data used to classify patients here does not allow for differentiation between “difficult-to-treat” and “severe” asthma, as this requires in-depth individual assessment.15 Data defining the quantitative relationship between “difficult-to-treat” and “severe” asthma is not available for Germany and is also scarce on an international level. On the other hand, as detailed above, we used a conservative approach for all definitions to avoid overestimation.
Conclusion
Around 54,000 patients with asthma treated according to GINA 4/5 in Germany, because of the asthma severity and/or difficult-to-treat asthma, had poor asthma control (OCS and/or SABA overuse), representing up to 15% of these patients in some regions. However, currently only 12,000 of the 625,000 patients with GINA 4/5 treatment are treated with biologicals suggesting a possible unmet need or treatment gap that may be due to underutilization of biologic treatments and the management of difficult-to-treat asthma using a treatable traits approach. Further data is needed to elucidate the reasons and to develop strategies to improve the management of these patients.
Abbreviations
GKV, gesetzliche Krankenversicherung; GP, general practitioner; ICS, inhaled corticosteroid; KV, kassenärztliche Vereinigung; LABA, long-acting β2-agonist; LAMA, long-acting muscarinic antagonist; LTRA, leukotriene-receptor antagonist; OCS, oral corticosteroid; PZN, Pharmazentralnummer; RP, respiratory physician; Rx, prescription; SABA, short-acting β2-agonist; SHI, statutory health insurance.
Acknowledgments
Funding for this analysis was provided by GSK. Editorial support (in the form of writing assistance, including preparation of the draft manuscript under the direction and guidance of the authors) was provided by Dr. A. Narkus, at MC Narkus GmbH Medical Consulting & Services, and was funded by GSK.
Disclosure
KCB and OS have no relevant competing interests to disclose. SH is a GSK employee and shareholder. KM reports speaker and/ or advisory fees from AstraZeneca, GSK, Novartis, Sanofi. CN received honoraria and advisory board fees from GSK, Sanofi, AstraZeneca and Novartis. DS received honoraria for lectures and/or consultancy from AstraZeneca, Bayer, Berlin-Chemie, Boehringer, Chiesi, GSK, Janssen, Novartis, Pfizer. RL is an employee of IQVIA. IQVIA is a technology service provider that carried out the database studies within the scope of a commercial engagement with GSK. DK reports personal fees from GSK. JCV has lectured for and received honoraria from AstraZeneca, Avontec, Bayer, Bencard, Bionorica, Boehringer-Ingelheim, Chiesi, Essex/Schering-Plough, GSK, Janssen-Cilag, Leti, MEDA, Merck, MSD, Mundipharma, Novartis, Nycomed/Altana, Pfizer, Revotar, Sandoz-Hexal, Stallergens, TEVA, UCB/Schwarz-Pharma, Zydus/Cadila and has participated in advisory boards for Avontec, Boehringer-Ingelheim, Chiesi, Essex/Schering-Plough, GSK, Janssen-Cilag, MEDA, MSD, Mundipharma, Novartis, Regeneron, Revotar, Roche, Sanofi-Aventis, Sandoz-Hexal, TEVA, UCB/Schwarz-Pharma and has received research grants from Deutsche Forschungsgesellschaft, Land Mecklenburg-Vorpommern, GSK, MSD and is a full time employee of the Universitätsmedizin Rostock. The authors report no other conflicts of interest in this work.
References
1. Virchow JC. Asthma - historical development, current status and perspectives. Pneumologie. 2010;64(9):541–549. doi:10.1055/s-0030-1255695
2. Papaioannou AI, Kostikas K, Zervas E, Kolilekas L, Papiris S, Gaga M. Control of asthma in real life: still a valuable goal?. Eur Respir Rev. 2015;24(136):361–369. doi:10.1183/16000617.00001615
3. Reddel HK, Boulet LP; Global Initiative for Asthma. Global strategy for asthma management and prevention; 2021. Available from: www.ginasthma.org.
4. Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43:343–373. doi:10.1183/09031936.00202013
5. Pepper AN, Renz H, Casale TB, Garn H. Biologic therapy and novel molecular targets of severe asthma. J Allergy Clin Immunol Pract. 2017;5(4):909–916. doi:10.1016/j.jaip.2017.04.038
6. Summary of product characteristics benralizumab. Available from: https://wwwemaeuropaeu.
7. Del Giacco SR, Bakirtas A, Bel E, et al. Allergy in severe asthma. Allergy. 2017;72:207–220. doi:10.1111/all.13072
8. Kaur R, Chupp G. Phenotypes and endotypes of adult asthma: moving toward precision medicine. J Allergy Clin Immunol. 2019;144:1–12. doi:10.1016/j.jaci.2019.05.031
9. Taube C, Bramlage P, Hofer A, Anderson D. Prevalence of oral corticosteroid use in the German severe asthma population. ERJ Open Res. 2019;5(4):00092–2019. doi:10.1183/23120541.00092-2019
10. Rathmann W, Bongaerts B, Carius H, Kruppert Y, Kostev K. Basic characteristics and representativeness of the German Disease Analyzer database. Int J Clin Pharmacol Ther. 2018;56:459–466. doi:10.5414/CP203320
11. Steppuhn H, Kuhnert R, Scheidt-Nave C. 12-Monats-Prävalenz von Asthma bronchiale bei Erwachsenen in Deutschland. J Health Monit. 2017;2(3). doi:10.17886/RKI-GBE-2017-052
12. Worth H, Criée CP, Vogelmeier CF, et al. Prevalence of overuse of short-acting beta-2 agonists (SABA) and associated factors among patients with asthma in Germany. Respir Res. 2021;22(1). doi:10.1186/s12931-021-01701-3
13. Janson C, Menzies‑Gow A, Nan C, Nuevo J, Papi A, Quint J. SABINA: an overview of short‑acting β2‑agonist use in asthma in European countries. Adv Ther. 2020;37:1124–1135. doi:10.1007/s12325-020-01233-0
14. Lommatzsch M, Wilmer C, Sauerbeck IS. Prevalence of the use of oral corticosteroids in asthma-a 3-year analysis in Germany. Eur Respir J. 2019;54:00092–2019
15. Hekking PPW, Wener RR, Amelink M, Zwinderman AH, Bouvy ML, Bel EH. The prevalence of severe refractory asthma. J Allergy Clin Immunol. 2015;135(4):896–902. doi:10.1016/j.jaci.2014.08.042
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.