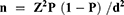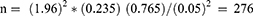Back to Journals » HIV/AIDS - Research and Palliative Care » Volume 13
Prevalence of Opportunistic Intestinal Parasitic Infections Among HIV/AIDS Patients Before and After Commencement of Antiretroviral Treatment at Felege Hiwot Referral Hospital: A Follow-up Study
Authors Getachew T, Hailu T , Alemu M
Received 4 May 2021
Accepted for publication 8 July 2021
Published 16 July 2021 Volume 2021:13 Pages 767—774
DOI https://doi.org/10.2147/HIV.S318538
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Bassel Sawaya
Tigest Getachew,1 Tadesse Hailu,2 Megbaru Alemu2
1Felege Hiwot Referral Hospital, Bahir Dar, Ethiopia; 2Department of Medical Laboratory Science, College of Medicine and Health Sciences, Bahir Dar University, Bahir Dar, Ethiopia
Correspondence: Tadesse Hailu Email [email protected]
Background: Coccidian parasites are opportunistic intestinal parasites that cause diarrhea in immunocompromised individuals. Although the impacts of coccidian infection are significant among HIV/AIDS infected cases, proper diagnosis and management of coccidian infection is limited in sub-Saharan Africa including Ethiopia.
Objective: The aim of this study was to determine the prevalence of coccidian parasitic infections among HIV/AIDS cases before and after commencement of antiretroviral treatment.
Methods: An institution-based longitudinal study was conducted among 304 randomly selected HIV/AIDS cases from February to July 2018 before and after commencement of antiretroviral therapy. A structured questionnaire was used to collect sociodemographic and associated factors data. Stool and blood samples were collected before and three months after treatment. Coccidian detection and CD4+ count were conducted via modified acid fast stain technique and fluorescence-activated cell scanning, respectively. Data were entered and analyzed using SPSS version 20. Descriptive statistics were used to compute coccidian prevalence. Logistic regression was used to compute possible association between associated factors and coccidian parasitic infection. Variables with P< 0.05 were considered to be statistically significant.
Results: Among 304 HIV/AIDS cases, prevalence of coccidian parasitic infection before and after antiretroviral treatment was 23.4% and 8.9%, respectively. Prevalence of Cryptosporidium spp. (19.7%) and Isospora belli (4.3%) before antiretroviral treatment were higher than Cryptosporidium spp. (7.9%) and Isospora belli (1.0%) after treatment. Drinking unprotected water (AOR: 7.41; 95%CI: 1.64– 33.45), poor knowledge of HIV/AIDS and coccidian parasite (AOR: 4.19; 95%CI: 1.69– 10.40), and CD4+ count below 200 cells/mm3 (AOR: 62.49; 95%CI: 25.32– 154.21) were significantly associated with coccidian infection.
Conclusion: Prevalence of coccidian parasites among HIV/AIDS cases decreases after antiretroviral treatment. Drinking unsafe water, limited knowledge of HIV/AIDS and coccidian parasite and low CD4+ cell count are factors associated with coccidian infection. Therefore, proper detection and treatment of coccidian parasites among HIV/AIDS cases should be prioritized.
Keywords: Cryptosporidium spp., Isospora belli, antiretroviral treatment, HIV/AIDS
Introduction
Coccidian parasites are opportunistic intestinal parasites (OIPs) that include Cryptosporidium spp., Isospora belli and Cyclospora cayetanensis which are the most common intestinal parasites infecting immunocompromised individuals. These parasitic infections are more common among infected cases of human immunodeficiency virus (HIV) and developed acquire immune deficiency syndrome (AIDS).1,2 An estimated nearly 80% of AIDS patients die of AIDS related illnesses including OIPs which usually occurs when CD4+ T cell count is below 200 cells/mm.3 Although coccidian caused self-limiting diarrhea in immunocompetent individuals, they cause more severe diarrhea among HIV/AIDS cases. For instance, HIV/AIDS patients with CD4+ T cell counts <100/µL may encounter chronic, persistent, and remarkably profuse diarrhea with significant fluid and electrolyte depletion with weight loss and abdominal pain.4–6
Previous reports regarding coccidian infections revealed that several factors like poor sanitation, unprotected source of water, lack of toilet facilities, lack of awareness about mode of transmission of coccidian parasites, poor environmental and personal sanitation practice and hygiene, poor socioeconomic status, overcrowding and absence of toilet are believed to be the main contributing factors for the high prevalence of coccidian infections in tropical and subtropical countries.7,8
In Ethiopia, most health institutions provide HIV/AIDS care detection and antiretroviral treatment (ART) services to improve the survival of HIV/AIDS cases. They routinely screen HIV/AIDS cases for coccidian parasitic infections; however, the direct saline microscopy diagnostic method has a low detection rate of coccidian parasites. As a result, coccidian infections are not properly diagnosed and these coccidian parasites still pose a serious challenge among HIV/AIDS cases. Due to the absence of sensitive routine diagnostic methods like modified acid fast stain technique, there is misdiagnosis, underreporting, and mistreatment and management of coccidian infections. Therefore, the aim of this study was to determine the prevalence of intestinal coccidian parasites and their associated factors among HIV seropositive individuals before and after the commencement of ART in Felege Hiwot Referral Hospital, Northwest Ethiopia.
Materials and Methods
Study Design, Period and Area
An institution-based longitudinal study was conducted from February 2018 to July 2018 at Felege Hiwot Referral Hospital ART clinic, Bahir Dar city, Northwest Ethiopia. Bahir Dar city is located 565 km from Addis Ababa at an altitude of 1788 meters above sea level. The hospital serves approximately 530,000 people and has more than 415 beds. The annual patient flow of the hospital is approximately 130,000. Currently, the total number of HIV/AIDS patients taking ART at the ART clinic in the hospital is 17,999. All HIV seropositive individuals who had not started antiretroviral treatment and are willing to participate and give consent were randomly selected and included in the study. However, those HIV seropositive individuals taking antiparasitic and/or antibiotic treatments two weeks prior to the data collection period were exclude from the study.
The sample size was determined by using previous study prevalence (23.5%) obtained in Fitche hospital,9 95% confidence interval (CI), and 5% precision.
By including 10% nonrespondent rate, the total sample size was 304.
Data Collection
A structured questionnaire was used to collect data on sociodemographic variables and associated factors to OIPIs infection. The questionnaire was filled by trained data collectors via a face to face interview. Stool and blood samples were collected by laboratory professionals at baseline before commencement of ART to check the respective coccidian parasites and CD4+ cell count. Antiretroviral therapy and cotrimoxazole were given based on the level of CD4+ cell count following the Ethiopian HIV/AIDS treatment guideline. Proper instruction how to collect stool samples was given for the participants, Training on coccidian parasites, how they are transmitted was given. A second round of stool and blood samples was collected three months after ART commencement. The stool samples were processed via formol ether concentration technique (FECT) followed by modified Ziehl–Neelsen techniques (MZNT).
Modified Ziehl–Neelsen Method
Firstly, a FECT was done by taking about one gram of stool sample and mixed in 10 mL saline and sieved with double layer of wet gauze directly into a 15 mL conical centrifuge tube. And then 10 mL of 10% formalin and 3 mL of ether were added into the tube. The tube was revolved at 2000 rpm for 3 min. The supernatant was decanted10 and a thin stool smear was done by taking a few drops of stool sample from the sediment. The smears were dried and fixed with methanol for 5 min and stained with carbol fuchsin for 15 min and washed with tap water. Acid alcohol (1%) was applied for 1–3 min to decolorize the preparation. Finally, the slides were stained with methylene blue for 1 min, and then washed with tap water and air dried. The slides were observed for coccidian using a microscope at 100× objectives.11
CD4+ Cell Count
About 2 mL of blood was collected via a vein puncture into tubes containing EDTA (ethylenediaminetetraacetic acid) anticoagulant to perform CD4+ cell count. About 100 µL of whole blood was mixed with 10 µL of each monoclonal antibody combination in separate tubes and incubated at room temperature for 20 min. Red blood cells were lysed by adding 2 mL of fluorescent activated cell sorter lysing solution. After overtaxing, tubes were incubated in dark at room temperature for 10 min. Then the CD4+ count was analyzed by using fluorescence-activated cell scanning count (FACS) following the manufacturer’s instruction.10
Data Quality Assurances
Training was given for data collectors prior to the actual data collection. The questionnaire was pretested to ensure applicability to the local context. Data supervision was conducted on a daily basis and the questionnaires were rechecked. Aseptic techniques and standard operating procedures were followed during sample collection, processing and identification of opportunistic intestinal parasites and measuring blood samples and counting the CD4+ cell count. Generally, the data quality was checked in the pre-analytical, analytical and postanalytical phases.
Data Analysis
Data analyses were performed using the SPSS version 20 (IBM Corporation, Armonk, NY, USA). The prevalence of coccidian infections and CD4+ count were computed by descriptive statistics. The factors associated with coccidian were computed with bivariate logistic regression. Variables with P<0.2 in the bivariate analysis were transferred to multivariate logistic regression to control potential confounders. The differences were considered to be statistically significant when P-value was <0.05.
Ethical Approval
Generally, our study followed the Declaration of Helsinki. Ethical clearance was obtained from the ethical review committee of Bahir Dar University College of Medicine Health Sciences (Reference no. IRB/03/007 dated April 4, 2018). A permission letter was also obtained from Amhara Regional Health Bureau and Felege Hiwot Referral Hospital. A written informed consent was obtained from each study participant. For study participants under the age of 18, informed written consent was also secured from their legal guardians before collecting the data. The participants had the right to refuse or withdraw from the study. This could not affect their treatment and follow-up. All aspects of the study were conducted according to Good Clinical Practice and Good Laboratory Practice guidelines. Confidentiality of the study participants’ information was securely stored and identified by study number. Any study participant who was positive for coccidian infection was referred doctors for further treatment.
Results
Sociodemographic Characteristics of Study Participants
A total of 304 HIV seropositive individuals were included. Of which, 188 (61.8%) were females. The mean (SD) age of the respondents was 34.83 (±10.77) years. The majority of the participants were urban residents 264 (86.8%), married 169 (55.6%), governmental employee 74 (24.3%), 76 (25%) college and above educational status and 139 (45.7%) in the age range 25–35 years (Table 1).
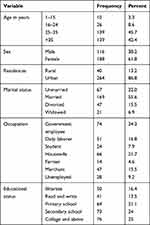 |
Table 1 Sociodemographic Characteristics of Pre-ART Individuals at Felege Hiwot Referral Hospital ART Clinic, 2018 |
Prevalence of Coccidian Infections
The overall prevalence of coccidian infections among HIV seropositive individuals before commencement of ART was 71 (23.4%). The prevalence of Cryptosporidium spp. and I. belli was 58 (19.1%) and 11 (3.6%), respectively. The prevalence of double infection of Cryptosporidium species and I. belli was 2 (0.7%). The prevalence of coccidian infections was higher among females 44 (14.5%), rural residents 48 (15.8%) and the age group of >35 years 36 (11.8%) (Table 2).
 |
Table 2 Distribution of Intestinal Coccidian Infection in Pre-ART HIV Seropositive Individuals Across Sociodemographic Variables at Felege Hiwot Referral Hospital ART Clinic, 2018 |
The overall prevalence of intestinal coccidian parasites among clients after three months of ART was 27 (8.9%) which comprises 24 (7.9%) Cryptosporidium spp. and 3 (1.0%) I. belli prevalence (Table 3).
 |
Table 3 Distribution of Intestinal Coccidian Parasites Among HIV/AIDS Cases Found on ART at Felege Hiwot Referral Hospital ART Clinic, 2018 |
The CD4+ Count Before and After ART
The mean (SD) CD4+ count was 230±101.6 and 346±183.7, respectively before and after commencement of ART. The prevalence of coccidian infections among participants who do not start ART and already started ART with CD4+ T cell <200 cells/mm3 was 62 (72.9%) and 25 (30.5%), respectively. Cryptosporidium spp. was the highest parasitic infection detected 50 (58.8%) before ART and 22 (26.8%) after ART among participants with CD4+ count <200 cells/mm3 (Table 4).
 |
Table 4 Prevalence of Coccidian Infections Among Pre-ART and on ART Cases in Relation to CD4+ Cell Count at Felege Hiwot Referral Hospital ART Clinic, 2018 |
Multivariate Analysis of Factors Associated with Intestinal Coccidian Parasite Infection
Pre-ART cases who used unprotected water source were 7.41 times at higher odds of coccidian infection than those who used tap water (AOR: 7.41; 95%CI: 1.64–33.45). Pre-ART cases who did not have HIV-OIP related knowledge were at 4.19 times higher odds of coccidian infection (AOR: 4.19; 95%CI: 1.69–10.40) than those who have HIV-coccidian related knowledge. Pre-ART cases who had less than 200 CD4+ cells/mm3 count were 62.49 times (AOR: 62.49; 95%CI: 25.32–154.21) more exposed than their counterparts (Table 5).
 |
Table 5 Bivariate and Multivariate Analysis of Associated Factors with Coccidian Infections Among Pre-ART Participants at Felege Hiwot Referral Hospital, 2018 |
Discussion
Coccidian parasitic infections are pronounced when the immunity is depleted. Factors decreasing the immune response including HIV/AIDS may contribute for the high prevalence of coccidian infections in the community. In the present study, the overall prevalence rate of coccidian infection among Pre-ART HIV seropositive individuals at 95%CI was 23.4% (18.7–28.5%). This result is higher than the earlier reports in different parts of Ethiopia: 15.4% from Jimma,2 (1.1%) in Dessie,12 17.7% in Arba Minch,13 2.2% in Harare14 and (15.4%) in Democratic Congo.15 The current result is also comparable with previous report (19%) in Cameroon.16 However, the prevalence of OIP infections among pre-ART in the present study is lower than the previous reports (69.7%) in Bahir Dar city17 and (76.7%) in Nigeria.18 The prevalence difference might be due to the geographic difference, sensitivity of diagnostic techniques, study participants’ immunity status, hygiene practice, socioeconomic difference, change in living conditions and awareness of patients on coccidian parasitic infection of the study participants.
The prevalence of Cryptosporidium spp. in pre-ART patients at 95%CI was 19.7% (15.4–24.7%) in the current study. This result is lower than reports (42.7%) in Nepal,19 but higher than (11%) in Ethiopia,20 (13.2%) in Southern Ethiopia21 and (3.1%) in Gondar.22 The difference might be due to the difference diagnostic method, the study period at which the data collected and personal hygiene of HIV infected cases.
The prevalence of I. belli infection at 95%CI among pre-ART was 4.3% (2.3–7.2%) in the present study. This result is lower than a previous report (15.5%) in Bahir Dar city,17 but it is higher than the report in Southern Ethiopia (2.2%).21 This result is also comparable from (3.9%) prevalence report in Southern Ethiopia.23 This difference in prevalence might be due to climatic condition, geographical distribution of the parasite, source of water for drinking and sanitation practice, knowledge on HIV related disease, lifestyle, education and immune status of the study participants.
The prevalence of coccidian parasite infection is decreased after the HIV cases started ART. In the current study, the prevalence of coccidian infection decreased after the commencement of ART. This low finding was similar to previous findings in Dessie12 and in Addis Ababa.24 This indicated that ART plays a great role in the reduction of OIP infections by boosting the immune cells.
Several risk factors including residence, source of water, knowledge on HIV-coccidian infection and level of CD4+ count were assessed whether they were associated with coccidian infections among HIV/AIDS cases. In the current study, prevalence of coccidian infections among pre-ART groups were found significantly associated with lower <500 cells/mm3 CD4+ cell counts. This result was in line with a previous study conducted in Addis Ababa.3 This indicated that higher CD4+ level generally clears the OIP infections.
The use of water from unprotected water source for drinking increases the possibility of acquisition of intestinal parasitic infections that have a feco-oral route of transmission. Drinking boiled water or pure water is a common problem in developing countries like Ethiopia where although it is important, it is high, especially in HIV patients. In the present study, HIV cases that drink unprotected water were 7.41 times more likely to have parasitic infection than those who used tap water. This result is supported by previous study report that using river water is a predictor of coccidian infections for HIV seropositive patients with more intestinal parasite than those using tap water.12
The odds of limited knowledge on the interaction of HIV/ADIS and intestinal parasite relation were almost 4.19 times more likely to be infected by coccidian parasites than others who had the knowledge. This finding is supported by previous study reports.7,25 Knowledge on the mode of transmission, prevention, and control measures and properly taking ART have an important effect to decrease coccidian infection among HIV/AIDS cases.
Strong immune response can kill parasitic infections especially OIPs. As the level of immune cell drop in the human system due to immune depleting mechanism like HIV/AIDS, people may suffer from OIPs infections. HIV infected individuals might also have low level of CD4+ counts.26 Those pre-ART HIV seropositive cases that had less than 200 CD4+ cell/mm3 count in the present study were 62.49 times more exposed for coccidian infections their counterparts. This finding was in line with previous reports.12,27 The justification might be due to ART preventing the coccidian infection by increasing the level of CD4+ count.
The limitation of the study: including study participants in a single institution and following the study participants for only three months and collecting data only twice.
Conclusions
Coccidian parasites including Cryptosporidium spp. and I. belli are the most important parasites identified in pre-ART and on ART HIV case. The prevalence of Cryptosporidium spp. and Isospora belli among HIV/AIDS cases decrease after the commencement of ART. Drinking unsafe water, poor knowledge on HIV and coccidian parasites infection and having low CD4+ count are the major factors associated with coccidian infections among HIV/AIDS cases. Therefore, early diagnosis of coccidian infections with sensitive diagnostic method like modified acid fast staining helps to properly manage HIV/AIDS cases and decreases the disease severity. Further studies with large sample size and area should be done.
Acknowledgments
We are happy to thank all the staff members of Felege Hiwot Hospital ART clinic nurses and the study participants who provided us stool and blood samples.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Zope A, Pai A, Baveja M. Opportunistic intestinal parasites in HIV infected indveduals and its correlation with the CD4 counts. RRJMHS. 2014;3:3.
2. Zeynudin A, Hemalatha K, Kannan S. Prevalence of opportunistic intestinal parasitic infection among HIV infected patients who are taking antiretroviral treatment at Jimma Health Center, Jimma, Ethiopia. Eur Rev Med Pharmal Sci. 2013;17:513–516.
3. Mengist HM, Taye B, Tsegaye A. Intestinal parasitosis in relation to CD4+T cells levels and anemia among HAART initiated and HAART naive pediatric HIV patients in a model ART center in Addis Ababa, Ethiopia. PLoS One. 2015;10(2):e0117715. doi:10.1371/journal.pone.0117715
4. Kulkarni S, Kairon R, Sane S, et al. Opportunistic parasitic infections in HIV/AIDS patients presenting with diarrhoea by the level of immune suppression. Indian J Med Res. 2009;131:63–66.
5. Xiao L, Ryan U. Molecular epidemiology. In: Fayer R, Xiao L, editors. Cryptosporidium and Cryptosporidiosis.
6. Kassu A, Tsegaye A, Wolday D, et al. Role of incidental and/or cured intestinal parasitic infections on profile of CD4+ and CD8+ T cell subsets and activation status in HIV-1 infected and uninfected adult Ethiopians. Clin Exp Immunol. 2003;132:113–119. doi:10.1046/j.1365-2249.2003.02106.x
7. Kumie A, Ali A. An overview of environmental health status in Ethiopia with particular emphasis to its organization, drinking water and sanitation: a literature survey. Ethiop J HealthDev. 2005;19:89–103.
8. Wafa L, Al-Mergin. Intestinal parasites infection among immuno-compromised patients in Riyadh, Saudi Arabia. Pak J Biol Sci. 2010;13(8):390–394. doi:10.3923/pjbs.2010.390.394
9. Adamu H, Wegayehu T, Petros B. High prevalence of diarrhoegenic intestinal parasite infections among non-ART HIV patients in Fitche Hospital, Ethiopia. PLoS One. 2013;8(8):e72634. doi:10.1371/journal.pone.0072634
10. Cheesbrough M. Medical Laboratory Manual for Tropical Countries.
11. Cama V, Gilman R, Vivar A, et al. Mixed Cryptosporidium infections and HIV. Emerg Infect Dis. 2006;12:1025–1028. doi:10.3201/eid1206.060015
12. Misaye A, Mulat D, Alemu A, Agersew A. Prevalence of intestinal parasites and associated risk factors among HIV/AIDS patients with pre-ART and on-ART attending Dessie Hospital ART clinic Northeast Ethiopia. AIDS Res Ther. 2013;10(7):1–9.
13. Alemu G, Alelign D, Abossie A. Prevalence of opportunistic intestinal parasites and associated factors among HIV patients while receiving ART at Arba Minch Hospital in Southern Ethiopia: a cross-sectional study. Ethiop J Health Sci. 2018;28(2):147–156. doi:10.4314/ejhs.v28i2.6
14. Merid T. Prevalence of opportunistic intestinal parasitic infections and associated risk factors among hiv sero-positive individuals at Hiwot Fana specialized university hospital, Harar town, eastern Ethiopia. AIDS Res Ther. 2014;11(12):5–14.
15. Wumba R, Longo-Mbenza B, Mandina M, et al. Intestinal parasites infections in hospitalized AIDS patients in Kinshasa, Democratic Republic of Congo. Parasite. 2010;17:321–328. doi:10.1051/parasite/2010174321
16. Nkenfou C, Nana C, Payne V. Intestinal parasitic infections in HIV infected and non-infected patients in a low HIV prevalence Region, West- Cameroon. PLoS One. 2013;8(2):514–579. doi:10.1371/journal.pone.0057914
17. Alemu A, Shiferaw Y, Getnet G, Yalew A, Addi Z. Opportunistic and other intestinal parasites among HIV/AIDS patients attending Gambi higher clinic in Bahir Dar city, North West Ethiopia. Asian Pac J Trop Med. 2011;4(8):661–665. doi:10.1016/S1995-7645(11)60168-5
18. Djieyep ACN, Djieyep FD, Pokam BT, David DL, Kamga HLF. The prevalence of intestinal coccidian parasites burden in HIV/AIDS patients on antiretroviral therapy in HIV centers in Mubi, Nigeria. Afr J Cln Exper Microbiol. 2014;15(3):165–172. doi:10.4314/ajcem.v15i3.8
19. Sucilathangam G, Velvizhi G, Palaniappan T. The prevalence of coccidian parasites in and around tirunelveli in HIV positive individuals and its correlation with the CD4 count. J Clin Diagnosis Res. 2011;5(Suppl 6):1182–1186.
20. Mohebali M, Yimam Y, Woreta A. Cryptosporidium infection among people living with HIV/AIDS in Ethiopia: a systematic review and meta-analysis. Pathog Glob Health. 2020;114(4):183–193. doi:10.1080/20477724.2020.1746888
21. Shimelis T, Tassachew Y, Lambiyo T. Cryptosporidium and other intestinal parasitic infections among HIV patients in southern Ethiopia: significance of improved HIV-related care. Parasit Vectors. 2016;9:270. doi:10.1186/s13071-016-1554-x
22. Eshetu T, Sibhatu G, Megiso M, et al. Intestinal parasitosis and their associated factors among people living with HIV at University of Gondar Hospital, Northwest-Ethiopia. Ethiop J Health Sci. 2017;27(4):411–420. doi:10.4314/ejhs.v27i4.12
23. Mariam ZT, Abebe G, Andargachew Mulu A. Opportunistic and other intestinal parasitic infections in AIDS patients, HIV sero-positive healthy carriers and HIV sero-negative individuals in southwest Ethiopia. East Afr J Public Health. 2008;5:3.
24. Dereje N, Moges K, Nigatu Y, Holland R. Prevalence and predictors of opportunistic infections among HIV positive adults on antiretroviral therapy (On-ART) versus Pre-ART In Addis Ababa, Ethiopia: a Comparative crosssectional study. HIV/AIDS Res Palliative Care. 2019;11:229–237. doi:10.2147/HIV.S218213
25. Lopez-Quintero C, Freeman P, Neumark Y. Hand washing among school children inBogota, Colombia. Am J Public Health. 2009;99:94–101. doi:10.2105/AJPH.2007.129759
26. Quesada-Lobo L. Key aspects of coccidia associated with diarrhea in HIV patients. Acta Méd Costarric. 2012;54:3.
27. Cerveja BZ, Tucuzo RM, Madureira AC, et al. Prevalence of intestinal parasites among HIV infected and HIV uninfected patients treated at the 1° De Maio health centre in Maputo, Mozambique. EC Microbiol. 2017;9(6):231–240.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.