Back to Journals » HIV/AIDS - Research and Palliative Care » Volume 13
Prevalence of Neurocognitive Impairment and Associated Factors Among People Living with HIV on Highly Active Antiretroviral Treatment, Ethiopia
Authors Wubetu AD , Asefa KK, Gebregiorgis BG
Received 19 December 2020
Accepted for publication 31 March 2021
Published 16 April 2021 Volume 2021:13 Pages 425—433
DOI https://doi.org/10.2147/HIV.S298141
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Roger Pinder
Abate Dargie Wubetu,1 Kokebie Kefelegn Asefa,2 Birhan Gebresillassie Gebregiorgis2
1Debre Berhan University, College of Health Science, Department of Psychiatry, Debre Berhan, Ethiopia; 2Debre Berhan University, College of Health Science, Department of Nursing, Debre Berhan, Ethiopia
Correspondence: Abate Dargie Wubetu
Debre Berhan University, College of Health Science, Department of Psychiatry Tel +251 910916569
Email [email protected]
Background: The burden of HIV is mainly found in Sub Saharan Africa. The HIV-associated neurocognitive impairment is found to be higher and it can exist at all stages of HIV. The HIV-associated neurocognitive impairment has a significant impact on a patient’s daily living and highly active antiretroviral treatment (HAART) adherence. Therefore, this study aimed to determine the prevalence and associated factors of HIV-associated neurocognitive impairment among adult people on HIV treatment.
Methods: A total of 423 people living with HIV/AIDS were planned to include in the study. A systematic random sampling technique was used to get the study participants. Binary logistic regression analysis was used to identify associated factors of HIV-associated neurocognitive impairment. Factors with a p-value of ≤ 0.2 on bivariate analyses were recruited for multivariate logistic regression analyses, and 95% CI at p-value < 0.05 was considered as statistically significant. Variance inflation factors for continuous variables and Spearman rank correlation for categorical variables were performed. There was no multicollinearity between suspected predictor variables. Model fitness was checked using Hosmer and Lemeshow Test, and its p-value was 0.45.
Result: A total of 422 individuals on HAART were included which gave a response rate of 99.8%. The prevalence of HIV-associated neurocognitive impairment was 41% (95% CI=36.3, 45.6). Older individuals, low monthly income, having comorbid depression and anxiety, have no communication about safe sexual intercourse, higher duration of HIV illness, and having poor social support were statistically significant associated factors of HIV neurocognitive impairment.
Conclusion: Two among five HIV patients on HAART treatment experienced HIV-associated neurocognitive impairment. It will be better if health professionals working at the HIV/TB clinic screen and consult HIV patients for psychiatric evaluation and treatment. Due attention should be given to HIV patients with associated factors.
Keywords: HIV, HAART, cognition, neurocognitive deficit, Ethiopia
Background
The human immunodeficiency virus (HIV) infected over 76 million people in the world. In the world, about 37.9 million people were living with HIV by the end of 2018. Sub-Saharan Africa remains among the hardest hit regions by the pandemic, with nearly one in every 25 adults (4.2%) living with HIV, accounting for nearly two-thirds of the global total HIV cases.1,2 The 75% burden of the Human Immune Deficiency Virus (HIV) exist in Sub-Saharan Africa. The sub-Saharan region contains only twelve percent of the world population.3
In Ethiopia, the prevalence rate of HIV infection reduced from 3.3% in 2000 to 0.9% in 2017 The number of HIV infections among adult Ethiopians was increased by 3748 HIV new infections from 2016 to 2017 and it became 722, 248 in 2017. The highest estimated prevalence of HIV infection in Ethiopia found in Addis Ababa (5%) and Gambella (4%).4
Neurocognitive impairment is a deficit in attention or concentration, memory, motor activities, and psychological functioning at the workplace.5 Neurological deficit is the crucial cause of morbidity and disability among people living with HIV. Based on the updated research on nosology of HIV-associated neurocognitive disorder classified into Asymptomatic Neurocognitive Impairment (ANI), HIV-associated Mild Neurocognitive Disorder (MND), and HIV-Associated Dementia (HAD).6 HIV-associated mild cognitive impairment affects up to 30% of PLWHIA. HIV-associated dementia is rare case and it affects up to 5% of HIV infected persons. HIV related cognitive impairment can happen at any stage of HIV. The severity of HIV-associated cognitive impairment might be complicated due to direct impact of the HIV and the ART drugs CNS toxicity.7
The prevalence of HIV-associated neurocognitive impairment ranges from 22% to 90%.8–14 Despite the problem is significant it is rarely studied in Ethiopia during the HAART era. Therefore, this study aimed to determine the prevalence of HIV-associated neurocognitive impairment among adult people living with HIV and on HAART and to identify factors associated with it.
Methods
Study Design, Period
The institutional-based cross-sectional study design was employed from 01 September 2019 to 01 September 2020. The study is part of a mega project titled the health-related impact of HIV/AIDS among people on HAART treatment in North Shoa Zone, Amhara, Ethiopia.
Study Setting
The study was conducted in selected public hospitals of North Shoa Zone, which provides ART HIV/AIDS treatment and services. Debre Berhan Town has located 695 km from Bahir- Dar, the capital of the region, and 130 km from Addis Ababa, the capital of Ethiopia. There are seven public hospitals in North Shoa Zone, one comprehensive and referral hospital, and six primary hospitals. During the data collection period, only three primary hospitals and the referral hospital were giving comprehensive HIV care and treatment, and all hospitals were included in the study. According to the North Shoa zonal health office report of 2017 a total number of 3406 HIV-positive peoples receiving clinical care.
Population
Source population: All adult HIV infected patients in public hospitals of North Shoa Zone in Amhara, Ethiopia.
Study population: All adult HIV infected patients on HAART in selected public hospitals of North Shoa Zone and who were available during the study period.
Inclusion criteria: The sample includes participants who can read and write, aged 18 years and above, and on HAART.
Exclusion criteria: Individuals who cannot respond due to the severity of the illness were excluded.
Sample Size Determination
In this study, the sample size was determined using the single population proportion formula. 50% proportion of HIV-associated neurocognitive impairment since there are no prevalence studies done by using Mini-Mental State Examination tool (MMSE), 95% confidence level, 5% margin of error, a 10% non-response rate was assumed to get the final sample size. The final sample size became 423 patients on HAART.
Sampling Procedures
A systematic random sampling technique was used to get study participants from each of the four study health institutions. The proportional allocation was used to allocate the final samples to each study area. The sampling fraction was determined by dividing the total study population who has monthly follow-up during the study period by the total sample size and participants were interviewed every 8th interval.
Data Collection Tools
A semi-structured questionnaire was used to collect data on socio-demographic characteristics, medical factors, and HIV illness-related factors. Comorbid medical disorders were reviewed in the patients’ chart record. Substance use, anxiety, and depression, current health status, perceived social support, and cognitive impairment variables were assessed by using the following tools as operationalized.
Substance use: Lifetime substance use of the substance was defined as lifetime use of at least one of specified substances after starting HAART and last month’s use of the substance was defined as the use of at least one of the specified substances in the previous one months before data collection.
Presence of anxiety and depression: Combined anxiety and depression symptoms were defined positive from the total sum of PHQ-4 scores, and rated as normal (0–2), mild (3–5), moderate (6–8), and severe. The presence of anxiety symptoms alone was suggested by a total score of ≥ 3 for the first 2 questions of PHQ-4, and depressive symptoms suggested by a total score of ≥ 3 for the last 2 questions of PHQ-4.15
Perceived social support: was assessed using the Oslo-3 item Social Support (OSS-3) scale to see the degree of social support and this scale is widely used in Ethiopia.16,17 OSS-3 in this study was scored according to total points ranging from 3–14; “poor support” 3–8, “moderate support” 9–11, and “strong support” 12–14.16
Current health status of PLWHA: was assessed by asking participants to rate their current health status according to a five-category index: HIV positive, with no symptoms, have symptoms, but have not had to change normal daily routines, have symptoms that have required to change parts of normal routines of daily activities (extra rest is not required during a normal day), because of symptoms, being in bed, or resting (less than half of waking hours), because of symptoms, being in bed, or resting (more than half of waking hours).18
Neurocognitive impairment: According to the Mini-Mental State Examination tool (MMSE), individuals living with HIV who score less than 25 out of the total score of 30 were considered to have a deficit in cognition. Also, individuals who score less than 13, 14–19, 21–24 were labeled with severe, moderate, and mild neurocognitive impairment respectively.19
Data Collectors and Data Quality Control
The principal investigator recruited four-degree holder nurses (one from each of the four study sites) for data collection. Data collectors were trained and oriented on how to use the questionnaire, the ethical principles of confidentiality, and data management before their involvement with data collection. The questionnaire was designed and modified appropriately. The questionnaire including all instruments used was translated into a local language. Amharic (participants interviewed with Amharic version) to be understood by all participants and translated back to English. The training was given to data collectors. A pre-test was done on 5% of the sample size before the start of actual data collection to test the simplicity and easy understandability of the questionnaire at a distinct primary health care facility and based on the finding from the pretest, the questioner was revised accordingly and time needed for an interview was estimated. The data collectors were supervised routinely by assigned supervisors and the completed questionnaires were checked daily by the principal investigators and assistant investigators for completeness and consistency.
Data Processing and Analysis
After data were checked for completeness and consistency, it was coded and entered into Epi Data 3.1. Then, data were exported to SPSS version 20 for analysis.
In descriptive statistics; tables, and frequency/percentage were used to present the information. Bivariate and multivariable binary logistic regression analysis was conducted to identify factors associated with HIV-associated neurocognitive deficit. Only factors with a p-value of ≤ 0.2 on bivariate analyses were recruited for multivariable logistic regression analyses and 95% CI at P-value < 0.05 was considered as statistically significant. The standard method of entry (enter method) was used to select variables. Collinearity diagnostics for continuous variables and Spearman rank correlation for categorical variables was performed and there was no multicollinearity or significant correlation between predictor variables. Model fitness was checked using Hosmer and Lemeshow Test, and its p-value was 0.45.
Ethical Considerations
Helsinki declaration for medical research involving human subjects was followed. Ethical clearance was obtained from the Institutional Health Research Review Committee (Ref. No. IHRERCB-0590/2019) of the college of health and medicine, Debre Berhan University. A permission letter was written for each study health institution and a permission letter was taken from the study institution administrator. Oral informed consent was taken from each study participant.
Results
Sociodemographic Characteristics of Patients on HAART
The majorities (86.7%) of the patients on HAART were from an urban area and the remaining (12.3%) were from a rural area. Almost sixty percent (60.2%) of patients on HAART were female. Nearly three-fourths (75.4%) of patients on HAART had a child and the maximum proportions (86%) were unemployed. The remaining (14%) were employed. Almost three-fourth (76.1%) of patients on HAART had communication about safe sexual intercourse with their sexual partner, (Table 1).
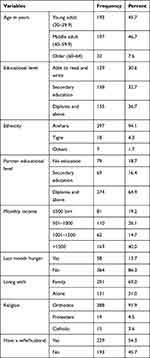 |
Table 1 Sociodemographic Characteristics of Patients on HAART, Ethiopia, 2020 (n=422) |
Medical Characteristics of Patients on HAART
From the total participants, almost 94% disclose their HIV status to their family or child. Almost twelve percent (11.6%) had an HIV positive child. Moreover, nearly thirty percent (29.9%) had an HIV positive family than a child. Less than one percent, (0.5%) of the patients on HAART treatment found in the fourth stage of the World Health Organization (WHO) classification. The minimum and the maximum CD4 count was 120 cells/dl (nadir CD4) and 1424 cells/dl respectively. The mean of the CD4 count was 512.25 cells/dl ± 246.876 standard deviations. For the majority of the patients on HAART treatment, the recent CD4 count was 500 cells/dl and above. Almost nine percent (8.8%) had been diagnosed with comorbid physical illness/opportunistic infection. Almost seven percent (7.3%) did not adhere to HAART treatment. The majority (98.8%) of patients’ duration of HIV infection was five years and above. The remaining 0.2% and 0.9% HIV infection was ≤ 1 year, and 2–4 years respectively, (Table 2).
 |
Table 2 Medical-Related Factors of Patients on HAART Treatment in Ethiopia, 2020 (n=422) |
Social Support and Mental Illness Characteristics of Patients on HAART
From the total patients living with HIV/AIDS (n=422), 50%, 20.1%, and 29.9% had poor, moderate, and strong social support respectively. Nearly five percent (4.7%) had a history of diagnosed psychiatric illness. Almost three percent (3.3%) had diagnosed with a family psychiatric disorder. Among the total participants (23.2%) had depression and anxiety. Of them, the minimum (4%) patients experience anxiety. Moreover, almost sixteen percent (15.9%) experienced depression. The same proportion (6.6%) had a history of last month and lifetime substance use.
Prevalence of HIV-Associated Neurocognitive Impairment
The prevalence of HIV-associated neurocognitive impairment was 41% (95% CI=36.3–45.6). Among them, 148 (35.1%) had a mild neurocognitive impairment, 22 (5.2%) had a moderate neurocognitive impairment, and 3 (0.7%) had severe neurocognitive impairment.
Associated Factors of Neurocognitive Impairment
Bivariate and multivariate binary logistic regression analysis was done to identify statistically significant factors of HIV-associated neurocognitive impairment among people on HAART treatment. Among the sixteen associated factors eligible for multivariate analysis, seven variables became significant in the final analysis.
Among sociodemographic characteristics of people on HAART treatment age, and family average monthly income contributes to the neurocognitive impairment. The results reveal that older participants were associated with a 6% increased odds of a diagnosis of HIV-associated neurocognitive impairment compared to the younger participants, 1.06 (95% CI=1.03, 1.08). Individuals who earn less than 500 Ethiopian Birr (ETB) per month had nearly five times higher odds to suffer neurocognitive impairment as compared with those who earn above 1500 ETB per month, 4.22 (95% CI=2.02,8.81). Moreover, individuals who earn 501–1000 ETB per month had nearly four times higher odds to suffer neurocognitive impairment as compared with those who earn above 1500 ETB, 3.93 (95% CI=2.09,7.36).
Medical factors also contribute to the prevalence of neurocognitive impairment among people on HAART. Individuals who had comorbid depression and anxiety had nearly six times higher odds to suffer neurocognitive impairment than those who did not have comorbidity, 5.51 (95% CI=1.81, 16.79). Individuals who had no communication about safe sexual intercourse had almost three times higher odds to develop neurocognitive impairment than those who had effective communication, 2.88 (95% CI=1.61, 5.16). Higher duration of HIV illness in a year associated with a 1% increase in HIV-associated neurocognitive impairment, 1.01 (95% CI=1.001, 1.02). Individuals on HAART who had poor social support had almost four times higher odds to develop neurocognitive deficit as compared with those who had strong social support, 3.65 (95% CI=1.86,7.17), (Table 3).
 |
Table 3 Bivariate and Multivariate Analysis to Identify Associate Factors of HIV-Associated Neurocognitive Deficit, Ethiopia, 2020 (n=422) |
Discussion
The prevalence of HIV-associated neurocognitive deficit was 41% (95% CI=36.3–45.6). HIV-associated neurocognitive impairment is associated with older age, low income, having depression and anxiety, having communication about safe sexual intercourse, having higher illness and treatment duration, and having poor social support. The current study prevalence in line with two studies done in Ethiopia 39.3% and 36.420,21 and Botswana 38%.22
This study is higher than studies done in Ethiopia (33.3%),8 South Asia (22.7%),9 and a systematic review and meta-analysis did across Sub-Saharan Africa countries.23 This discrepancy might happen due to the study design and population. Such as the study was done in Southern Asia includes only the adult population on HAART. Moreover, systematic review and meta-analysis studies investigate the pooled prevalence of the dependent variable (neurocognitive deficit) and may affect the prevalence.
Moreover, it is also lower than studies done in Kenya (81.1%),10 and southern Ethiopia (67.1%),24 Malawi (70%),11 India (90.1%),12 Northern Italy (47.1%),13 and United States (52%).14 This variation might be due to sociodemographic variation; tools used, and study period. Most studies listed here are done almost five years back. Therefore, this may increase the prevalence of HIV-associated neurocognitive impairment as treatment qualities are increasingly modified.
The results reveal that older participants were associated with 6% increased odds of a diagnosis of neurocognitive impairment compared to the younger participants. The association supported by the studies done in Ethiopia20,25), Kenya,10 and southern Asia.9 The possible justification might be due to HIV-associated neurocognitive impairment may be enhanced by the effect of brain aging.
Individuals who earn less than 500 Ethiopian birrs (ETB) per month had nearly five times higher odds to suffer neurocognitive impairment as compared with those who earn above 1500 ETB per month. Moreover, individuals who earn 501–1000 ETB per month had nearly four times higher odds to suffer neurocognitive impairment as compared with those who earn above 1500 ETB. Having a higher monthly income may allow the patient to fulfill basic needs like nutrition. Therefore, patients could get a balanced diet, and the brain will function well.
Individuals who had comorbid depression and anxiety had nearly six times higher odds to suffer neurocognitive impairment than those who did not have comorbidity. The association might be due to having comorbid/associated mental illness that could worsen a neurocognitive impairment. Depression had pseudo cognitive impairment and it may overestimate the association.
Individuals who had no communication about safe sexual intercourse had almost three times higher odds to develop neurocognitive impairment than those who had effective communication. This may allow the patient to adhere to HAART treatment and make life easy. This allows the patient to control the viral load and low severity of HIV infection promotes good cognition.
Higher duration of HIV illness in a year is associated with a 1% increase in HIV-associated neurocognitive impairment. This association supported by the study done in southern Ethiopia,24 and Kenya.10 This might be due to the advanced stage of the HIV illness, the impact of the virus itself, and ART drugs.
Individuals on HAART who had poor social support had almost four times higher odds to develop neurocognitive impairment as compared with those who had strong social support. Individuals may support patients living with HIV by remembering the medication time and can give material support. These factors may allow the patient to adhere to HAART treatment and improve their cognition.
Limitation
Since this study is done in selected hospitals in North Shoa Zone, the neurocognitive impairment might not apply to all PLWHA in Ethiopia. This study only includes HIV patients on HAART and may underestimate the prevalence of neurocognitive impairment as HAART can reduce the problem. Moreover, since the sub-cortical processes are primarily affected, the prevalence of neurocognitive impairment can be underestimated because the milder forms of neurocognitive impairment cannot be reliably detected.
Conclusion
Two among five HIV patients on HAART treatment were developed HIV-associated neurocognitive impairment. Neurocognitive impairment hurt the patient’s overall quality of life and also HAART adherence. It will be better if health professionals working at the HIV/TB clinic screen and consult HIV patients for psychiatric evaluation and treatment. Due attention should be given to HIV patients with associated factors.
Abbreviation
HAART, highly active antiretroviral therapy; HAND, HIV-associated neurocognitive deficit.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval
Helsinki declaration for medical research involving human subjects was followed. Ethical clearance was obtained from the Institutional Health Research Review Committee (Ref. No. IHRERCB-0590/2019) of the college of health and medicine, Debre Berhan University. A permission letter was written for each study health institution and a permission letter was taken from the study institution administrator. Verbal informed consent was taken from each study participant. The informed verbal consent process was approved by Debre Berhan University Institutional Ethics Review Board.
Acknowledgment
We would like to thank Debre Behan University for funding this study and giving ethical clearance. We would like to acknowledge the study hospitals and the study participants.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was funded by Debre Berhan University. The title is the subtheme of the mega-project title as the health-related impact of HIV/AIDS in North Shoa Zone. The funding institution had a role in data collection, analysis, and write-up.
Disclosure
The authors declare that they have no conflicts of interest.
References
1. Unaids J Fact sheet—latest global and regional statistics on the status of the AIDS epidemic. Geneva: UNAIDS; 2017.
2. HIV/AIDS JUNPo. UNAIDS report on the global AIDS epidemic 2013. Geneva: UNAIDS; 2013: 201.
3. UNAIDS. Global AIDS update; 2016:16.
4. Kibret GD, Ferede A, Leshargie CT, Wagnew F, Ketema DB, Alebel A. Trends and spatial distributions of HIV prevalence in Ethiopia. Infect Dis Poverty. 2019;8(1):90. doi:10.1186/s40249-019-0594-9
5. Clifford DB, Ances BM. HIV-associated neurocognitive disorder. Lancet Infect Dis. 2013;13(11):976–986. doi:10.1016/S1473-3099(13)70269-X
6. Antinori A, Arendt G, Becker J, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69(18):1789–1799. doi:10.1212/01.WNL.0000287431.88658.8b
7. Sadock BJ, Sadock VA. Kaplan and Sadock’s Synopsis of Psychiatry: Behavioral Sciences/Clinical Psychiatry. lippincott williams & wilkins; 2011.
8. Belete T, Medfu G, Yemiyamrew E. Prevalence of HIV associated neurocognitive deficit among HIV positive people in Ethiopia: a cross sectional study at Ayder Referral Hospital. Ethiop J Health Sci. 2017;27(1):67–76. doi:10.4314/ejhs.v27i1.9
9. Chan LG, Kandiah N, Chua A. HIV-associated neurocognitive disorders (HAND) in a South Asian population-contextual application of the 2007 criteria. BMJ Open. 2012;2(1):e000662. doi:10.1136/bmjopen-2011-000662
10. Mohamed AA, Oduor C, Kinyanjui D. HIV-associated neurocognitive disorders at Moi teaching and referral hospital, Eldoret, Kenya. BMC Neurol. 2020;20(1):1–11. doi:10.1186/s12883-020-01857-3
11. Kelly CM, van Oosterhout JJ, Ngwalo C, et al. HIV associated neurocognitive disorders (HAND) in Malawian adults and effect on adherence to combination anti-retroviral therapy: a cross sectional study. PLoS One. 2014;9(6):e98962. doi:10.1371/journal.pone.0098962
12. Achappa B, Priyadarshni S, Madi D, et al. Neurocognitive dysfunction among HIV positive patients using International HIV dementia scale. Asian J Med Sci. 2014;5(4):61–64. doi:10.3126/ajms.v5i4.8724
13. Focà E, Magro P, Motta D, et al. Screening for neurocognitive impairment in HIV-infected individuals at first contact after HIV diagnosis: the experience of a large clinical center in Northern Italy. Int J Mol Sci. 2016;17(4):434. doi:10.3390/ijms17040434
14. Heaton R, Clifford D, Franklin D, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75(23):2087–2096. doi:10.1212/WNL.0b013e318200d727
15. Kroenke K, Spitzer RL, Williams JB, Löwe B. An ultra-brief screening scale for anxiety and depression: the PHQ–4. Psychosomatics. 2009;50(6):613–621. doi:10.1176/appi.psy.50.6.613
16. Dalgard OS, Dowrick C, Lehtinen V, et al. Negative life events, social support and gender difference in depression. Soc Psychiatry Psychiatr Epidemiol. 2006;41(6):444–451. doi:10.1007/s00127-006-0051-5
17. Dalgard O. Community health profile as tool for psychiatric prevention. Promot Ment Health. 1996;5:681–695.
18. Martikainen P, Aromaa A, Heliövaara M, et al. Reliability of perceived health by sex and age. Soc Sci Med. 1999;48(8):1117–1122. doi:10.1016/S0277-9536(98)00416-X
19. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi:10.1016/0022-3956(75)90026-6
20. Salahuddin M, Manzar MD, Hassen HY, et al. Prevalence and predictors of neurocognitive impairment in ethiopian population living with HIV. HIV AIDS (Auckl). 2020;12:559.
21. Tsegaw M, Andargie G, Alem G, Tareke M. Screening HIV-associated neurocognitive disorders (HAND) among HIV positive patients attending antiretroviral therapy in South Wollo, Ethiopia. J Psychiatr Res. 2017;85:37–41. doi:10.1016/j.jpsychires.2016.10.016
22. Lawler K, Mosepele M, Ratcliffe S, et al. Neurocognitive impairment among HIV-positive individuals in Botswana: a pilot study. J Int AIDS Soc. 2010;13(1):15. doi:10.1186/1758-2652-13-15
23. Habib AG, Yakasai AM, Owolabi LF, et al. Neurocognitive impairment in HIV-1-infected adults in Sub-Saharan Africa: a systematic review and meta-analysis. Int J Infect Dis. 2013;17(10):e820–e31. doi:10.1016/j.ijid.2013.06.011
24. Debalkie Animut M, Sorrie MB, Birhanu YW, Teshale MY. High prevalence of neurocognitive disorders observed among adult people living with HIV/AIDS in Southern Ethiopia: a cross-sectional study. PLoS One. 2019;14(3):e0204636. doi:10.1371/journal.pone.0204636
25. Mossie TB, Tegegne MT. HIV dementia among HIV positive people at Debre markos hospital, Northwest Ethiopia. Am J Psychol Neurosci. 2014;2(2):18–24. doi:10.11648/j.ajpn.20140202.11
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
