Back to Journals » Infection and Drug Resistance » Volume 15
Prevalence of Multidrug-Resistant TB Among Smear-Positive Pulmonary TB Patients in Banadir, Somalia: A Multicenter Study
Authors Dirie AMH , Çolakoğlu S, Abdulle OM, Abdi BM, Osman MA, Shire AM, Hussein AM
Received 16 August 2022
Accepted for publication 5 December 2022
Published 10 December 2022 Volume 2022:15 Pages 7241—7248
DOI https://doi.org/10.2147/IDR.S386497
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Abdirahman Mohamed Hassan Dirie,1 Sedat Çolakoğlu,1 Osman Muhyadin Abdulle,2 Bashir Mohamud Abdi,3 Mohamed Abdi Osman,4 Abdiaziz Mohamud Shire,5 Abdinafic Mohamud Hussein6
1Pulmonology Department, Somalia Mogadishu Turkey Recep Tayyip Erdogan Training and Research Hospital, Mogadishu, Banadir, Somalia; 2Manager of the Multi-Drug Resistant Center in Southern Somalia, Mogadishu, Banadir, Somalia; 3HIV Program manager, Ministry of Health, Somali Federal Government, Mogadishu, Banadir, Somalia; 4Infectious and Microbiology Department, Somalia Mogadishu Turkey Recep Tayyip Erdogan Training and Research Hospital, Mogadishu, Banadir, Somalia; 5National TB program Manager, Somali Federal Government, Ministry of Health, Mogadishu, Banadir, Somalia; 6Cardiovascular Surgery Department, Somalia Mogadishu Turkey Recep Tayyip Erdogan Training and Research Hospital, Mogadishu, Banadir, Somalia
Correspondence: Abdirahman Mohamed Hassan Dirie, Tel +252615479778, Email [email protected]
Background: Tuberculosis (TB) is an infectious disease that is the second most common cause of death from a single infectious agent. TB infection affects anyone, regardless of age, gender, and ethnicity. Drug-resistant TB is a serious public health problem, which needs treatment with a second-line anti-TB drug and it includes poly-drug resistance (PDR), multi-drug resistance (MDR), and extensive drug resistance (XDR). The goal of this research is to find out the prevalence of MDR TB among pulmonary TB patients in Banadir, Somalia.
Methods: This was a multicenter retrospective review of data involving 1732 smear-positive pulmonary TB patients visiting Banadir TB centers between July 1, 2019 and June 30, 2020. Demographic, clinical, and drug susceptibility data were retrieved from TB treatment cards. The data were analyzed using Statistical Package for Social Sciences (SPSS) software (IBM SPSS Statistics version 26).
Results: All 1732 pulmonary TB cases were previously diagnosed by the Gene Xpert MTB/RIF test. Among them, 70.4% (1219/1732) were males. The mean age was 31.77 years. Overall, the prevalence of drug resistance TB was 10.56% (183/1732). The MDR TB was 1.96%, poly-drug resistance (PDR) was 0.12%, and extensive drug resistance was 0.06%.
Conclusion: This study showed a prevalence of MDR-TB among pulmonary TB patients, which is similar to some of the eastern African countries.
Keywords: TB, drug-resistance, poly-drug resistance, MDR, XDR
Introduction
Tuberculosis (TB) is defined as an infectious disease, which causes ill health and is one of the leading causes of death worldwide. Before the COVID-19 pandemic in 2019, TB was the most common cause of death from a single infectious disease.1 TB can affect anyone, regardless of age, sex, religion, and ethnicity. The highest burden is on adult males, who account for 56% of all TB cases, compared to their adult female counterparts, who accounted for 32%, and children for 12% less than 15 years.2
Resistance to both isoniazid and rifampicin is defined as multidrug-resistant TB (MDR-TB), and it is of greatest concern that it requires treatment with second-line drugs.3 Internationally, the burden of MDR-TB or RR-TB (MDR/RR-TB) is static. In the last 10 years, the best estimation of the proportion of newly diagnosed MDR/RR-TB was about 3–4%, and those who had previously been treated for TB were about 18–21%.1
TB is a serious public health problem around the world. The World Health Organization (WHO) estimates that Mycobacterium TB (MTB) infects one-third of the world’s population or roughly 2 billion individuals.4,5
Globally, an estimated 10.0 million (range, 8.9–11.0 million)6 people became ill with TB in 2019, a figure that has been steadily declining in recent years, probably due to a decline in case detection in the COVID-19 pandemic era.2 The WHO uses five categories to classify cases of drug-resistant TB: isoniazid-resistant TB, Rifampicin resistance (RR) TB, and multidrug resistance (MDR) TB, pre-extensively drug-resistant (pre-XDR) TB, and extensive drug resistance (XDR) TB.1,7
Globally, in 2020, 71% (2.1/3.0 million) of people with smear-positive pulmonary TB were tested for rifampicin resistance, up from 61% (2.2/3.6 million) in 2019 and 50% (1.7/3.4 million) in 2018.1 Three countries, namely China, the Russian Federation, and India, the most populous countries, carry the greatest burden of MDR/RR-TB, together accounting for more than 45% of the world’s total cases.4 For more than 10 years, the prevalence of newly diagnosed MDR/RR-TB has remained at about 3–4% and those previously treated for TB have remained at about 18–21%.1
A single-center study in Djibouti, a country with 800,000 people, conducted between April 2010 and April 2011, showed a high level of (11.4%) MDR-TB among 180 TB positive cases.8 China, the Democratic Republic of the Congo, India, Indonesia, Nigeria, Pakistan, the Philippines, the Russian Federation, South Africa, and Vietnam are among the countries that account for roughly 70% of the global gap between the estimated global incidence of MDR/RR-TB each year and the number of people enrolled in treatment in 2020.1
Somalia is a low-income country in East Africa with a high burden of TB. In a nationwide survey in 2011, multidrug-resistant TB (MDR TB) was found to be in 5.2% and 40.8% of patients with new and previously treated TB, respectively.9 National surveillance of anti-TB resistance is essential to assist countries in planning the scale-up of patient management.10 Active pulmonary TB mostly presents with symptoms of productive cough, dyspnea, weight loss, and night sweats, but rarely, it might present with only a mass on the chest as an empyema necessitans in immune competent patients.11
The prevalence and patterns of MDR in the population of Somalia have not been reported recently in the academic literature. The aim of this study was to determine the prevalence of MDR among pulmonary TB patients in the Banadir, the most populated region in Somalia.
Materials and Methods
Study Area
This study was a multicenter, retrospective review of medical records of TB patients treated in the Banadir Region of Somalia. The Banadir Region is the region that contains the capital city of Somalia and its surrounding areas, which consist of 17 districts. The population of this region is about three million people.
The national TB program of the Ministry of Health has 11 centers for TB in the Banadir region. Ten of them are centers designed for the treatment of drug-sensitive TB patients, and one of these centers (ie, the Forlanini or Laserreto TB Center) is designed for the treatment of drug-resistant TB. There is a Gene Xpert MTB/RIF machine for the detection of rifampicin resistance in every center, and every suspected TB case is subjected to this test.
Any rifampicin-resistant case is urgently referred to the Drug-Resistant Center in which LPA and culture are performed to investigate Isoniazid and second-line anti-TB resistance status.
The system is highly decentralized, and qualified healthcare professionals and volunteers provide TB services like physical examination, smear microscopy, culture, chest X-ray, Gene Xpert MTB/RIF assay, line probe assay (LPA), and Lowenstein-Jensen (LJ) culture.
The method of diagnosis of drug resistant TB was done by using the Gene Xpert MTB/RIF test first, and all rifampicin-resistant patients were transferred to the drug-resistant center for LPA and LJ culture tests.
Study Population
All pulmonary TB patients who were registered in TB centers in the Banadir Region from July 1, 2019 to June 30, 2020 and had a positive sputum test were included in this study. These patients were 1732 smear-positive cases who were registered in the TB centers of Benadir region during one year from July 1, 2019 to June 30, 2020.
Criteria of Selection
There is no sampling method used in this study, instead, all eligible smear-positive pulmonary TB cases were counted and the data was utilized. The inclusion criteria were TB smear-positive patients with drug sensitivity test results determined by the Gene Xpert MTB/RIF PCR, LPA, and LJ culture who were residents in the Banadir region at the time of the diagnosis. The exclusion criteria were as follows: all extrapulmonary TB cases; cases without drug sensitivity tests; smear negative patients; transferred patients from other regions; and those whose residence area was not in the Banadir Region.
The HIV test was a compulsory test for all patients who were diagnosed as TB patients. Treatment was delivered free of charge and under the supervision of direct observation therapy (DOT).
Data Collection
The data was retrieved from the registrar book of the TB centers, and it was entered into SPSS application system directly.
Data Analysis
The data was entered into the Statistical Package for Social Sciences (SPSS) software (IBM SPSS Statistics version 26) for analysis. The percentage of those with MDR/RR-TB in the study population was determined by standard deviation.
The relationship between MDR/RR-TB and independent variables like demographics and clinical entities was investigated using Chi-Square and Pearson correlation, as well as multivariate logistic regression analysis with a 95% confidence interval; a p-value less than 0.05 was considered a statistically significant cut-off value.
Ethical Consideration
This research was carried out retrospectively by utilizing routinely documented patient data from registrars of the Banadir TB centers, and informed consent from the patients was not required.
The Medical Research Ethics Committee of the Somalia Mogadishu Turkey Recep Tayyip Erdogan Training and Research Hospital provided ethical clearance (Ref No. MSTH/4999/284, on 12/12/2020). The National TB Program of the Somali Ministry of Health granted permission to conduct the study (Ref No. FMOH/NP/009/2022, on January 10, 2022).
The whole data was utilized anonymously without discovering the identity of the patients, but we used only the ID number of the TB centers.
WHO Definitions
Isoniazid-resistant TB is defined as an MTB strain resistant to isoniazid, RR-TB is defined as MTB resistant to rifampicin, MDR-TB is defined as an MTB strain resistant to at least rifampicin and isoniazid, and pre-extensively drug-resistant TB (pre-XDR-TB) is defined as an MTB strain resistant to rifampicin and any fluoroquinolone. XDR-TB is TB that is resistant to rifampicin, plus any fluoroquinolone, plus at least one of the drugs bedaquiline and linezolid.7,10,12
Results
The relevant information about the patients, including demographic data (age, sex, and residence) and clinical data (HIV status and smear status), was obtained from the registers of TB centers.
In this retrospective record analysis study, we collected 3392 TB cases. After excluding 244 cases that came from other regions, the rest were 3148 cases that were from our study area.
A total of 1732 eligible smear-positive cases were extracted and analyzed in this study as eligible cases for the study, while other 1416 cases were extrapulmonary cases, smear negative cases, and radiologically treated cases.
Among 1732, 89.43% (1549/1732) had Rifampicin sensitive Mycobacterium TB (MTB) and 10.57% (183/1732) were Rifampicin Resistant (RR TB) cases (Figure 1).
 |
Figure 1 Number of patients with drug sensitive TB and those with DR TB. |
One hundred and eighty-three (183) patients who tested positive for drug-resistant pathogens were resistant to at least one of the first-line anti-TB drugs. All of the 183 drug-resistant cases were resistant to rifampicin.
The proportion of males and females in this study was 70.4% (1219/1732) and 29.6% (513/1732), respectively, with mean, median, and mode ages of 31.77, 27, and 20 years, respectively, without statistical significance (P = 0.59) (Figure 2).
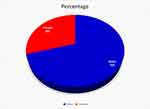 |
Figure 2 Gender of all TB patients in the study. |
Among the 183 drug-resistant cases, 81.4% (149/183) were newly diagnosed DR cases and 18.6% (34/183) of them were previously treated patients. Among the previously treated cases, 61.7% (N = 21; 11.5% of the total DR cases) relapsed, 29.4% (N = 10; 5.5% of the total DR cases) were first-line treatment failed cases, and 8.8% (N = 3; 1.6% of the total DR) were lost to follow-up cases (Table 1).
 |
Table 1 Registration Groups of the MDR TB Patients |
The age of our patients ranged from 1 to 90 years, with a mean, median, and mode of 31.77, 27, and 20 years, respectively. More than two-thirds of the smear-positive MTB patients (73.2%; N = 1267/1732) were under the age of forty, and more than half of them (55.5%; N = 961/1732) were teenagers and young adults with an age range between 10 and 29 years (Table 2).
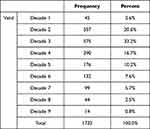 |
Table 2 Age Groups of All Pulmonary TB Patients in the Study |
About two-thirds of drug-resistant subjects (66.7%; N = 122/183) were under the age of 30 years, and more than 84.2% (N = 154/183) of them were under the age of 40 years (Table 3).
 |
Table 3 Age Groups of All Rifampicin Resistant TB Patients |
The male proportion of people who have smear-positive TB was more than that of the female (70.4% versus 29.6%, respectively). A similar proportion of men and women (71.6% versus 28.4%, respectively) had drug-resistant isolates. However, these differences were not statistically significant (P = 0.46). So, we did not get a statistically significant relationship between drug resistance and the age of the patients.
Overall, the TB/HIV co-infection rate in this study was 2% (34/1732). We have not analyzed the relationship between drug resistance development and previous treatment because of a lack of documentation that differentiates previously treated patients from newly diagnosed TB patients in some TB centers.
Discussion
The prevalence of MDR in our study was 1.96%. This is similar to a study conducted in Kenya between September 2015 and August 2016, which found 1.5% of patients to have multi-drug resistance TB.13 A similar prevalence of 1.69% of MDR was reported in a cross-sectional study that was carried out at Mulago national referral hospital in Kampala, Uganda,14 and in Zimbabwe, the overall prevalence of MDR-TB was 2.0%.15
Our study, when compared to the literature, showed a five-times lower prevalence than that reported in seven regional TB laboratories in Ethiopia from July 2017 to June 2018, where the resultant prevalence of MDR-TB was 11.6%.16 It was also lower than that reported in Addis Ababa, Ethiopia, from August 1, 2017 to January 5, 2018, which showed a prevalence of MDR of 11.54%.17
It is lower than that reported from a Botswana public health facility-based study between January 2013 and December 2014, which noted an MDR/RR-TB prevalence of 5.4%.4 In Ghana, MDR prevalence was reported as being 3.2%.18 Overall, the DR-TB prevalence in this study was 10.56%, which is lower than that reported in a national survey conducted in 2010–2011 in Somalia, which showed a DR TB prevalence of 18.6%.9
In Zimbabwe, a national survey reported a prevalence of RR TB of 2%.15 A retrospective review of data in Dalian, Liaoning Province of China, conducted between January 2012 and December 2015, reported a higher MDR prevalence of 10.1%.19,20
In Makkah, Saudi Arabia, the prevalence of drug resistance among TB patients admitted to one hospital from January 2009 to January 2019 was found to be 17.1%,21 which is slightly higher than that in our study. Although it was not statistically significant, the proportion of newly diagnosed DR cases was three times higher than that previously treated (78.3% and 21.7%, respectively), and this was a coincidence with that reported in the Ghana National Survey (72.2% and 27.8%, respectively)18 and Hangzhou, China, in 2011, when the proportion of newly diagnosed and previously treated was 76.3% and 23.7%, respectively, and also in 2015, the proportion was 76.9% and 23.1% (new cases and previously treated cases, respectively).7
The proportion of previously treated patients should be high in countries with a high burden of TB. However, the reason for the higher proportion of newly diagnosed patients was not clear, but it may be related to an error in the documentation or some patients may have hidden their previous TB treatment.
More than two-thirds of our study population were males (70.4%) and less than one-third were females (29.6%), which is the same as the proportion reported in Ghana, with a male and female proportion of 69.6% and 30.4%, respectively.18 Egypt22 reported the same male and female proportions of 67.5% and 32.5%, respectively. In Tigray, Ethiopia, reported similar results (65.3% males and 34.7% females).23
The majority of our study population were young adults, which is consistent with other literature. Overall, the TB/HIV co-infection rate in this study was 2% (34/1732) and this result co-exists with a recent study conducted in our country which reported HIV prevalence among TB patients at 1.5%.24
The reason for the lower MDR prevalence among TB patients in Banadir, Somalia, was not clear to us, but it may be related to the shortage of LPA or phenotypic DST availability in the country, which was available only in three regions in the country at large.
There were several limitations to our study, including that available demographic variables were limited to age, gender, residence, HIV status, and drug sensitivity. However, we did not get enough documented data for previously and newly treated patient categories in non-DR TB patients, smoking history, marital status, and presentation symptoms.
Extensive research is needed to determine the impact, risk factors, and outcomes of drug-resistant TB and related factors such as rural or urban residence, education level, marital status, smoking history, and other comorbidities. Comprehensive research about patterns of resistance to TB, MDR-TB, Poly-DR TB, and XDR TB is needed.
Conclusion
The prevalence of the MDR in this country is low, but it is similar to the neighboring countries. The majority of the TB patients and MDR patients were males. The MDR prevalence was higher in newly diagnosed patients when compared to previously treated patients. More than half of our patients were teenagers and young adults, with an average age group of 10 to 39 years.
Data Sharing Statement
Data supporting the findings of this study are also available upon request from the corresponding author.
Ethical Approval and Consent to Participate
We obtained ethical approval from our hospital’s Medical Research Ethical Committee and the National TB Program before beginning this study, and both waived informed consent from study participants because this was a retrospective study.
Acknowledgments
We thank Banadir TB centers’ staff, staff of the National TB Program and the education department of our hospital for their support. We would like to send special thanks to Dr. Hafsa Abdullahi Ahmed, director of Finsoma TB Center, and Dr. Ali Akhyar, Director of Saa’id TB Center, for their special support in the data collection period.
Author Contributions
All authors made a significant contribution to this work, whether in the conception, study design, execution, acquisition of data, analysis and interpretation, also all of them took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
There are no funding sources for this work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. World Health Organization. Global Tuberculosis Report [Internet]; 2021. Available from: https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2021.
2. World Health Organization (WHO). WHO Report on TB 2020 [Internet]. Vol. 1. World Health Organization; 2020.
3. World Health Organization (WHO). Operational handbook on tuberculosis [Internet]. WHO operational handbook on tuberculosis Module 5; 2022. 45. Available from: https://apps.who.int/iris/bitstream/handle/10665/340256/9789240022614-eng.pdf.
4. Tembo BP, Malangu NG. Prevalence and factors associated with multidrug/rifampicin resistant tuberculosis among suspected drug resistant tuberculosis patients in Botswana. BMC Infect Dis. 2019;19(1):1–8. doi:10.1186/s12879-019-4375-7
5. Rudnicka E, Napiera P, Pod A, Smolarczyk R, Grymowicz M. Since January 2020 Elsevier has created a COVID-19 resource centre with free information in English and Mandarin on the novel coronavirus COVID- 19. The COVID-19 resource centre is hosted on Elsevier Connect, the company’s public news and information; 2020.
6. Howard NC, Khader SA. Immunometabolism during Mycobacterium tuberculosis Infection. Trends Microbiol. 2020;28(10):832–850. doi:10.1016/j.tim.2020.04.010
7. Li Q, Zhao G, Wu L, et al. Prevalence and patterns of drug resistance among pulmonary tuberculosis patients in Hangzhou, China. Antimicrob Resist Infect Control. 2018;7(1):4–9. doi:10.1186/s13756-018-0348-7
8. Boyer-Cazajous G, Martinaud C, Déhan C, et al. High prevalence of multidrug resistant tuberculosis in Djibouti: a retrospective study. J Infect Dev Ctries. 2014;8(2):233–236. doi:10.3855/jidc.3837
9. Sindani I, Fitzpatrick C, Falzon D, et al. Multidrug- Resistant. Emerg Infect Dis. 2013;19(3):1–3. doi:10.3201/eid1901.120393
10. Yao C, Guo H, Li Q, et al. Prevalence of extensively drug-resistant tuberculosis in a Chinese multidrug-resistant TB cohort after redefinition. Antimicrob Resist Infect Control. 2021;10(1):1–8. doi:10.1186/s13756-021-00995-8
11. Dirie AMH, Özdemir H, Hussein AM, Warsame AM. Empyema necessitans mimicking mesothelioma; unusual presentation of active pulmonary TB in immuno-competent state. Ann Med Surg. 2022;1:75.
12. World Health Organization. Global Tuberculosis Report 2021 [Internet]. Geneva: World Health Organization; 2021. Available from. https://www.who.int/publications/i/item/9789240037021.
13. Otieno OC, Nyamache AK, Nonoh J, Amukoye E. Prevalence and detection of drug resistant mutations in mycobacterium tuberculosis among patients visiting selected health centres in Nairobi, Kenya. BMC Infect Dis. 2018;19:1–7.
14. Kateregga NJ, Atuheire C, Ntambi S, Ocaka D, Ndukui GJ, Wampande E. Prevalence of multidrug resistant mycobacterium tuberculosis and risk factors among youth attending MDR-TB unit in mulago hospital. J Bacteriol Mycol. 2018;5(9):1088.
15. Timire C, Metcalfe JZ, Chirenda J, et al. Prevalence of drug-resistant tuberculosis in Zimbabwe: a health facility-based cross-sectional survey. Int J Infect Dis. 2019;87:119–125. doi:10.1016/j.ijid.2019.07.021
16. Diriba G, Kebede A, Tola HH, et al. Surveillance of drug resistance tuberculosis based on reference laboratory data in Ethiopia. Infect Dis Poverty. 2019;8(1):4–9. doi:10.1186/s40249-019-0554-4
17. Sinshaw W, Kebede A, Bitew A, et al. Prevalence of tuberculosis, multidrug resistant tuberculosis and associated risk factors among smear negative presumptive pulmonary tuberculosis patients in Addis Ababa, Ethiopia. BMC Infect Dis. 2019;19(1):1–15. doi:10.1186/s12879-019-4241-7
18. Sylverken AA, Kwarteng A, Twumasi-Ankrah S, et al. The burden of drug resistance tuberculosis in Ghana; results of the First National Survey. PLoS One. 2021;16(6):1–14. doi:10.1371/journal.pone.0252819
19. Lv XT, Lu XW, Shi XY, Zhou L. Prevalence and risk factors of multi-drug resistant tuberculosis in Dalian, China. J Int Med Res. 2017;45(6):1779–1786. doi:10.1177/0300060516687429
20. He GX, Zhao YL, Jiang GL, et al. Prevalence of tuberculosis drug resistance in 10 provinces of China. BMC Infect Dis. 2008;8:1–8. doi:10.1186/1471-2334-8-166
21. Sambas MFMK, Rabbani U, Al-Gethamy MMM, et al. Prevalence and determinants of multidrug-resistant tuberculosis in Makkah, Saudi Arabia. Infect Drug Resist. 2020;13:4031–4038. doi:10.2147/IDR.S277477
22. Emam SA, Kasem EM, Sedhom AE. Characteristics of multidrug resistant tuberculosis in minia, Egypt. Medico Legal Updat. 2020;20(1):446–452.
23. Welekidan LN, Skjerve E, Dejene TA, et al. Characteristics of pulmonary multidrug-resistant tuberculosis patients in Tigray Region, Ethiopia: a cross-sectional study. PLoS One. 2020;15(8):1–20. doi:10.1371/journal.pone.0236362
24. Dirie AMH The prevalence of HIV among tuberculosis patients in Benadir, Somalia. Retrospective multi-center study The prevalence of HIV among tuberculosis patients in Benadir. Somalia: Retrospective multi-center study; 2022.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
