Back to Journals » Research and Reports in Tropical Medicine » Volume 10
Prevalence of intestinal parasites and its risk factors among food handlers in food services in Nekemte Town, West Oromia, Ethiopia
Authors Eshetu L, Dabsu R , Tadele G
Received 6 September 2018
Accepted for publication 5 February 2019
Published 8 May 2019 Volume 2019:10 Pages 25—30
DOI https://doi.org/10.2147/RRTM.S186723
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Mario Rodriguez-Perez
Legesse Eshetu,1 Regea Dabsu,2 Geletta Tadele2
1Department of Microbiology, Nekemte Regional Laboratory, Nekemte, Ethiopia; 2Department of Medical Laboratory Sciences, Institute of Health Sciences, Wollega University, Nekemte, Ethiopia
Background: Food-borne diseases are a public-health problem in developed and developing countries. The World Health Organization (WHO) estimated that in developed countries, up to 30% of the population suffers from food borne diseases each year and more in developing countries, where up to 2 million deaths are estimated per years.
Objective: To assess the prevalence of intestinal parasites and its associated risk factors among food handlers in Nekemte town.
Methods: A cross-sectional study was conducted in Nekemte from April to May, 2016. A total of 240 food handlers were selected using a simple random-sampling technique from hotels, bars, and restaurants. Data were collected using pretested questions and stool-specimen examination for intestinal parasites. For intestinal parasites, stool-sample examinations were done using wet-mount and concentration methods. Data analysis was done using SPSS version 20. Associations among intestinal parasites and risk factors were determined using logistic regression and P
Results: The prevalence of intestinal parasites in this study was 52.1%. Entamoeba histolytica/dispar was the most predominant parasite (56.8%), followed by Ascaris lumbricoides (26.4%), Taenia saginata (16%), and hookworm (16.8%). Hygienic practice such as hand washing after toilet by water, hand washing after toilet by water and soap, trimming of finger nail, wearing proper working clothes and shoes were statistically significant with intestinal parasitic infection if not regularly performed (P<0.05).
Conclusion: The prevalence of intestinal parasites in this study was high, with single double, and triple infection. Food handlers should practice safe food preparation and food service to reduce the probability of intestinal parasite infection.
Keywords: food handlers, intestinal parasite, Nekemte town
Introduction
In developing countries, where there are poor regulations for food hygiene, food handlers are appointed in food and drinking establishments without investigating their health status for the common intestinal parasite.1 The majority of asymptomatic individuals for parasitic infections can be considered dangerous to society, because such food handlers routinely practice their jobs without giving due attention to the transmission of infections. Intestinal parasites can be transmitted to consumers directly or indirectly through food, water, nails, and fingers from food handlers.2,3
Studies have been done on food handlers to investigate the prevalence of intestinal parasites and associated risk factors. A study in west Iran indicated that among food handlers, about 9% stool specimens were positive for different intestinal parasites, which included G. lamblia 2.9%, Entamoeba coli 4.3%, Blastocystis spp. 1.4%, and Hymenolepis nana 0.5%. A valid health card, awareness of transmission of intestinal parasites, and participation in training courses in environmental health with intestinal parasite were negatively associated factors.4
Also, in a study done in Gambia, about 250 (46.3%) food handlers were intestinal parasite carriers. From the identified parasites, the majority were Entamoeba histolytica/dispar (150 [46%]), followed by G. lamblia (52 [16%]), E. coli (40 [12.3%]), Entamoeba hartmanii 20 ([6.1%]), Strongyloides spp. (18 [5.5%]), Ascaris lumbricoides (14 [4.3%]), Iodamoeba bütschlii (nine [2.8%]), and Taenia spp. (six [1.8%]). Among the risk factors, living with domestic animals, lack of training in food handling, and handwashing practices was associated risk factors of intestinal parasite infections.5
In a study done at Jimma University Specialized Hospital cafeteria food handlers, among 148 samples, 33% were positive for one or more intestinal parasites, of which the most prevalent identified was A. lumbricoides (16%), followed by E. histolytica/dispar (4.3%). Parasitic infection and food handlers who did not practice handwashing after defecation or before serving food were positively associated.6 According to studies done in southern Ethiopia, about 36% of food handlers were found to be positive for different intestinal parasites, with the most abundant being E. histolytica/dispar (14%), followed by A. lumbricoides (9.27%).7
Therefore, the carrier states of humans are of concern to food-manufacturing and food-service institutions, because of the perceived risk of contamination of food by infected food handlers and the risk of food-borne disease outbreaks. The prevalence of these intestinal parasites is different in different areas of the country. In addition to geographical conditions, participation in different awareness programs might be another factor that needs investigation in this study area. This study aimed to assess the prevalence of intestinal parasites and associated risk factors among food handlers in Nekemte town from April to May, 2016.
Methods
The study was conducted in Nekemte town, East Wollega zone, west Oromia, Ethiopia from April to May, 2016. Nekemte is located about 331 km from the capital city, Addis Ababa, the specific location 90°14’ north latitude and 36°30’ east longitude, and has an altitude range of 1,960–2,170 m above sea level. Nekemte is classified into six sub-administrative cities, with a total population of 104,806 and an area of 1,962 ha. In Nekemte, there were 55 hotels (Kasso sub-city 13, Darge sub-city 1, Bakkanisa Kasse subcity 25, Chelaleki sub-city 12, Burka Jato sub-city four) and 51 bars and restaurants (Darge 5, Bakkanisa Kasse 11, Chelaleki 12, Burka Jato 18, Bakka Jamaa 5) during the study period (data lists obtained from each subcity administration office). A total of 527 food handlers were working in these food-service establishments (obtained from Nekemte Town Health Office, Regulatory Department).
A cross-sectional study design was used. Source populations were all food handlers who prepare and serve food in the kitchens of different hotels, bars and restaurants found in Nekemte. All food handlers preparing and/or serving food for consumers in hotels, bars and restaurants found in Nekemte during the study period were included. A total of 240 food handlers were included in the study, selected using a simple random-sampling technique. To determine sample size from each food-service establishment (55 hotels and 51 bar and restaurants), proportional random allocation was used.
To collect data, structured questions on background information and risk-factor assessment were developed to a standardized level by translation to the local language (Afan Oromo). To check the correctness of the questions and need for modification, 24 (10%) of the required sample size were checked in the nearest place Diga town which is nearest to Nekemte. With pretested questions, study participants were interviewed to collect data on sociodemographic and related risks factors: certification of food training (it is food service–management certification that helps to ensure that food-service operators take proper precautions to minimize the spread of food-borne illness) and medical checkup (diagnosis of health status of food handlers in health institutions at least every 3 months).
After the questionnaire had been completed, appropriate instructions were given to the food handlers on how to collect stool specimens. Each food-handler specimen-collection container was labeled with a specific code for the purpose of identification. Stool samples provided by participants were processed for direct microscopy and concentration. Using an applicator stick, a fresh specimen was emulsified with normal saline and iodine solution on the slides and became ready for microscopic examination. Then, cysts, trophozoites, and eggs of intestinal parasites were examined directly under microscopy. Stool samples were emulsified in formol water, the suspension discarded to remove large fecal particles, ether or ethyl acetate added, and the mixed suspension centrifuged. Cysts and eggs were fixed and concentrated for microscopic examination.
In addition, a small portion of fresh stool samples were processed for detection of opportunistic parasites using the Ziehl–Neelsen method. Thin smears were prepared directly from sediments of concentrated stools and allowed to air-dry. Slides were then fixed with methanol for 5 minutes and stained with carbol fuchsin for 30 minutes. After slides had been washed in tap water, they were decolorized with acid alcohol for 1–3 minutes and stained with methylene blue for 1 minute. Slides were then washed in tap water and observed under light microscopy with a magnification of 100×.8
For data quality, training was given to two senior laboratory professionals on how to complete the structured questionnaire with accuracy. Data quality was ascertained through review of all data-collection formats and follow-up of all stages of quality control in data collection. All stages of laboratory-test processes were managed according to available job aids. The accuracy of test results in identification of intestinal parasites was increased by conducting two smear examinations using physiological saline and using iodine solution. Additionally, one formalin-concentrated smear examination and one stained slide by modified acid-fast stain using formalin-concentrated samples was examined. Data analysis was done using SPSS version 20. Association between intestinal parasites with risk factors was determined usinglogistic regression and P<0.05 was considered significant.
The study was approved by the Wollega University Research and Ethics Review Board in accordance with the Declaration of Helsinki. Then, an official permission letter was obtained from Wollega University and Nekemte municipality. Data were collected after written informed consent had been obtained from all study participants. For those aged <18 years, their parents were contacted for their assent to participate in the study and provide written informed consent. An ethical issue that may have arisen from the results of this study was further circumvented by ensuring that the names of establishments and individuals were not mentioned. Being in collaboration with the town health office, food handlers who were positive for helminthes infections were treated free of charge. Those positive for protozoa were given prescriptions to be treated.
Results
From the total food handlers, about 159 (66.3%) were in the age group of 20–40 years. Mean age of food handlers was 24 years(range 12–59 years). Of 240 participants, 109 (45.4%) were male and 131 (54.6%) were female. One hundred and twenty four ( 51.7%) of food handlers had educational status of grade 7–12. Only 20 (8.3%) food handlers had not had any formal education. The percentage of parasite infectivity decreased proportionally with age category among the food handlers: <20 years 54.2%, 20–40 years 51.6%, and >40 years 44.4% intestinal parasites were detected. In sum, 54.1% of males and 50.4% females were infected with intestinal parasites. For the education level, >12 years 52.9%, 7–12 years 50.0%, 1–6 years 58.1%, and of the illiterate 45.0% were infected with intestinal parasites. Sociodemographic factors, such as age, sex, and education level, had no statistically significant association with intestinal parasitic infection (P>0.05, Table 1).
  | Table 1 Sociodemographic factors of food handlers in food services in relation to parasite positivity in Nekemte town from April to May, 2016 (n=240) |
The prevalence of intestinal parasites in this study was 125 of 240 (52.1%). Among those positive individuals, 104 of 125 (83.2%) had a single infection, 19 of 125 (15.2%) double infection, and two of 125 (1.6%) triple infection (Figure 1). E. histolytica/dispar was the most predominant parasite (71 of 125 [56.8%]), followed by A. lumbricoides (33 of 125 [26.4%]), hookworm (21 of 125 [16.8%]) and Taenia saginata (20 of 125 [16%]). G. lamblia (two of 125 [1.6%]) and S. mansoni (one of 125 [0.8%]) were found less commonly (Table 2).
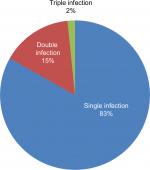  | Figure 1 Type of parasitic infection among food handlers in Nekemte town from April to May, 2016 (n=240). |
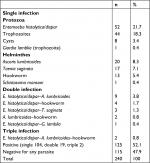  | Table 2 Types of parasite recovered from food handlers in food services in Nekemte town from April to May, 2016 (n=240) |
The wet-mount method is more effective for protozoan infection (100%) than helmenthic infection (81%). In contrast, the concentration method is more effective for helmenthic infection (100%) than protozoan infection (18%), due to the destruction of trophozoites during centrifugation. From a total of 240 food handlers examined, the consistency of stool specimens showed statistically significant association (P=0.015) with intestinal parasite infection. Among those taking medical checkups, 45.2% were positive for intestinal parasites, and of those not taking medical checkups, 53.5% were positive for intestinal parasites. Of those who had been certified and those who had not, 63.2% and 51.1% were infected with intestinal parasites, respectively. Of those with >2 service year, 1–2 service year, and <1 service year, 46.2%, 44.0%, and 60.8% were infected with intestinal parasites, respectively (Figure 2).
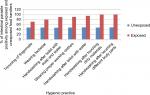  | Figure 2 Intestinal parasite positivity (infection) among unexposed and exposed categories of hygienic practice among food handlers. |
There was statistical significance between personal hygienic conditions of food handlers and intestinal parasitic infection ( all P<0.05) ( Table 3). Most of those who did not use hygienic practices were infected by intestinal parasites greatly: those who did not wash their hands after touching different body parts 100%, after touching dirty materials 100%, and after using the toilet 94.7%, and those who did not wear proper working clothes 90%. Most hygienic practices, such as handwashing with water after using the toilet by, handwashing with water and soap after using the toilet, trimming finger nails, and wearing proper working clothes and shoes were significantly associated with intestinal parasitic infection if not regularly performed (P<0.05, Table 3).
  | Table 3 Hygiene practices of food handlers in food services in relation to parasite positivity in Nekemte town from April to May, 2016 (n=240) |
Discussion
The prevalence of intestinal parasites in this study was 52.1% (125 of 240). This prevalence was higher than a study done in Nigeria.9 This may be due to differences in sociodemographic factors. This study was somewhat closer to a study done in Bahir Dar town, northwest Ethiopia, in which the prevalence of intestinal parasites among food handlers was 41.1%.10 The prevalence of intestinal parasites in this study is comparable to a study conducted in the Mekelle University student cafeteria (49.4%).11 The present prevalence shown in the current study was higher than studies conducted in Amritsar, India (12.9%),12 Eastern Province, Saudi Arabia (9.3%),13 and Sai-Yok District, Kanchanaburi Province, Thailand (10.3%).14
Among positive cases, 104 of 125 (83.2%) were a single infection, 19 of 125 (15.2%) double infection, and two of 125 (1.6%) triple infection among food handlers examined. The results of this study are similar to a study conducted in Jubail, Saudi Arabia, in which single infection was 83.94%, double infection 11.51%, and triple infection 1.55%.15
In the present study, E. histolytica/dispar was the most predominant parasite (71 of 125 [56.8%]), followed by A. lumbricoides (33 of 125 [26.4%]), hookworm (21 of 125 [16.8%]), and T. saginata (20 of 125 [16%]). G. lamblia (two of 125 [1.6%]) and S. mansoni (one of 125 [0.8%]) were less prevalently found. In contrast to this, in a study conducted at a tertiary-care hospital in Nigeria, E. histolytica (4.5%) (next to G. lamblia [9%]) was the second-most predominant parasite detected.9 This difference might be due to geographic and sociodemographic differences among the food handlers. Our results are also in agreement with the study conducted in Bahir Dar town, northwest Ethiopia, in which E. histolytica (12.76%) and A. lumbricoid (11.7%) were the predominant parasites10 and also similar to the study conducted at Gondar University, northwest Ethiopia, in which A. lumbricoid (6.5%) and E. histolytica (6.0%) were the predominant parasites.16
This research has revealed that most hygienic practices, such as handwashing with water after toilet use, handwashing with water and soap after toilet use, handwashing after touching dirty materials, trimming of finger nails, wearing of footwear, wearing proper working clothes, and handwashing after touching different body parts had statistically significant values (P<0.05) as a determinant factor for intestinal parasite infection. This study result shows similarity with other studies11,17,18 that identified personal hygiene like poorly kept nails, dirty working clothes, lack of footwear, and handwashing practices as the determinant factors for intestinal parasite infection.
The scope of this study was limited to hotels, bars, and restaurants. Food handlers working in universities, hospitals, and butchers and those who sell coffee and tea were not included, which needs further study. There was no technique used to differentiate E. histolytica and E. dispar.
Conclusion
The prevalence of intestinal parasites in this study was 52.1%. Among these positive cases, 82.4% were a single infection, 16% double infection, and 1.6% triple infection. E histolytica/dispar was the most predominant parasite (56.8%), followed by A. lumbricoides (26.4%), T. saginata (16.8%), and hookworm (16.8%). This research has revealed that hygienic practices, such as handwashing, trimming fingernails, wearing footwear, and wearing proper working clothes, were determinant factors for reduction of intestinal parasite infection. Food handlers should practice safe handling of food in preparation and service.
Acknowledgment
We would like to acknowledge all of the food-handler participants, data collectors, and health extension workers in the study area, and also Wollega University for funding this study.
Disclosure
The authors report no conflicts of interest in this work.
References
Mudey AB, Kesharwani N, Mudey GA, Goyal RC, Dawale AK, Wagh VV. Health status and personal hygiene among food handlers working at food establishment around a rural teaching hospital in Wardha district of Maharashtra, India. Glob J Health Sci. 2010;2(2):198. | ||
Saeed HA, Hamid HH. Bacteriological and parasitological assessment of food handlers in the Omdurman area of Sudan. J Microbiol Immunol Infect. 2010;43(1):70–73. | ||
Tessema AG, Gelaye KA, Chercos DH. Factors affecting food handling practices among food handlers of Dangila town food and drink establishments, North West Ethiopia. BMC Public Health. 2014;14(1):571. | ||
Kheirandish F, Tarahi MJ, Ezatpour B. Prevalence of intestinal parasites among food handlers in Western Iran. Rev Inst Med Trop Sao Paulo. 2014;56(2):111–114. | ||
Jallow HS, Kebbeh A, Sagnia O, et al. High prevalence of intestinal parasite carriage among food handlers in the Gambia. Int J Food Sci Biotech. 2017;2(No. 1):1–5. | ||
Girma H, Beyene G, Mekonnen Z. Prevalence of intestinal parasites among food handlers at cafeteria of Jimma University Specialized Hospital, Southwest Ethiopia. Asian Pac J Trop Dis. 2017;7(8):467–471. | ||
Mama M, Alemu G. Prevalence and factors associated with intestinal parasitic infections among food handlers of southern Ethiopia: cross sectional study. BMC Public Health. 2015;16:105. | ||
Cheesbrough M. District Laboratory Practice in Tropical Countries. Cambridge: Cambridge University Press. 2006. Part II. | ||
Zaglool DA, Khodari YA, Othman RAM, Farooq MU. Prevalence of intestinal parasites and bacteria among food handlers in a tertiary care hospital. Niger Med J. 2011;52(4):266. | ||
Abera B, Biadegelgen F, Bezabih B. Prevalence of Salmonella typhi and intestinal parasites among food handlers in Bahir Dar Town, Northwest Ethiopia. Ethiop J Health Dev. 2010;24(1). | ||
Nigusse D, Kumiem A. Food hygiene practices and prevalence of intestinal parasites among food handlers working in Mekelle university student’s cafeteria, Mekelle. Global Adv Res J Soc Sci. 2012;1(4). | ||
Senthilkumar B, Prabakaran G. Multidrug resistant Salmonella typhi in asymptomatic typhoid carriers among food handlers in Namakkal district, Tamil Nadu. Indian J Med Microbiol. 2005;23(2):92. | ||
Qodri MH, Ai-Gamdi MA, Al-Harfi RA. Asymptomatic Salmonella, Shigella and Intestinal Parasites Among Primary School Children in the Eastern Province. J Family and Community Med. 1995;2(2):36–40. | ||
Teera K, Wanna M, Supaporn N, et al. Parasitic and enteric bacterial infections among food handlers in Tourist-area restaurants and Educational-institution Cafeterias, Sai-Yok district, Kanchanaburi Province, Thailand. Trop Med Parasitol. 2011;34. | ||
Al-Ghamdi KS. Parasitic and bacterial infestation among food handlers in Jubail, eastern region of Saudi Arabia. J Family Community Med. 1996;3(2):64–70. | ||
Birhaneselassie M, Williams D. A study of Salmonella carriage among asymptomatic Food-Handlers in southern Ethiopia. Int J Nutr Food Sci. 2013;2(5):243–245. | ||
Mohan U, Mohan V, Raj K. A study of carrier state of S. Typhi, intestinal parasites & personal hygiene amongst food handlers in Amritsar city. Indian J Community Med. 2006;31(2):60–61. | ||
Smith SI, Agomo CO, Bamidele M, Opere BO, Aboaba OO. Survey of food handlers in bukas (a type of local restaurant) in Lagos, Nigeria about typhoid fever. Health. 2010;2(8):951–956. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
