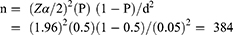Back to Journals » Research and Reports in Tropical Medicine » Volume 12
Prevalence of Intestinal Parasites and Associated Factors Among Psychiatric Patients Attending Felege Hiwot Comprehensive Specialized Referral Hospital, Northwest Ethiopia
Authors Agmas A, Alemu G , Hailu T
Received 4 March 2021
Accepted for publication 15 April 2021
Published 4 May 2021 Volume 2021:12 Pages 51—61
DOI https://doi.org/10.2147/RRTM.S308666
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Mario Rodriguez-Perez
Aster Agmas,1 Getaneh Alemu,2 Tadesse Hailu2
1Department of Parasitology, Felege Hiwot Comprehensive Specialized Referral Hospital, Bahir Dar, Ethiopia; 2Department of Medical Laboratory Science, Bahir Dar University, Bahir Dar, Ethiopia
Correspondence: Getaneh Alemu
Department of Medical Laboratory Science, Bahir Dar University, PO Box: 79, Bahir Dar, Ethiopia
Tel +251 922842776
Email [email protected]
Background: Intestinal parasitic infections are among the major public health problems in resource-poor countries such as Ethiopia. Certain segments of the population such as psychiatric patients are believed to be at higher risk of infection because of their poor hygiene practices. However, information on the prevalence and contributing factors among psychiatric patients is limited in Ethiopia.
Methods: A facility-based cross-sectional study was conducted among 432 psychiatric patients attending Felege Hiwot Comprehensive Specialized Referral Hospital, Northwest Ethiopia from May to July 2020. Participants were selected using a systematic random sampling technique. Data on socio-demography and associated factors were collected using a pre-tested structured questionnaire. Stool sample was collected and processed for parasitological examination using direct wet mount, modified Richie’s concentration, Kato-Katz and modified Ziehl-Neelsen staining techniques following standard protocols. Data were entered and analyzed using Statistical Package for the Social Sciences software version 20. The prevalence of intestinal parasitosis and associated factors were computed by descriptive statistics and binary logistic regression at 95% confidence interval (CI), respectively. Association between variables was considered statistically significant only if P-value < 0.05 at 95% confidence level.
Results: A total of 168 (38.9%) participants were positive for at least a single species of intestinal parasite. Hookworm, Entamoeba histolytica/Entamoeba dispar and Ascaris lumbricoides were the most frequent parasites, detected in 74 (17.1%), 47 (10.9%) and 37 (8.6%) participants, respectively. Double infection was detected in 16 (9.5%) participants where 8 (4.8%) had hookworm and Ascaris lumbricoides co-infections. Not washing raw fruits and vegetables before eating (adjusted odds ratio = 8.402; 95% CI: 3.055– 23.109; P < 0.001) and having a psychiatric diagnosis other than bipolar disorder (adjusted odds ratio = 3.104; 95% CI: 1.334– 7.222; P = 0.009) were significantly associated with intestinal parasitic infections.
Conclusion: The prevalence of intestinal parasites among psychiatric patients was significant. Therefore, improving hygiene practice and implementing regular screening for intestinal parasitic infection among psychiatric patients are recommended so as to control parasite transmission and improve the health of psychiatric patients.
Keywords: intestinal parasites, psychiatric patients, Bahir Dar
Background
Intestinal parasitic infections (IPIs) are common public health problems globally with the majority of cases and deaths occurring in tropical countries.1 Annually, 16 million deaths occur due to parasitic diseases worldwide.2 Soil-transmitted helminths (STHs) infect 24% of the world’s population.3 Among protozoan parasites, Entamoeba histolytica and Giardia duodenalis infect 500 million and 280 million people worldwide, respectively.4,5 In Ethiopia, intestinal parasites (IPs) are widely distributed and affect various segments of the population.6
Transmission of IPIs is significantly linked to poor hygiene and sanitary conditions, tropical climate and poor access to safe water. Moreover, person to person transmission is much more common in overcrowded living conditions and institutions such as schools, day care centers, orphanages and rehabilitation centers.7 Mentally ill individuals are considered to be vulnerable to IPIs as many people with such disabilities cannot be trained for proper health behaviors.8,9 As a result, they do not practice hygiene and sanitary activities and IPIs have been a major problem in rehabilitation centers for psychiatric patients.10
Intestinal parasitic infections are known to cause diarrhea, nausea, vomiting, anorexia, abdominal pain and tenesmus.11,12 Chronic infections can bring serious complications such as prolonged diarrhea, anemia, reduced physical and mental development and malnutrition.7,12 Considering the burden of intestinal parasites (IPs), the Federal ministry of health has been taking strong measures in order to control these diseases. The health extension, water sanitation and hygiene (WASH), and mass drug administration (MDA) programs for selected parasites have been implemented strategies.13 These programs primarily target school-age children, who are believed to be at higher risk of infection and development of complications. However, other vulnerable and high-risk segments of the population such as psychiatric patients have been forgotten and left untreated by MDA for STHs.
Some characteristics of psychiatric patients such as poor mobility, poor cooperation with hygienic measures, and frequent interaction with each other contribute to easy transmission of IPs.14 The prevalence of IPs among these patients has been reported to range from 5% to 35.2% or even more.15,16 In addition to their direct health impact, IPIs might complicate a psychiatric health condition as chronic helminth infections add to the underlying cause in affecting cognitive development. Therefore, intervention of IP transmission among psychiatric patients contributes for better prognosis of such patients. This will be more important in IP endemic areas such as the Amhara region of Ethiopia where more than half of the general population harbors at least one pathogenic IP.17
Besides, factors contributing for IP transmission should also be determined. Despite this, there is a paucity of such data in Ethiopia in general and in the study area in particular. Hence, the present study aimed to assess the prevalence of intestinal parasites and associated factors among psychiatric patients attending Felege Hiwot Comprehensive Specialized Referral Hospital (FHCSRH) Psychiatry clinic.
Methods and Materials
Study Design, Area and Period
An institution-based cross-sectional study was conducted among psychiatric patients attending FHCSRH, Bahir Dar City, Northwest Ethiopia from May to July 2020. Bahir Dar City is the capital of Amhara National Regional State. The city is at an altitude of 1800 m above sea level and 560 km away in the northwest direction from Addis Ababa, the capital city of Ethiopia. The city has three governmental and three private hospitals, of which FHCSRH is the largest hospital serving around 2.4 million patients per year. The hospital has a psychiatric clinic which gives both inpatient and outpatient services. According to FHCSRH 2019/2020 report, the Psychiatry clinic serves more than 48,000 outpatients and 800 admitted patients per half year.
Sample Size Determination and Sampling Technique
The sample size (n) was calculated using a single population proportion formula. Since there are no previous data for the study area, we used a prevalence of 50% (P = 0.5), 95% CI (Z α/2 = 1.96) and 5% margin of error (d = 0.05) to calculate the sample size.
After adding 15% (58) for non-respondents, the final sample size was 442. Based on data from the hospital records, around 900 psychiatric patients were expected to visit the hospital psychiatric clinic during the data collection period. Hence, systematic random sampling technique with K-value of 2 (900/442) was used to select participants. The first participant was selected on the appointment registration expected to attend on the starting day of the study through a lottery method. Then, every 2nd eligible patient was recruited in the study. All mentally disabled patients who (or whose caregivers) volunteered and could give consent/assent for participation were included in the study. Those participants who were unconscious, critically ill or unable to respond to research questions and those taking anti-parasitic drugs within the last 2 weeks before data collection were also excluded.
Data Collection
Questionnaire Data
Data on socio-demographic characteristics, clinical data and WASH related factors for IPIs were collected using a structured questionnaire. The Amharic version of the questionnaire was administered through face to face interview by trained psychiatric nurses.
Stool Collection and Processing
Study participants were consulted to collect approximately 2 g of stool sample using a wide mouth, leak proof and clean stool cup. Each sample was labeled with a unique code and transported to the Medical Microbiology and Parasitology Laboratory of College of Medicine and Health Sciences immediately after collection. A direct wet mount smear was prepared by mixing a matchstick head amount of fresh stool (2 mg) with a drop of 0.85% saline followed by a drop of iodine. Smears were examined for helminths egg or larvae and protozoan trophozoites and cysts with the low-power (10×) and high power (40x) objectives of a microscope.18
Kato-Katz smear was prepared from each stool sample by placing a small amount of stool on newspaper and a piece of nylon screen was pressed on top and some of the stool was sieved through the screen to accumulate on the top. The upper surface of the screen was scraped with a spatula to collect the sieved stool. A template with a hole capacity of 41.7 mg was placed on a microscope slide and the sieved stool was added with the spatula until the hole of the template was completely filled and excess stool was removed from the edge of the hole. The template was removed carefully and the stool remaining on the slide was covered with a pre-soaked cellophane strip with glycerol. Slides were inverted and pressed against the cellophane strip to evenly spread the stool and then placed on a bench with cellophane upwards to enable evaporation of water while glycerol cleared the stool debris. Smears were microscopically examined within 1 h for the detection of hookworm ova and the examination was repeated after 1 h for the detection of other helminths ova.
Stool from each participant was also processed by the modified Ritchie’s fecal concentration method. First, 2 mL of 10% formalin and 1 mL of ethyl acetate were added to the Ritchie’s tube, respectively. Then 0.5 g of the fecal sample was added and the tube was screwed shut. After a minute, the cover was removed and the filtration concentration unit was introduced and centrifuged at 1000×g for 3 min. The supernatant was discarded and part of the sediment was examined using 10× and 40× objectives.
Modified Ziehl-Neelsen staining technique was used for the detection and identification of intestinal coccidian oocysts. Stool smear was prepared, air-dried, and fixed with absolute methanol for 3 min. Then, the preparation was stained with carbol fuchsin for 15 min and the stain was washed off with tap water. After washing, it was decolorized with 1% acid alcohol for 15 s then again washed off with tap water. Finally, the preparation was stained with 0.5% methylene blue for 30 s, washed off with tap water and dried by placing on a rack, and then the smear was examined for oocysts, using 40x and 100x objectives of a microscope.
Data Quality Control
To maintain the data quality, training was given to data collectors. The questionnaire was translated to Amharic and re-translated back to English. Standard operating procedures were strictly followed during specimen collection, transportation, processing and parasite detection. About 10% of the Kato-Katz slides were randomly selected and checked by a third technician who was blind to the initial results. All the reagents used for processing of stool were checked every week for contamination. The principal investigator checked all aspects of data collection every day.
Statistical Analysis
Data were checked for completeness, entered and analyzed using Statistical Package for Social Science software version 20. Data were summarized as frequencies, prevalence and mean using descriptive statistics. Bivariate logistic regression was used to assess associations between dependent and independent categorical variables. Multivariate logistic regression analysis was then followed for variables with P <0.2 in the bivariate analysis to determine the association between IPIs and associated factors. Association between variables was considered statistically significant only if P <0.05 at 95% CI. The strength of association between exposure and outcome variables were measured through adjusted odds ratios (AOR).
Results
Socio-Demographic Characteristics of Study Participants
Among the total sample size of 442 psychiatric patients, data from 432 participants were complete for analysis. The remaining 10 participants were unable to bring a stool sample. From the total of 432 patients, 231 (53.5%) were male. The participants’ age ranged from 15 to 69 years old with mean age of 34.2 (±11.48 standard deviation) years. Three hundred (69.4%) of the psychiatric patients were urban inhabitants and 164 (38.0%) were with no formal occupation. Regarding their educational status, 198 (45.8%) participants did not attend formal education while 234 (54.2%) had attended primary school or above. Out of the 432 participants, 263 (60.9%) were single and 286 (66.2%) came from households with three or more family members (Table 1).
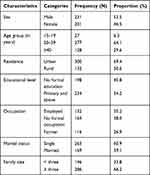 |
Table 1 Socio-Demographic Characteristics of Psychiatric Patients Attending FHCSH, Northwest Ethiopia from May to July 2020 |
Clinical Data of Study Participants
Regarding the causes of mental problem, 115 (26.6%), 64 (14.8%), 57 (13.2%), and 46 (10.6%) participants had schizophrenia, epilepsy, substance disorder, and anxiety disorder, respectively. More than half (52.2%) of the participants were diagnosed within the previous one year (Table 2).
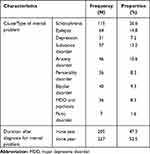 |
Table 2 Causes of Mental Illness and Duration Since Diagnosis of Psychiatric Patients Attending FHCSH, Northwest Ethiopia from May to July 2020 |
Study Participants’ WASH Practice
Among the 432 study participants, 415 (96.1%) and 432 (100%) washed their hands after soil contact and before meals, respectively. Two hundred and sixty-six (61.6%) of the participants used a latrine and the majority (364; 84.3%) of them washed their hand after toilet. Two hundred and eighty-seven (66.4%) and 394 (91.2%) participants used piped water for washing and drinking, respectively. The majority of psychiatric patients (413; 95.6%) had no swimming habit and 259 (59.7%) of the participants frequently wear shoes. Three hundred and ninety (90.3%) participants got their food at home and 181 (41.9%) patients eat raw fruit/vegetables (Table 3).
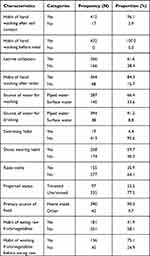 |
Table 3 Utilization of WASH and Related Factors by Psychiatric Patients Attending FHCSH, Northwest Ethiopia from May to July 2020 |
Prevalence of Intestinal Parasitic Infections
Among 432 participants, 168 (38.9%; 95% CI: 34.3–43.5) tested positive at least for a single species of IP by one or more of the diagnostic methods. Eight parasite species, namely hookworm, Ascaris lumbricoides, Trichuris trichiura, Schistosoma mansoni, E. histolytica/E. dispar, G. duodenalis, Cryptosporidium spp., and Hymenolepis nana were detected. Among these IPIs, hookworm was the most prevalent, detected in 74 (17.1%) participants followed by E. histolytica/dispar in 47 (10.9%), A. lumbricoides in 37 (8.6%), and G. duodenalis in 13 (3.0%) (Table 4). Among 168 (38.9%) IP infected psychiatric patients, 16 (9.5%) were co-infected by two parasite species. Of these, hookworm and A. lumbricoides co-infections were predominantly detected in 8 (4.8%) participants (Table 5).
 |
Table 4 Intestinal Parasites Positivity Based on Different Diagnostic Methods in Psychiatric Patients Attending FHCSH, Northwest Ethiopia from May to July 2020 |
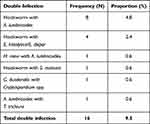 |
Table 5 Double Infection of IPs Among Psychiatric Patients Attending FHCSRH, Northwest Ethiopia from May to July 2020 |
Factors Associated with Intestinal Parasitic Infections Among Psychiatric Patients
In the multivariate logistic regression analysis, washing raw fruits/vegetables before consumption of them raw had a statistically significant association with IPIs among psychiatric patients (AOR = 8.402; 95% CI: 3.055–23.109; P <0.001). The prevalence of IPs among participants who do not wash fruits/vegetables before consuming them raw was 55.6% (25/45) while the prevalence was only 18.4% (25/136) among participants who consumed washed fruits/vegetables. All other considered socio-demographic and WASH related factors were not significantly associated with IPIs in the multivariate logistic regression analysis (Table 6).
 |
Table 6 Logistic Regression of Factors Associated with IPIs Among Psychiatric Patients Attending FHCSRH, Northwest Ethiopia from May to July 2020 |
Association Between Type of Mental Disorder and Intestinal Parasitic Infections
In bivariate logistic regression, being a psychiatric patient caused by other than bipolar disorder was significantly associated with IPIs (COR = 3.286, 95% CI: 1.418–7.611, P= 0.006). Similarly in multivariable logistic regression analysis, participants with a mental problem caused by other than bipolar disorder had a statistically significant association with IPIs (AOR = 3.104, 95% CI: 1.334–7.222, P = 0.009) (Table 7).
 |
Table 7 Bivariate and Multivariate Logistic Regression of Cause of Mental Disorder with Parasitic Infections at FHCSRH, Northwest Ethiopia from May to July 2020 |
Discussion
Addressing the magnitude of IPIs and their associated factors among psychiatric patients is necessary to design appropriate intervention strategies. The high prevalence of IPs in the present study (38.9%, 95% CI: 34.3–43.5) suggested that mental illness directly influences hygiene and sanitary practices. The present finding was in line with a previous study result of 38.4% from Nigeria.19
The present prevalence was higher than findings of 12.45%, 13.5%, 29.5% and 23% from Tanzania,20 Ghana,21 Iran22 and Italy,8 respectively. On the contrary, the present result was lower than study findings of 56.7% and 43.5% from Bahir Dar23 and Egypt,24 respectively. Smaller sample size in the previous studies might account for such differences. For instance, studies in Tanzania,20 Ghana,21 Italy8 and Iran22 recruited only 233, 111, 125 and 173 participants, respectively. Small sample size might result in high sampling error which in turn makes the data less representative of the source population. Variation in age group of the study participants may also contribute to differences in IP prevalence. Laboratory methods used also have a significant role in the difference of IP prevalence across studies. In the present study, a combination of direct wet mount, Kato-Katz, Richie’s concentration and modified Ziehl Neelsen staining were used, which increases the detection rate of microscopy.
Species level analysis revealed that hookworm (17.1%) was the most predominant parasite in the present study. This finding is higher than prevalence of 6.2% and 8.4% in Bahir Dar23 and Nigeria,19 respectively. However, it was lower than prevalence of 37.93% in Tanzania.20 Variations in transmission level across study areas and shoe wearing habit of the population could contribute to the differences. Moreover, variations in sanitation practices and laboratory methods used are responsible factors. In the present study, the second most prevalent intestinal helminth was A. lumbricoides (8.6%) which is in line with the study result of 9.6% in Bahir Dar.23 However, the present prevalence of A. lumbricoides was lower than previous results of 15.3% and 10.4% from Nigeria19 and Italy,8 respectively. The difference might be due to the variation in WASH implementation and endemicity of A. lumbricoides.
The prevalence of S. mansoni infection (1.4%) in the present study was lower than 41.38% prevalence from Tanzania.20 This might be due to variation in the local transmission, frequency of water contact and number of study participants recruited. Only 4.4% of the participants had frequent swimming habit in the present study which contributes to a low prevalence of S. mansoni. On the other hand, the present prevalence was higher than a previous finding of 1.0% in Bahir Dar.23 This variation might be due to difference in the diagnostic techniques, in that Kato-Katz technique was not performed in the previous study.
The prevalence of E. histolytica/E. dispar (10.9%) in the current study was in line with a previous study result of 10.34% in Tanzania.20 On the contrary, the prevalence of E. histolytica/E. dispar in this study was lower compared with findings of 14.4% from Bahir Dar.23 However, it was higher than results of 7.4% and 5.5% from Nigeria19 and Egypt,24 respectively. The prevalence of G. lamblia in the present study was 3.0% which was in line with a previous finding of 3.0% in Nigeria.19 However, it was lower than prevalence of 5.8% and 4.0% from Bahir Dar23 and Northern Iran,22 respectively. Variations in local implementation of WASH activities might be responsible for such differences. Mental health problems are associated with alteration of the immune function meaning that frequency of opportunistic infections are expected to increase. This is supported by previous studies from Ghana21 and Egypt24 where Cryptosporidium spp. were the most frequent parasites found in mentally ill patients. However, examination of Ziehl Neelsen stained stool smears, in the present study, revealed that only three (0.7%) participants were infected with Cryptosporidium spp. It is difficult to explain this because none of the participants had diarrhea and we did not evaluate the immune status of participants.
In the present study psychiatric patients who eat unwashed raw fruits/vegetables were 8.4 times at higher risk of acquiring IP compared with those who eat washed raw fruits/vegetables. This is supported by a study done in Bahir Dar, which showed that the risks of being infected by IPs are increased by 8 times among psychiatric patients consuming unwashed raw fruits/vegetables. Infective stages of many IPs can be collected from raw fruits/vegetables which results in ingestion of parasites while eating.25 Therefore, it is justifiable that psychiatric patients with a habit of consuming unwashed raw fruits/vegetables are at higher risk of parasitic infections. Psychiatric patients with bipolar disorder are known for keeping good personal hygiene and giving value for themselves. This puts them at lower risk for IPIs as confirmed in the present study, in that participants with mental problem other than bipolar disorder were 3.104 times at higher risk of infection compared with those with bipolar disorder.
Of the total infected psychiatric patients, the prevalence of IPs was higher in males than females. However, the difference was not statistically significant, which was similar to findings from Tanzania.20 Contrary findings were reported in recent studies from Italy,8 Ghana21 and Nigeria19 where males were significantly at higher risk of IPIs compared with females. Occupational and socio-cultural variations, beyond the mental problem, account for these differences in the exposure level of male and females in different geographic settings. Similar to sex, all other considered factors, as shown in Tables 6 and 7, were not significantly associated with IPIs, may be due to response bias and undetermined confounders. Because they have mental problems, we could not be sure that the patients respond to each question based on the practical truth and their day to day experiences. This demands a further cohort study in order to obtain a definitive conclusion about factors associated with IPIs among psychiatric patients.
Limitations of the Study
A single stool sample was collected and examined from all study participants due to logistic problems. This might underestimate the true prevalence of IPs among psychiatric patients in the study area.
Conclusions
The prevalence of IPIs among psychiatric patients was significant in the study area. Hookworm was one of the most predominantly identified IPs followed by E. histolytica/E. dispar. Consumption of unwashed raw fruits/vegetables and a psychiatric diagnosis other than bipolar disorder were the factors significantly associated with IPIs. Therefore, these findings should be a wakeup call for policymakers to give attention for psychiatric patients in setting a strategy to control and intervene in IP transmissions. Psychiatry health-care providers should continuously give health education in order to improve the hygiene practices of psychiatric patients. Psychiatry health-care providers should also implement regular screening and treatment for IPIs among psychiatric patients. Other health-care providers should also create awareness about the health impact and means of prevention for their clients, since other population groups will act as sources of infection for psychiatric patients.
Abbreviations
AOR, adjusted odds ratio; CI, confidence interval; COR, crude odds ratio; FHCSRH, Felege Hiwot Comprehensive Specialized Referral Hospital; IPs, intestinal parasites; IPIs, intestinal parasite infections; MDA, mass drug administration; STHs, soil transmitted helminths; WASH, water sanitation and hygiene.
Data Sharing Statement
The original data for this study is available from the corresponding author.
Ethics Approval and Consent to Participate
This study was conducted in accordance with the Declaration of Helsinki - Ethical principles for medical research involving human subjects. The research was carried out after obtaining ethical approval from the institutional review committee of College of Medicine and Health Sciences, Bahir Dar University. Moreover, support letter was obtained from Amhara National Regional State Health Bureau. Permission letter was also secured from FHCSRH. Informed written consent or assent was obtained from participating patients or caregivers, respectively. According to the research ethics guideline of Bahir Dar University, informed verbal consent is accepted to conduct researches where the biological sample collection doesn’t include invasive procedures. Hence, the verbal consent for the present study was approved by the institutional review committee. Laboratory results were promptly communicated to the clinic for treatment of IP positive participants.
Acknowledgments
We thank the ethical review committee of Bahir Dar University, College of Medicine and Health Sciences for giving ethical approval. We also thank Amhara Public Health Institute and FHCSRH for giving permission support during data collection. Our regards also go to the study participants for participating in the study.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
Bahir Dar University funded for data collection. No fund was obtained for data analysis and manuscript preparation.
Disclosure
The authors declare that they have no conflicts of interest for this work.
References
1. Haque R. Human intestinal parasites. J Health Popul Nutr. 2007;25(4):387.
2. Schmunis GA, López‐Antuñano FJ. World‐wide importance of parasites. In: Topley & Wilson’s Microbiology and Microbial Infections; 2010. doi:10.1002/9780470688618.taw0167
3. World Health Organization. Soil-transmitted helminth infections fact sheet. Available from: https://www.who.int/news-room/fact-sheets/detail/soil-transmitted-helminth-infections.
4. Ayed LB, Sabbahi S. Entamoeba histolytica. In: Rose JB, Jimenez-Cisneros B, editors. Global Water Pathogen Project. Michigan: Michigan University, E. Lansing, MI, UNESCO; 2017.
5. Laishram S, Kang G, Ajjampur SSR. Giardiasis: a review on assemblage distribution and epidemiology in India. Indian J Gastroenterol. 2012;3(1):3–12. doi:10.1007/s12664-012-0161-9
6. Mekonnen B, Erko B, Legesse M. Prevalence of intestinal parasitic infections and related risk factors among street dwellers in Addis Ababa, Ethiopia. J Trop Dis. 2014;2(2):1–7. doi:10.4172/2329-891X.1000132
7. Bartsch SM, Hotez PJ, Asti L, et al. The global economic and health burden of human Hookworm infection. PLoS Negl Trop Dis. 2016;10(9):e0004922. doi:10.1371/journal.pntd.0004922
8. Gatti S, Lopes R, Cevini C, et al. Intestinal parasitic infections in an institution for the mentally retarded. Ann Trop Med Parasitol. 2000;94(5):453–460. doi:10.1080/00034983.2000.11813564
9. Anvarian M. The prevalence of intestinal protozoa in patients referred to Tabriz Imam Reza Hospital from January 2010 to December 2010. Adv Environ Biol. 2011;5(7):1916–1919.
10. Sharif M, Daryani A, Asgarian F, Nasrolahei M. Intestinal parasitic infections among intellectual disability children in rehabilitation centers of northern Iran. Res Dev Disabil. 2010;31(4):924–928. doi:10.1016/j.ridd.2010.03.001
11. Victoria A Worm Disease Profile of Primary School Children.
12. Feleke BE. Nutritional status and intestinal parasite in school age children: a comparative cross-sectional study. Int J Pediatr. 2016;1:1–8. doi:10.1155/2016/1962128
13. Negussu N, Mengistu B, Kebede B, et al. Ethiopia schistosomiasis and soil-transmitted helminthes control programme: progress and prospects. Ethiop Med J. 2017;55(1):75–80.
14. Humphreys M. How four once common diseases were eliminated from the American South. Health Aff. 2009;28(6):1734–1744. doi:10.1377/hlthaff.28.6.1734
15. Bleakle YH. Disease and development: evidence from hookworm eradication in the American South. Q J Econ. 2007;122(1):73–117. doi:10.1162/qjec.121.1.73
16. Hotez P. Hookworm and poverty. Ann N Y Acad Sci. 2008;1136(1):38–44. doi:10.1196/annals.1425.000
17. Aiemjoy K, Gebresillasie S, Stoller NE, et al. Epidemiology of soil-transmitted helminth and intestinal protozoan infections in preschool-aged children in the Amhara region of Ethiopia. Am J Trop Med Hyg. 2017;96(4):866–872. doi:10.4269/ajtmh.16-0800
18. Cheesbrough M. District Laboratory Practice 2nd Edition in Tropical Countries. Cambridge University Press; 2006.
19. Eze N, Abah A, Ezeoru D. Intestinal parasitic infections among patients of psychiatric hospital Rumuigbo, Rivers State, Nigeria. Int J Trop Dis Health. 2019;37(2):1–8. doi:10.9734/ijtdh/2019/v37i230163
20. Nyundo AA, Munisi DZ, Gesase AP. Prevalence and correlates of intestinal parasites among patients admitted to Mirembe National Mental Health Hospital, Dodoma, Tanzania. Hindawi J Parasitol Res. 2017;2017:1–6. doi:10.1155/2017/5651717
21. Duedu KO, Karikari YA, Attah SK, Ayeh-Kumi PF. Prevalence of intestinal parasites among patients of a Ghanaian psychiatry hospital. BMC Res Notes. 2015;8(1):651. doi:10.1186/s13104-015-1634-6
22. Saeidinia A, Tavakoli I, Naghipour MR, et al. Prevalence of Strongyloidesstercoralis and other intestinal parasites among institutionalized mentally disabled individuals in Rasht, northern Iran. Iran J Parasitol. 2016;11(4):527–533.
23. Fentahun A, Asrat A, Bitew A, Mulat S. Intestinal parasitic infections and associated factors among mentally disabled and non-disabled primary school students, Bahir Dar, Amhara regional state, Ethiopia, a comparative cross-sectional study. BMC Infect Dis. 2019;19(1):549. doi:10.1186/s12879-019-4165-2
24. Rhongbutsr IP, Saichua P, Navaphongpaveen K, Taylor A, Leelawongtawon R, Kitvatanachai S. Intestinal parasitic infections in students at a school for handicapped children in KhonKaen Province, Thailand. Thammasat Med J. 2010;10(4):406–410.
25. El-Nadi NAF, Omran EK, Ahmed NS, Fadel EF. Current status of intestinal parasites among elementary school children in Sohag, Egypt. J Adv Parasitol. 2017;4(2):33–40. doi:10.17582/journal.jap/2017/4.2.33.40
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.