Back to Journals » Infection and Drug Resistance » Volume 16
Prevalence of HIV Transmitted Drug Resistance in Nanjing from 2018 to 2021
Authors Su Y, Qi M, Zhong M, Yu N, Chen C, Ye Z, Cheng C, Hu Z, Zhang H, Wei H
Received 30 September 2022
Accepted for publication 16 December 2022
Published 2 February 2023 Volume 2023:16 Pages 735—745
DOI https://doi.org/10.2147/IDR.S391296
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Suresh Antony
Yifan Su,1 Mingxue Qi,1 Mingli Zhong,2 Nawei Yu,1 Chen Chen,1 Zi Ye,1 Cong Cheng,1 Zhiliang Hu,1 Hongying Zhang,3 Hongxia Wei1
1Department of Infectious Disease, The Second Hospital of Nanjing Affiliated to Nanjing University of Chinese Medicine, Nanjing, People’s Republic of China; 2Department of Epidemiology and Biostatistics, School of Public Health, Nanjing Medical University, Nanjing, People’s Republic of China; 3Nanjing Center for Disease Control and Prevention Affiliated with Nanjing Medical University, Nanjing, People’s Republic of China
Correspondence: Hongxia Wei, Department of Infectious Disease, The Second Hospital of Nanjing, Nanjing University of Chinese Medicine, Nanjing, 210003, People’s Republic of China, Email [email protected] Hongying Zhang, Email [email protected]
Background: Transmitted drug resistance (TDR) is a major challenge in the clinical management of acquired immunodeficiency syndrome (AIDS). Therefore, this study aimed to investigate the epidemic characteristics of and risk factors for human immunodeficiency virus (HIV)-1 TDR in Nanjing from 2018 to 2021 to provide support for clinical management.
Methods: The HIV-1 Pol gene was amplified by nested reverse transcription polymerase chain reaction from venous blood of 1190 HIV-infected patients who did not receive antiviral therapy, and the amplified product was sequenced using an in-house sequencing method. The sequencing result was compared with the HIV drug resistance database from Stanford University to elucidate the rates of antiviral drug resistance and distribution of drug-resistant mutation sites. Factors associated with TDR were evaluated using a logistic regression model.
Results: Detection of drug resistance at the gene level was successful in 1138 of 1190 HIV-1-infected patients (95.6%), and the overall 4-year drug resistance rate was 8.2% (93/1138). The drug resistance rate was higher for non-nucleoside reverse transcriptase inhibitors (NNRTIs; 6.7%) than for nucleoside reverse transcriptase inhibitors (NRTIs; 2.5%) or protease inhibitors (PIs; 0.1%) (χ2 = 83.907, P< 0.0001). The most common NNRTI-related mutation was V179D/E followed by K103N. M184V was the dominant NRTI-associated mutation, and M46L/I was the most prevalent PI-associated mutation. A CD4+ T cell count of < 50 cells/μL was significantly associated with an increased risk of TDR (OR=3.62, 95% CI: 1.38– 9.51, P=0.009).
Conclusion: The prevalence of TDR in the city of Nanjing from 2018 to 2021 was at a moderate epidemic risk according to World Health Organization standards. Continuous monitoring of TDR can inform clinical diagnosis and treatment. Patients with advanced disease and a low CD4+ T lymphocyte count are more likely to have TDR in Nanjing.
Keywords: HIV-1, transmitted drug resistance, mutation sites
Introduction
Since the first case of acquired immunodeficiency syndrome (AIDS) was discovered in the United States in 1981, AIDS has spread around the world and become a serious public health concern. The Joint United Nations Program on HIV/AIDS (UNAIDS) estimated that there were 38.4 million people were living with HIV, 1.5 million new cases, and about 650,000 deaths from HIV-related diseases in 2021.1 Presently, antiretroviral therapy (ART) is the main method of treatment for AIDS. The preferred regimen is two nucleoside reverse transcriptase inhibitors (NRTIs) plus one non-nucleoside reverse transcriptase inhibitor (NNRTI)/protease inhibitor (PI)/integrase inhibitor (INSTI).2 The recommended regimens in China were tenofovir plus lamivudine or emtricitabine as NRTIs, efavirenz or ripiverin as NNRTIs, ripiverin/ritonavir as PIs and dolutegravir or raltegravir as INSTIs.2
The National Free Antiretroviral Treatment Program (NFATP) was launched in six provinces in central China in 2003 and subsequently expanded to 31 provinces and autonomous regions in 2006, improving the access of patients living with HIV to antiretroviral treatment.3,4 Previous research showed that the use of ART in China reduced AIDS-related deaths from 39.3% in 2002 to 14.2% in 2009.5 However, the continuous use of ART in China has led to an increase in the drug resistance rate in newly-treated patients living with HIV,6 especially the resistance to NNRTIs.7 A nationwide cross-sectional survey conducted in 2015 found that the average prevalence of transmitted drug resistance (TDR) was 3.7%,8 and a significant increase in TDR in different regions was reported by other studies during subsequent years.9,10 The presence of drug resistance in patients before treatment results in a reduced viral suppression rate, impaired immune recovery, further accumulation of drug resistance, and increases in the number of new HIV infections and risk of death.11,12 Furthermore, resistant mutations in pregnant women need prompt diagnosis and treatment because they can be passed from mother to child if the mother is not virologically suppressed, which complicates management of the infant.13 Therefore, close monitoring and early detection of TDR in ART initiators is crucial to avoid antiviral treatment failure and other negative effects. Additionally, a prior study found that the changes in HIV drug resistance rates over time varied between different regions.14 Previous investigations reported that the prevalence of TDR was 6.0% in sub-Saharan Africa,15 7.8% in Greece,16 10.7% in Hungary,17 and 14.0–17.5% in the United States.18,19 However, the prevalence of TDR in the city of Nanjing in recent years is unknown. Therefore, this study aimed to estimate the prevalence of TDR in Nanjing during 2018–2021 and elucidate the factors associated with TDR to provide up-to-date information that may help support clinical decision making.
Methods
Study Design and Participants
This study included outpatients attending the Second Hospital of Nanjing from January 2018 to December 2021. The inclusion criteria were: (i) outpatient attending the Second Hospital of Nanjing; (ii) HIV infection confirmed by an HIV antibody test; (iii) had not received any previous antiviral treatment; and (iv) genotypic drug resistance was detected before treatment. Baseline data such as sex, age, marital status, route of transmission, CD4+ T lymphocyte count, and viral load (HIV ribonucleic acid [RNA] level) were collected from the patients who were included in the study. All patients provided written informed consent, and the study was approved by the Medical Ethics Committee of the Second Hospital of Nanjing (approval number: 2018-LY-kt027; approval date: 9 May, 2018). The study was conducted in accordance with the Declaration of Helsinki.
Detection of HIV Genotype Resistance
A 10-mL sample of peripheral blood was collected from the HIV-positive patients, and plasma was separated by centrifugation. Viral load was evaluated using a commercial kit (COBAS® AmpliPrep/COBAS® TaqMan® HIV-1 Test version 2.0; Roche Diagnostics International, Risch-Rotkreuz, Switzerland). HIV-1 RNA was extracted using an automated nucleic acid extraction system (Shuoshi Company, Taizhou, Jiangsu, China). Using the complete genome (9719 bp) of the international standard strain of HXB2 as a reference, PCR was used to amplify the full length of the protease gene (codons 1–99) and the first 300 amino acids (codons 1–300) of the reverse transcriptase gene. The primers used for the first round of PCR were: lateral upstream primer, F1a:5′-TGAARGAITGYACTGARAGRCAGGCTAAT-3′ and F1b:5′-ACTGARAGRCAGGCTAATTTTTTAG-3′; lateral downstream primer, RT-R1: 5′-ATCCCTGCATAAATCTGACTTGC-3′. The primers used for the second round of PCR were: medial upstream primer, PRT-F2: 5′-CTTTARCTTCCCTCARATCACTCT-3′, medial downstream primer, RT-R2: 5′-CTTCTGTATGTCATTGACAGTCC-3′. The reaction conditions for the first round of PCR were: 50°C for 45 min; 94°C for 2 min; 50 cycles of 94°C for 15s, 55°C for 20s and 72°C for 2 min; 72°C for 10 min; and holding temperature of 4°C. The reaction conditions for the second round of PCR were: 94°C for 4 min; 40 cycles of 94°C for 15s, 55°C for 20s and 72°C for 2 min; 72°C for 10 min; and holding temperature of 4°C. The amplified products were sequenced using an in-house method based on traditional Sanger sequencing,20 and the sequences were corrected and spliced using CExpress software. The resulting full sequences were analyzed using the Stanford HIVDB software provided on the Stanford University HIV Drug Resistance Database (http://HIVDB.stanford.edu/) to confirm the genotyping and to identify the drug resistance mutations. The resistance level of HIV-1 to each antiviral drug was divided into five grades according to the scoring criteria from the Stanford website: sensitive, potentially low resistance, low resistance, moderate resistance, and high resistance. In this study, patients with low, medium or high drug resistance (according to the Stanford website) were classified as having TDR, in accordance with the World Health Organization’s (WHO’s) HIV Drug Resistance Report 2021.21
Statistical Analysis
The data were analyzed using SPSS 26.0 (IBM Corp., Armonk, NY, USA). Quantitative data are described as absolute numbers and rates. Univariate and multivariate logistic regression analyses were used to identify factors associated with HIV-1 TDR, and the χ2 test was used to analyze drug resistance. A P-value <0.05 was considered statistically significant.
Results
Demographic Characteristics
From 2018 to 2021, a total of 2151 HIV-infected outpatients attended the Department of Infectious Diseases of the Second Hospital of Nanjing and started antiretroviral therapy. The genotypic resistance testing rates in these 2151 patients during 2018, 2019, 2020 and 2021 were 8.5%, 46.1%, 74.7%, and 83.7%, respectively. In total, 1190 of the 2151 HIV-1-infected patients (55.3%) were tested for genotype resistance, and successful sequencing was achieved in 1138 of these 1190 cases (95.6%, 1138/1190). Therefore, the final analysis included 1138 HIV-infected patients (Table 1). The majority of the 1138 newly-treated patients with HIV-1 were male (93.6%). Most of the participants were aged 20–40 years-old (64.8%), with only 13 patients (1.1%) aged <18 years-old, and the median age was 31 years-old. The main route of transmission was homosexual transmission (68.5%), but there was an increase in the rate of heterosexual transmission over time (from 15.8% in 2018 to 23.2% in 2021). The median CD4+ T lymphocyte count was 288 cells/μL, and the median HIV RNA (viral load) was 41,800 copies/mL.
 |
Table 1 Demographic Characteristics of 1138 Newly-Treated Patients Living with HIV Who Successfully Underwent Genotype Resistance Testing |
TDR Prevalence
Among the 1138 patients who were tested for genotypic resistance, the overall TDR prevalence rate was 8.2% (93/1138), and the drug resistance rate was significantly higher for NNRTIs (6.7%, 76/1138) than for NRTIs (2.5%, 29/1138) or PIs (0.1%, 1/1138) (χ2=83.907, P<0.0001). Resistance to both NNRTIs and NRTIs was observed in 1.1% (13/1138) of the patients, and simultaneous resistance to all three classes of drugs (NNRTIs, NRTIs and PIs) was not identified in this study population.
The TDR prevalence increased progressively from 2018 to 2020, reaching a peak of 9.4%, before falling in 2021 (Figure 1). Among the different types of antiviral drug, NNRTIs had the highest drug resistance rate in all four years analyzed, reaching a peak prevalence of 7.49% in 2020 (Figure 1). The NRTI drug resistance rate fell during the four-year period and remained below 4% from 2019 to 2021 (Figure 1). PIs had the lowest drug resistance rates among the different types of antiviral drug (Figure 1).
 |
Figure 1 Epidemic trend of the different types of antiviral TDR from 2018 to 2021. |
Analyses of Drug Resistance Levels and Drug Resistance Mutation Sites
Drug resistance levels were evaluated for 13 commonly used antiviral drugs. These drugs included five NNRTIs, namely efavirenz (EFV), etravirine (ETR), nevirapine (NVP), ripiverin (RPV) and doravirine (DOR); five NRTIs, namely abacavir (ABC), zidovudine (AZT), emtricitabine (FTC), lamivudine (3TC) and tenofovir (TDF); and three PI drugs, namely atazanavir/ritonavir (ATV/r), denavir/ritonavir (DRV/r) and ripiverin/ritonavir (LPV/r).
The drug resistance level and TDR prevalence for each of these antiviral drugs are shown in Figure 2 and Table 2. EFV and NVP had the highest drug resistance rates among the NNRTI drugs. Of the 76 patients, 40.8% (31/76) developed resistance to the NNRTI class drugs, EFV, NVP, and RPV, and the resistance rates for these three agents were similar (χ2=4.572, P=0.102). Relatively large proportions (89.8% and 82.8%) of patients had drug resistance to EFV and NVP at a medium to medium-high level, while 69.8% of patients exhibited resistance to RPV at a low level. ETR had the lowest drug resistance rate (1.49%, 17/1138) among the NNRTI drugs, and the resistance rate for ETR was significantly lower than that for NVP (5.6%, 64/1138) (P<0.0001). The overall drug resistance rate for NRTIs was lower than that for NNRTIs, with ABC having the highest drug resistance rate of 1.4% (16/1138) followed by AZT (1.2%, 14/1138) and FTC (1.1%, 13/1138). The drug resistance rate of 3TC, which is commonly used in Nanjing, was 1.1% (12/1138), and this would be considered medium-high drug resistance. Only one patient had high-level resistance to AZT, and the rest exhibited low-level resistance. Resistance to PIs was detected in only one patient in the study and manifested as both low resistance to ATV/r and moderate resistance to LPV/r. No DRV/r resistant strains were detected in this study.
 |
Table 2 Drug Resistance and TDR Prevalence for Different Antiviral Drugs |
 |
Figure 2 Frequency and degree of resistance of different antiviral drugs. |
Fifteen NRTI-related mutation sites, 14 NNRTI-related mutation sites, and 8 PI-related mutation sites were identified in this study (Figure 3). The drug-resistant mutation rate was 18.5% (210/1138), and the most common NNRTI-related mutation site was V179D/E with a mutation frequency of 7.8% (89/1138), followed by K103N (1.7%, 20/1138), V106I (1.5%, 17/1138), and G190A (0.6%, 7/1138). M184V (0.5%, 6/1138), D67DAG (0.4%, 5/1138) and K65R (0.4%, 5/1138) were the main NRTI-related mutations, while M46L/I (0.5%, 6/1138) was the main PI-related mutation.
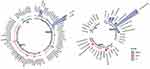 |
Figure 3 The drug-resistant mutation sites detected in newly-treated patients living with HIV. |
Risk Factors Associated with TDR
Factors independently associated with HIV-1 TDR were evaluated using univariate and multivariate logistic regression analyses, using gender, age, marital status, route of transmission, CD4+ T lymphocyte count and HIV RNA as independent variables. Gender, age, route of transmission, marital status and HIV RNA showed no significant differences among the groups. Patients with a CD4+ T lymphocyte count <50 copies/mL were more likely to have TDR than patients with a CD4+ T lymphocyte count ≥500 copies/mL (P=0.009) (Table 3).
 |
Table 3 Analysis of the Factors Associated with HIV-1 Transmissible Drug Resistance |
Discussion
A total of 1138 newly-treated HIV patients with no prior history of ART who attended our institution during 2018–2021 were successfully genotyped for baseline drug resistance, and the overall 4-year drug resistance rate was 8.2%. The resistance rates in Nanjing were 7.9%, 8.4%, 9.4% and 6.9% in 2018, 2019, 2020 and 2021, respectively, which were higher than those in Jiangsu province during 2009–201122 (2.1% in 2009, 4.0% in 2010, and 4.1% in 2011). The resistance rate was also higher than those in other regions of China during the same period, such as 6.7% in Beijing,23 4.6% in Guangdong,24 and 4.9% in Shenyang.25 According to WHO guidelines, HIV drug resistance rates of <5%, 5–15% and >15% are considered low, medium, and high prevalence, respectively.26 Therefore, the overall TDR prevalence rate of 8.2% in Nanjing would be considered moderate prevalence but is far higher than the overall TDR level in South China.27 Since prior studies have reported that the prevalence of TDR differs between geographical regions and is increasing,27 a likely reason for the higher rate in Nanjing than in other geographical regions is that our study evaluated TDR prevalence more recently than previously published investigations.
In this study, the drug resistance rate was highest for NNRTIs among the three types of antivirals. The trend in the drug resistance rate for NNRTIs between 2018 and 2021 was similar to that for the overall TDR rate, indicating that the prevalence of TDR was dominated by drug resistance to NNRTI drugs. The overall TDR prevalence and NNRTI resistance rate both exhibited a downward trend between 2020 and 2021. Since the decrease only occurred in 2021, it is impossible to judge whether the downward trend is a long-term phenomenon, and continuous monitoring of drug resistance in newly-treated patients living with HIV in this region should be recommended. Additionally, with the emergence of a new-generation integrase inhibitors such as dolutegravir (DTG), more patients are adopting INSTI-based antiviral regimens. The use of INSTI drugs in the Nanjing area has increased gradually in recent years (10.5% in 2018, 25.3% in 2019, 36.7% in 2020, and 54.4% in 2021 in this study population). DTG has a high resistance barrier and can reduce the transmission of HIV.28 However, integrase-related drug resistance mutations were not evaluated in the present research, and this is one of the limitations of our study. As antiviral treatment regimens continue to improve, more attention should be paid to INSTI drug resistance in newly-treated patients.
NNRTIs have a low resistance barrier, and NNRTI drug resistance has exhibited a rising trend in Africa, Asia and other regions.29 The WHO recommends switching first-line antiretroviral therapy from NNRTI-based antiviral regimens to non-NNRTI-based regimens if the NNRTI drug resistance rate is ≥10%.30 Although the NNRTI resistance rate in Nanjing did not reach a high level, NNRTIs should be considered with caution when initiating treatment in this region given the currently limited selection of NNRTI drugs in China. The main NNRTI-related mutation site was V179D/E, which is consistent with previous survey results in southwest China.31 The existence of this mutation site can lead to potential low resistance to multiple NNRTI drugs. The combination of V179D and K103R significantly reduces the sensitivity to EFV and NVP, which are widely used as first-line NNRTI drugs in Nanjing. The most common NRTI-related mutation in this study was M184V, which results in high resistance to 3TC and FTC and low resistance to ABC.32 Although the resistance rate to 3TC, a commonly used NRTI drug in Nanjing, was only 1.1%, the most prevalent NRTI-related mutation sites in this region were M184V/I and K65R, which can reduce the susceptibility to 3TC. Therefore, NRTI-related mutations still need to be taken seriously. We detected few PI-related mutations, and the main mutation site identified was M46L/I, in agreement with survey results from Shanghai and Chongqing.9,33 As PIs have a relatively high drug resistance barrier and are often used as second-line treatments in China,34 the current drug resistance level is significantly lower for PIs than for NNRTIs or NRTIs. This suggests that non-NNRTI drugs, such as PI-based antiviral drugs, could be the preferred regimen when resistance to NNRTIs is experienced.
The drug-resistance mutation rate in this study reached as high as 18.5%, and the prevalence of potential low drug resistance caused by the V179D/E mutation was 10.3%. However, it was unclear whether this affected the efficacy of antiviral drugs. We are currently collecting follow-up information for a population with potential drug resistance who were enrolled during 2019–2021 and treated with antiviral therapy for 1 year, with the aim of evaluating the effect of potential drug resistance mutations on antiviral efficacy as well as observing changes in mutation sites during the course of treatment.
This study also analyzed the risk factors for TDR. A CD4+ T lymphocyte count <50 /μL was a risk factor for TDR, suggesting that ART initiation in advanced HIV infection was more likely to be affected by drug resistance. Most studies have not detected a significant association between CD4+ T cell count and TDR.9,35,36 However, one investigation concluded that patients with HIV carrying a TDR mutation had a lower average CD4+ T cell count than patients with non-drug resistant HIV (373/mm3 vs 496/mm3, P=0.013).37 The mechanism underlying the association between low CD4+ T cell count and TDR remains unestablished. However, one possibility is the crucial role of host immune in control of HIV-138 and treatment-naïve patients with lower CD4+ T cell counts may have been infected with HIV for a longer period of time, allowing the virus to accumulate mutations as it rapidly replicated.39 Further studies will be needed to characterize in detail the relationship between TDR and CD4+ T cell count.
This study investigated the TDR prevalence rate and drug resistance characteristics of HIV-1 in Nanjing in recent years. Nanjing Second Hospital is the only designated hospital for AIDS treatment in Nanjing, which enabled us to include a comprehensive sample of patients with AIDS receiving treatment in this region, and this is an advantage of the present study. The prevalence of drug resistance in newly-treated patients can lead to failure of antiviral therapy and the accumulation of drug resistance in the population. In Nanjing, pre-treatment drug resistance testing of patients infected with HIV began to be formally promoted in 2018, and the drug resistance testing rates were 8.5%, 46.1%, 74.7% and 83.7%, respectively, during the four-year study period. However, the prevalence of TDR in the Nanjing area has not been well elucidated recently, and this study has contributed data for recent years. Our study also has the following disadvantages. First, subtypes of patients were not included in this study, so it is not possible to understand their prevalence and association with TDR. Furthermore, drug resistance detection did not include INSTI-related drug resistance mutations, whereas the utilization rate of INSTI drugs has been increasing gradually in recent years, and the secondary mutation site E157Q/T97A, which confers INSTI resistance, has been detected in newly-treated patients in China.40 Finally, cofounders such as level of education and occupation were not adjusted in this study, which would be addressed in future studies.
Conclusion
The prevalence of TDR in Nanjing during 2018–2021 was at a medium level and dominated by drug-resistance to NNRTIs. Continuous monitoring of TDR prevalence in ART-naive patients living with HIV is crucial to evaluate the efficacy of current major antiviral drugs and help formulate effective personalized treatments for patients living with HIV. However, more attention should be paid to drug resistance in patients with advanced disease and a low CD4+ T lymphocyte count.
Abbreviations
3TC, lamivudine; ABC, abacavir; ATV/r, atazanavir/ ritonavir; AZT, zidovudine; CRF, circulating recombinant form; CI, confidence interval; DTG, dolutegravir; DOR, doravirine; DRV/r, denavir/ritonavir; EFV, efavirenz; ETR, etravirine; FTC, emtricitabine; IDU, intravenous drug use; LPV/r, lopinavir/ ritonavir; NNRTI, non-nucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; NVP, nevirapine; OR, odds ratio; PCR, polymerase chain reaction; PI, protease inhibitor; RPV, rilpivirine; TDF, tenofovir disoproxil fumarate; TDR, transmitted drug resistance.
Data Sharing Statement
The datasets analyzed during the current study are available from the corresponding author Hongxia Wei upon reasonable requests.
Ethics Approval and Consent to Participate
The study protocol was approved by the Medical Ethics Committee of The Second Hospital of Nanjing (approval number: 2018-LY-kt027; approval date: 9 May, 2018), and all subjects provided written informed consent. The study was conducted in accordance with the Declaration of Helsinki.
Acknowledgments
We thank all the participants in the study. We also thank the staff of The Second Hospital of Nanjing for facilitating access to the relevant medical records. And we are grateful to the AIDS Healthcare Foundation for their support of our work.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the 2020 Annual Medical Research Project of Jiangsu Commission of Health, awarded to Hongxia Wei [Grant# ZDA 2020014], and the Key Project supported by Medical Science and Technology Development Foundation, Nanjing Department of Health, awarded to Hongxia Wei (Grant# ZKX 22040) and awarded to Hongying Zhang (Grant# ZKX 19048).
Disclosure
The authors declare that there are no competing interests associated with this study.
References
1. HIV/AIDS UNPo. Global HIV & AIDS statistics — fact sheet. Available from: https://www.unaids.org/en/resources/fact-sheet.
2. AIDS and Hepatitis C Professional Group, Society of Infectious Diseases, Chinese Medical Association; Chinese Center for Disease Control and Prevention. Chinese guidelines for diagnosis and treatment of HIV/AIDS (2021 edition). Zhonghua Nei Ke Za Zhi. 2021;60(12):1106–1128. Chinese. doi:10.3760/cma.j.cn112138-20211006-00676
3. Zhang FJ, Pan J, Yu L, Wen Y, Zhao Y. Current progress of China’s free ART program. Cell Res. 2005;15(11–12):877–882. doi:10.1038/sj.cr.7290362
4. Zhang F, Haberer JE, Wang Y, et al. The Chinese free antiretroviral treatment program: challenges and responses. AIDS. 2007;21(Suppl 8):S143–S148. doi:10.1097/01.aids.0000304710.10036.2b
5. Zhang F, Dou Z, Ma Y, et al. Effect of earlier initiation of antiretroviral treatment and increased treatment coverage on HIV-related mortality in China: a national observational cohort study. Lancet Infect Dis. 2011;11(7):516–524. doi:10.1016/S1473-3099(11)70097-4
6. Zhang F, Liang B, Liang X, et al. Using molecular transmission networks to reveal the epidemic of pretreatment HIV-1 drug resistance in Guangxi, China. Front Genet. 2021;12:688292. doi:10.3389/fgene.2021.688292
7. Hamers RL, Rinke de Wit TF, Holmes CB. HIV drug resistance in low-income and middle-income countries. Lancet HIV. 2018;5(10):e588–e596. doi:10.1016/S2352-3018(18)30173-5
8. Zhao S, Feng Y, Hu J, et al. Prevalence of transmitted HIV drug resistance in antiretroviral treatment naïve newly diagnosed individuals in China. Sci Rep. 2018;8(1):12273. doi:10.1038/s41598-018-29202-2
9. Wang Z, Zhang M, Zhang R, et al. Diversity of HIV-1 genotypes and high prevalence of pretreatment drug resistance in newly diagnosed HIV-infected patients in Shanghai, China. BMC Infect Dis. 2019;19(1):313. doi:10.1186/s12879-019-3927-1
10. Xu Y, Peng X, Peng X, et al. Characterization of HIV-1 subtypes and transmitted drug resistance among treatment-naive HIV-infected individuals in Zhejiang, China, 2014–2017. Arch Virol. 2018;163(8):2233–2237. doi:10.1007/s00705-018-3839-1
11. Phillips AN, Stover J, Cambiano V, et al. Impact of HIV drug resistance on HIV/AIDS-associated mortality, new infections, and antiretroviral therapy program costs in Sub-Saharan Africa. J Infect Dis. 2017;215(9):1362–1365. doi:10.1093/infdis/jix089
12. Inzaule SC, Hamers RL, Noguera-Julian M, et al. Clinically relevant thresholds for ultrasensitive HIV drug resistance testing: a multi-country nested case-control study. Lancet HIV. 2018;5(11):e638–e646. doi:10.1016/S2352-3018(18)30177-2
13. Yeganeh N, Kerin T, Ank B, et al. Human immunodeficiency virus antiretroviral resistance and transmission in mother-infant pairs enrolled in a large perinatal study. Clin Infect Dis. 2018;66(11):1770–1777. doi:10.1093/cid/cix1104
14. Riou J, Dupont C, Bertagnolio S, et al. Drivers of HIV-1 drug resistance to non-nucleoside reverse-transcriptase inhibitors (NNRTIs) in nine Southern African countries: a modelling study. BMC Infect Dis. 2021;21(1):1042. doi:10.1186/s12879-021-06757-6
15. Rhee SY, Kassaye SG, Barrow G, Sundaramurthi JC, Jordan MR, Shafer RW. HIV-1 transmitted drug resistance surveillance: shifting trends in study design and prevalence estimates. J Int AIDS Soc. 2020;23(9):e25611. doi:10.1002/jia2.25611
16. Kantzanou M, Karalexi MA, Papachristou H, Vasilakis A, Rokka C, Katsoulidou A. Transmitted drug resistance among HIV-1 drug-naïve patients in Greece. Int J Infect Dis. 2021;105:42–48. doi:10.1016/j.ijid.2021.02.043
17. É Á, Müller V, Mezei M, et al. Transmitted drug resistance in newly diagnosed and treatment-naïve HIV type 1-infected patients in Hungary. J Glob Antimicrob Resist. 2020;20:124–130. doi:10.1016/j.jgar.2019.07.014
18. Chan W, Ly W. Surveillance of transmitted HIV drug resistance among newly diagnosed, treatment-naive individuals at a county HIV clinic in Santa Clara County. Heliyon. 2019;5(9):e02411. doi:10.1016/j.heliyon.2019.e02411
19. Ross LL, Shortino D, Shaefer MS. Changes from 2000 to 2009 in the prevalence of HIV-1 containing drug resistance-associated mutations from antiretroviral therapy-naive, HIV-1-infected patients in the United States. AIDS Res Hum Retroviruses. 2018;34(8):672–679. doi:10.1089/aid.2017.0295
20. Ye J, Hao M, Xing H, et al. Characterization of subtypes and transmitted drug resistance strains of HIV among Beijing residents between 2001–2016. PLoS One. 2020;15(3):e0230779. doi:10.1371/journal.pone.0230779
21. Organization WH. HIV drug resistance report 2021; 2021. Available from: https://reliefweb.int/sites/reliefweb.int/files/resources/9789240038608-eng.pdf.
22. Guo H, Xu X, Hu H, et al. Low prevalence of the transmitted HIV-1 drug resistance among newly diagnosed HIV-1 individuals in Jiangsu Province, China during 2009–2011. BMC Public Health. 2015;15:120. doi:10.1186/s12889-015-1489-8
23. Li R, Song C, Chen D, et al. Prevalence of transmitted drug resistance among ART-naïve HIV-infected individuals, Beijing, 2015–2018. J Glob Antimicrob Resist. 2022;28:241–248. doi:10.1016/j.jgar.2022.01.017
24. Lan Y, Li L, He X, et al. Transmitted drug resistance and transmission clusters among HIV-1 treatment-naïve patients in Guangdong, China: a cross-sectional study. Virol J. 2021;18(1):181. doi:10.1186/s12985-021-01653-6
25. Wang Z, Zhao B, An M, et al. Transmitted drug resistance to tenofovir/emtricitabine among persons with newly diagnosed HIV infection in Shenyang city, Northeast China from 2016 to 2018. BMC Infect Dis. 2021;21(1):668. doi:10.1186/s12879-021-06312-3
26. World Health Organization. Global strategy for the surveillance and monitoring of HIV drug resistance; 2012. Available from: https://www.who.int/hiv/pub/drugresistance/drug_resistance_strategy/en/.
27. Zuo L, Liu K, Liu H, et al. Corrigendum to “Trend of HIV-1 drug resistance in China: a systematic review and meta-analysis of data accumulated over 17 years (2001–2017)” [EClinicalMedicine 18 (2020) 100238]. EClinicalMedicine. 2021;33:100696. doi:10.1016/j.eclinm.2020.100696
28. Scarsi KK, Havens JP, Podany AT, Avedissian SN, Fletcher CV. HIV-1 integrase inhibitors: a comparative review of efficacy and safety. Drugs. 2020;80(16):1649–1676. doi:10.1007/s40265-020-01379-9
29. Gupta RK, Gregson J, Parkin N, et al. HIV-1 drug resistance before initiation or re-initiation of first-line antiretroviral therapy in low-income and middle-income countries: a systematic review and meta-regression analysis. Lancet Infect Dis. 2018;18(3):346–355. doi:10.1016/S1473-3099(17)30702-8
30. World Health Orgnization. Guidelines on the public health response to pretreatment HIV drug resistance; 2021. Available from: https://www.who.int/publications/i/item/9789241550055.
31. Pang X, Tang K, He Q, et al. HIV drug resistance and HIV transmission risk factors among newly diagnosed individuals in Southwest China. BMC Infect Dis. 2021;21(1):160. doi:10.1186/s12879-021-05854-w
32. Clutter DS, Jordan MR, Bertagnolio S, Shafer RW. HIV-1 drug resistance and resistance testing. Infect Genet Evol. 2016;46:292–307. doi:10.1016/j.meegid.2016.08.031
33. Liu M, He XQ, Deng RN, et al. Pretreatment drug resistance in people living with HIV: a large retrospective cohort study in Chongqing, China. HIV Med. 2022;23 Suppl 1(Suppl1):95–105. doi:10.1111/hiv.13253
34. Obeng BM, Bonney EY, Asamoah-Akuoko L, et al. Transmitted drug resistance mutations and subtype diversity amongst HIV-1 sero-positive voluntary blood donors in Accra, Ghana. Virol J. 2020;17(1):114. doi:10.1186/s12985-020-01386-y
35. Song YX, Xin RL, Li ZC, et al. Prevalence of transmitted drug resistance among HIV-1 treatment-naive patients in Beijing. Epidemiol Infect. 2018;146(3):339–344. doi:10.1017/S0950268817003016
36. Ye J, Hao M, Xing H, et al. Transmitted HIV drug resistance among individuals with newly diagnosed HIV infection: a multicenter observational study. AIDS. 2020;34(4):609–619. doi:10.1097/QAD.0000000000002468
37. Ding Y, Chen M, Wang J, et al. Increase in HIV-1-transmitted drug resistance among ART-naïve youths at the China-Myanmar border during 2009 ~ 2017. BMC Infect Dis. 2021;21(1):93. doi:10.1186/s12879-021-05794-5
38. Brumme ZL, Brumme CJ, Heckerman D, et al. Evidence of differential HLA class I-mediated viral evolution in functional and accessory/regulatory genes of HIV-1. PLoS Pathog. 2007;3(7):e94. doi:10.1371/journal.ppat.0030094
39. Liu DJ, Feng MX, Liu M. 中国未接受抗病毒治疗的人类免疫缺陷病毒/获得性免疫缺陷综合征(HIV/AIDS)人群HIV原发耐药的Meta分析 [Primary drug resistance of human immunodeficiency virus (HIV)among the treatment-naive individuals with HIV in China: a meta-analysis]. Beijing Da Xue Xue Bao Yi Xue Ban. 2015;47(3):474–482. Chinese.
40. Liu L, Dai L, Yao J, et al. Lack of HIV-1 integrase inhibitor resistance among 392 antiretroviral-naïve individuals in a tertiary care hospital in Beijing, China. AIDS. 2019;33(12):1945–1947. doi:10.1097/QAD.0000000000002282
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
