Back to Journals » Journal of Blood Medicine » Volume 11
Prevalence of Hepatitis B Virus and Hepatitis C Virus Among Blood Donors in Nekemte Blood Bank, Western Oromia, Ethiopia: Retrospective 5 Years Study
Authors Abebe M , Alemnew B , Biset S
Received 23 September 2020
Accepted for publication 12 December 2020
Published 31 December 2020 Volume 2020:11 Pages 543—550
DOI https://doi.org/10.2147/JBM.S282099
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Martin H Bluth
Milkias Abebe,1 Birhan Alemnew,2 Sirak Biset3
1Department of Medical Laboratory Sciences, Institute of Health Sciences, Wollega University, Nekemte, Ethiopia; 2Department of Medical Laboratory Sciences, College of Health Sciences, Wolidia University, Woldia, Ethiopia; 3Department of Medical Microbiology, School of Biomedical and Laboratory Sciences, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia
Correspondence: Milkias Abebe
Department of Medical Laboratory Sciences, Institute of Health Sciences, Wollega University, P. O. Box: 395, Nekemte, Ethiopia
Tel +251912863004
Email [email protected]
Background: Hepatitis B virus and hepatitis C virus are the greatest threats to blood safety for the recipient. This study aimed to determine the seroprevalence and trends of HBV and HCV infections among blood donors over a period of 5 years at Nekemte blood bank, Ethiopia.
Methods: A retrospective study was conducted from January 2015 to December 2019 at Nekemte blood bank. The recorded blood donors’ history and laboratory tests were reviewed by data collectors analyzed with Statistical Package for the Social Sciences version 20 software. A p-value of less than 0.005 was considered statistically significant.
Results: A total of 17,810 consecutive blood donors were screened between January 2015 and December 2019. The seroprevalence of HBV and HCV was 3.06% and 0.64%, respectively. The prevalence of HBV was significantly associated with male (AOR: 2.51; 95% CI: 1.17, 2.91), unmarried (AOR: 2.81; 95% CI: 1.79, 2.51) and rural (AOR: 2.11; 95% CI: 1.17, 3.05) blood donors. The prevalence of HCV was significantly associated with blood donor those were male (AOR: 3.01; 95% CI: 1.17, 3.91), within 45– 65 years of age (AOR: 3.56: 95% CI: 1.14, 3.99) and unmarried (AOR: 3.14; 95% CI: 1.65, 3.96).
Conclusion: The current study shows the prevalence of hepatitis B virus was higher among study participants. However, the prevalence of HCV was low compared to the study conducted in other countries in Africa, a substantial percentage of the blood donors harbor HCV infections. Therefore, it is recommended to increase awareness of people (particularly on unmarried, male and rural resident) on modes of transmission and prevention of infection could help in reducing the burden of both HBV and HCV.
Keywords: blood donor, prevalence, transfusion transmitted infection
Introduction
Blood transfusion service is mandatory and a therapeutic procedure, as there is no genuine substitution to save the life of many patients who suffer from the loss of blood. Globally, more than eighty-one million units of blood are donated by blood donors each year. However, the presence of blood-borne infections in blood cells of asymptomatic donors is the major cause of transmitting infectious agents through blood transfusion.1 These transfusions transmissible infectious diseases have long-term consequences on the recipients, families, and general communities since the infected person represents a pool for the infection and can transmit the disease during its asymptomatic period which can contribute to an ever-widening pool of infection in the population.2 The most commonly encountered transfusion infections from the viral origin are Hepatitis B Virus (HBV) and Hepatitis C Virus (HCV). Therefore, the World Health Organization (WHO) recommends that all blood collected should be tested for major transfusion transmissible infections (TTIs) caused by these pathogens prior to donation.3
African countries require the greatest amount of blood transfusions worldwide while simultaneously facing great suffering and pain from serious TTIs. Proper donor selection and checkup of the donated blood for TTIs are essential to reduce the transmission of these infections. Conversely, the safety of blood for transfusion in a low-income country is still uncertain because of the increasing prevalence of viral infections (HBV and HCV) in the communities, inability of the laboratory test to detect persons infection in the window period, shortage of expert professionals, poverty, population growth, urbanization and insecure environments.4,5
Hepatitis B Virus is highly contagious because it can be able to transmit easily from one person to another by blood transfusion. Globally, estimated 257 and 71 million peoples were living with chronic HBV and HCV infections, respectively. Moreover, it was estimated that about 1.34 million deaths were attributed to hepatitis.3 Furthermore, transfusion of contaminated blood causes up to 16 million new infections with Hepatitis B and 5 million new infections with Hepatitis C every year in the world.6 Findings from a study conducted in India also indicated that the magnitude of HBV and HCV among blood donors was 1.76% and 0.19%, respectively,7 whereas 0.51% and 0.25% of blood donors in China were positive for HBC and HCV infection, respectively.8 Besides, 12.5% of patients who receive blood transfusion are at risk of post-transfusion Hepatitis in sub-Saharan Africa.9 Another study conducted in Ethiopia revealed that HBV is highly prevalent among blood donors (8.4%) next to immigrants (11.0%).10 It is also documented that viral hepatitis is the most predominant viral TTI than HIV and HCV among blood donors in Ethiopia.1,11–13
The high demand for blood transfusion as a result of frequent road traffic accidents, children suffering from malaria and anemia, surgical, and obstetric blood loss heightened the problems of blood safety in Ethiopia. Thus, continuous monitoring of the magnitude of transfusion transmissible infections in blood donors is important for optimizing donor recruitment strategies to minimize infectious disease transmission. Therefore, this study was conducted to determine the seroprevalence and associated risk factors of HBV and HCV from 2015 to 2019 among blood donors at Nekemte blood bank.
Materials and Methods
Study Area
The data were collected from the Nekemte blood bank. The blood bank is situated in the Nekemte town, East Wollega Zone, which is located 326 km from, the capital city of Ethiopia, Addis Ababa. The blood bank mainly provides services to 16 hospitals that are found in the Wollega zone.
Study Design
A retrospective study of blood donor data recorded on Nekemte Blood Bank from January 2015 to December 2019 was undertaken.
Study Population
Voluntary non-remunerated blood donors who were attended at Nekemte blood bank during 5 years period and those were screened for hepatitis B and hepatitis C infection. Blood donors who were physically fit, aged between 18 and 65 years, and had a body weight greater than 50 Kg were included in the study. However, blood donors who were anemic (hemoglobin level: ≤12.5g/dl for females and ≤ 13.5g/dl for males) and apparently unhealthy or malnourished individuals were excluded.
Laboratory Examination
All blood donors’ samples were tested for HBsAg and anti-HCV using Wantai AiD™ HBsAg Enzyme Linked Immuno-Sorbent Assay (ELISA) and Wantai AiD™ anti-HCV ELISA test kit, respectively, developed by Beijing Wantai Biological Pharmacy Enterprise Co., Ltd. China laboratory diagnosis.
Data Collection and Statistical Analysis
All complete data on socio-demographic variables (age, sex, residence, marital status, occupation, educational status and blood group) and laboratory test results during the study period were retrieved from the blood donors register at Nekemte Blood Bank using the data extraction format by laboratory technicians. Donors who visited the blood bank twice or more were identified, and their first visit was considered with the aim to control bias by indication. Data were entered into Epi Info software (version 7), and then transferred to SPSS version 20 software for analysis. Logistic regression was applied to identify the potential risk factors. Candidate variables at p-value <0.25, in bivariate analysis, were entered into multivariable logistic regression. Backward model selection method was used. The degree of association between dependent and independent variables was assessed using an adjusted OR with 95% CI at p-value <0.05. The Hosmer and Lemeshow test were used to check model fitness at a p-value of 0.05.
Results
Demographic Characteristics of Blood Donors
A total of 17,810 blood samples donated to the Nekemte Blood Blank Center from January 2015 to December 2019 were retrieved. Of the total, the majority (70.1%) of the donors were males, 64.3% were within 18–30 years of age, 74.4% were unmarried, 59.4% were students, and 70% were urban blood donors (Table 1).
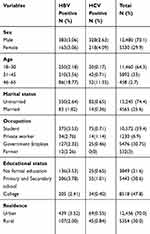 |
Table 1 Prevalence of HBV and HCV in Different Socio-Demographic Variable Among Blood Donor at Nekemte Blood Bank from January 2015 to December 2019 |
Seroprevalence of HBV and HCV
The seroprevalence of HBV and HCV was 3.06% (546/17,810) and 0.64% (114/17,810) respectively. Both HBV and HCV infection was highest among male blood donor, college students, donor within the age group 46–65 years. Furthermore, the highest prevalence of HBV was among married blood donors, whereas for HCV, it was highest among unmarried donors (Table 1).
Trends of HBV and HCV Seroprevalence
The seroprevalence of HBV was increased from 2.98% in 2015 to 3.46% in 2019 but decreased to 2.05% in 2017. Regarding HCV the prevalence was increased further from 0.37% in 2015 to 3.64% in 2017 then decreased further back to 0.34% in 2018 and 0.46% in 2019 (Table 2).
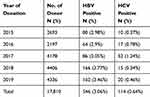 |
Table 2 Seroprevalence of HBV and HCV Infection with Respect to Donation Year Among Blood Donors at Nekemte Blood Bank from January 2015 to December 2019 |
Seroprevalence and Associated Factors of HBV and HCV
A multivariate logistic regression analysis was undertaken to determine associated risk factors with HBV and HCV infection. Blood donors who were male were two times more likely (AOR: 2.51; 95% CI: 1.17, 2.91) to be infected with HBV than female blood donors. In this analysis, study participants who were in the age group of 46–65 years were two times more likely (AOR: 2.18; 95% CI: 1.16, 2.01) to be infected with HBV than those who were in the age group of 18–30 years. Blood donors who were unmarried were three times more likely (AOR: 2.81; 95% CI: 1.79, 2.51) to be infected with HBV than females. Regarding residence blood donors, rural donors were two times more likely (AOR: 2.11; 95% CI: 1.17, 2.05) to be infected with HBV than urban blood donors (Table 3). Furthermore, the overall prevalence of HCV significantly higher among males (AOR: 3.01; 95% CI: 1.17, 3.51) and unmarried (AOR: 3.14; 95% CI: 1.65, 3.96) compared to their counterparts. It was also significantly higher among the age groups of 46–65 years who were three times more likely (AOR: 3.56; 95% CI: 1.14, 3.99) to be infected with HCV than those who were in the age group of 18–30 years (Table 4).
 |
Table 3 Logistic Regression of HBV with Socio-Demographic Characteristics of Blood Donor at Nekemte Blood Bank from January 2015–2019 |
 |
Table 4 Logistic Regression of HCV with Socio-Demographic Characteristics of Blood Donor at Nekemte Blood Bank from Jan 2015 to Dec 2019 |
Discussion
Blood transfusion is an important component of health care in which millions of lives are being saved each year through this procedure.14 In the present study majority of the donors (70.1%) were males and were aged between 18 and 30 years. Similar findings were reported from Gondar.15 This is due to Ethiopia women are usually housewives and this may lead them to avoid outdoor activities. Moreover, women have lower hemoglobin levels and a higher number of vasovagal reactions. This may cause a high rate of refusal for women donors.16 In the current study, the total seroprevalence of HBV and HCV showed an increment with respect to year even though it does not show a trend. Consistently an increment in the seroprevalence had been reported previously from Gondar12 and Bahir Dar study, which was conducted from 2014 to 2018.17 This might be as a result of the overall increment in the seroprevalence of this infection in the community since it is assumed that blood donors are representative of the community. Besides, the sensitivity of the test methods used to screen donated blood is improved as a result of changes in policies and strategies that governments have enforced to control TTIs.
The seroprevalence of HBV in the current study was 3.06% which was comparable with a study done by Kebede et al 3.05%17 and Biadgo et al 3.6%12 in Ethiopia. However, the present study was lower than previously conducted studies in a different region of Ethiopia which were 9.5% Wolaita Sodo,18 4.67% in Dire Dawa,2 4.4% in Harar,19 4.1% in Gondar20 and 10.9% in Jigjiga.1 Besides, the current study was also lower than studies from another African country which were 4.1% in Nigeria,21 9.7% in Sierra Leone,22 14.75% in Mali,23 and 14.3% in Ghana.24 This is probably because of the differences in the geographical distribution of the infection in the society, population differences regarding social behavior, lifestyle, socioeconomic status, and level of awareness in different regions of the country. Moreover, differences in specificity and sensitivity of screening tests used at different sites during the time of screening might be the cause of variations. On another hand, the current study was higher than a report from Eritrea which was 2.58%,25 0.6% in Namibia,26 0.46% in Nepal,27 1.93% in India,14 0.13% in Iran28 and 0.87% in China.29 The difference might be due to different risky behaviors at different geographical locations, the capacity of the primary screening test, and the method of laboratory diagnostic test.
The present study revealed that male blood donors were two times more likely to be infected with HBV than females. A consistent result has been reported from a number of studies from different regions of Ethiopia in Harar,19 Dire Dawa,30 North Shewa,31 Bahir Dar,32 Gondar,12 Jigjiga,1 and studies from other countries such as Ghana30 and Egypt.16 This might be due to the fact males are approximately 1.5 times more likely to develop chronic HBV infection than females as a result of the slower plasma disappearance rate for HBsAg in males compared to females.33 Moreover, the gender differences in behavioral risk factors such as having multiple sex partners could attribute to an increase in the prevalence of HBV among male donors. Besides, a plausible explanation of this disproportionate male to female seropositivity ratio may be related to the fact that females are better diagnosed in Ethiopia due to prenatal care. Therefore, a higher proportion of female blood donors may be aware of their seronegative status of HBV.34 The current study also showed that unmarried donors were more likely to be infected with HBV. Similar findings have been reported from a study conducted in the different study areas.4,35–37 The relatively lower HBV seropositivity among donor those were married might be attributed to marriage stable the sexual network (a group of individuals connected through sexual contact). Moreover, unmarried people have a probability of a wider sexual network, leading to more sexual partners, which in turn elevate their risk of acquiring HBV. Additionally, the prevalence of HBV was significantly higher among rural populations. This outcome agrees with a similar study conducted in other countries.16,22 Rural donors can be the attributing factor for the high prevalence of HBV due to their poor awareness regarding the mode of transmission and prevention, low educational status, and access to medical care is more limited in rural communities compared to urban. Furthermore, in this study, higher prevalence of HBV was detected in study participants within the age group 31–35 years and 45–65 years compared to age group 18–30 years. Similar studies in other countries have shown that age to be one of the independent predictors of acquiring HBV infection.30,38–40 This would probably be the birth cohort effect and presumptively due to a lack of immunization against HBV in their times.39,41
The overall prevalence of HCV in the current study was 0.64% which was comparable with the study done in Ethiopia by Yohannes et al which was 0.6%42 and 0.6% by Birhaneselassie.13 The present study was slightly higher than studies conducted in South Ethiopia which was 0.2%,17 0.32% in Central North Ethiopia,31 0.4% in Jijiga, Ethiopia,1 and 0.3% in Eritrea.34 However, the prevalence was lower when compared to previously conducted studies in a different region of Ethiopia 0.8%, 1.6%, 13.3%11,12,20 and in another country 3.4% Sudan,43 1.5% Tanzania,44 4% in Ghana45 and 4.3% in Egypt.46 These variations in the prevalence of HCV infection in different parts of the world could be a result of a combination of several factors including, different risky behaviors at different geographical locations, the quality of the primary screening test, and the effectiveness of their program to select blood donors.
In the current study, higher prevalence of HCV was associated with study participants in the age group of 31–35 years and 45–65 years compared to study participants in 18–30 years. This outcome might be due to patients of older age at the time of infection and impaired immune system are at increased risk of developing chronic HCV infection.47 Additionally, male blood donors were three times more likely to be infected with HCV infection than female donors. This result was agreed with studies done in another study area.27,48 The findings could be due to some risk behaviors of males such as outside socialization, multiple sex relationships and habit of drinking alcohol than females. Furthermore, it may also be due to fewer females donating blood; hence, fewer females are screened compared to males. Besides, unmarried blood donors were more likely to have HCV infection than married study participants in the current study. The result was an agreement with studies conducted in another study area.4,36,37,49 This might be due to unmarried blood donors might have a wider group of individuals connected through sexual contact, leading to more sexual partners, which in turn increase their risk of acquiring HCV compared with those married.
This study has some limitations; due to a retrospective blood donation card were review it might not include some variables. Additionally, all the test results did not give positive serological result during the window period. Besides, the method of laboratory analysis does not include molecular analysis, which is a more confirmatory test. However, this study tried to give better information on the magnitude, trends and some associated factors of HBV and HCV since it used a large sample size and a long year of blood donors’ data.
Conclusion
The current study shows the prevalence of hepatitis B virus was higher among study participants. However, the prevalence of HCV was low compared to the study conducted in other countries in Africa, a substantial percentage of the blood donors harbor HCV infections. Therefore, it is recommended to strictly the choice of blood donors and screening their blood sample using standard methods to ensure the safety of blood for the recipient. Besides, increasing awareness of people (particularly on unmarried, male and rural resident) on modes of transmission and prevention of infection could help in reducing the burden of both HBV and HCV.
Data Sharing Statement
All data generated or analyzed during this study were included in this article.
Ethics Approval and Consent to Participate
This research was conducted after obtaining ethical clearance from the Wollega University, Department of Medical Laboratory Sciences ethical review committee. All data and samples obtained from them were kept confidential by using codes instead of any personal identifiers and were meant only for the purpose of the study. However, because of the nature of the study (retrospective review of blood donors’ records), informed consent was not applied from the study subjects. The study was conducted in accordance with the Declaration of Helsinki.
Acknowledgments
We would like to thank the staff of Nekemte blood bank.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Mohammed Y, Bekele A. Seroprevalence of transfusion transmitted infection among blood donors at Jijiga blood bank, Eastern Ethiopia: retrospective 4 years study Infectious Diseases. BMC Res Notes. 2016;9(1):129. doi:10.1186/s13104-016-1925-6
2. Ataro Z, Urgessa F, Wasihun T. Prevalence and trends of major transfusion transmissible infections among blood donors in Dire Dawa Blood bank, Eastern Ethiopia: retrospective study. Ethiop J Health Sci. 2018;28(6):701–710.
3. WHO. Blood safety and availability fact sheet. 2019;1–7. Available from: https://www.who.int/news-room/fact-sheets/detail/blood-safety-and-availability.
4. Negash M, Ayalew M, Geremew D, Workineh M. Seroprevalence and associated risk factors for HIV, Hepatitis B and C among blood Donors in South Gondar District blood Bank, Northwest Ethiopia. BMC Infect Dis. 2019;19:430. doi:10.1186/s12879-019-4051-y
5. De Xie D, Li J, Chen JT, et al. Seroprevalence of human immunodeficiency virus, hepatitis B virus, hepatitis C virus, and treponema pallidum infections among blood donors on Bioko Island, Equatorial Guinea. PLoS One. 2015;10(10):1–9.
6. Zaheer HA, Waheed U. Blood safety system reforms in Pakistan. Blood Transfus. 2014;12(4):452–457.
7. Kumar A, Sharma S, Gangane N, Ingole N. Seroprevalence of Transfusion Transmissible Infections (TTIs) among blood donors in a tertiary care hospital, central India: a prospective study. Muller, J MED Sci Res. 2014;5(2):113–116. doi:10.4103/0975-9727.135737
8. Zheng X, Ding W, Li G, et al. Seroprevalence of transfusion-transmissible infectious agents among volunteer blood donors between 2006 and 2012 in Zhejiang, China. Blood Transfus. 2015;13(3):401–410.
9. Bartonjo G, Oundo J, Ng’ang’a Z. Prevalence and associated risk factors of transfusion transmissible infections among blood donors at Regional Blood Transfusion Center Nakuru and Tenwek Mission Hospital, Kenya. Pan Afr Med J. 2019;34:1–13. doi:10.11604/pamj.2019.34.31.17885
10. Belyhun Y, Maier M, Mulu A, Diro E, Liebert UG. Hepatitis viruses in Ethiopia: a systematic review and meta-analysis. BMC Infect Dis. 2016;16:761. doi:10.1186/s12879-016-2090-1
11. Dessie A, Abera B, Wale F. Seroprevalence of major blood-borne infections among blood donors at Felege Hiwot referral hospital, Northwest Ethiopia. Ethiop J Heal Dev. 2007;21(1):1–3.
12. Biadgo B, Shiferaw E, Woldu B, Alene KA, Melku M. Transfusion-transmissible viral infections among blood donors at the North Gondar district blood bank, northwest Ethiopia: a three year retrospective study. PLoS. 2017;12(7):e0180416. doi:10.1371/journal.pone.0180416
13. Birhaneselassie M. Prevalence of transfusion-transmissible infections in donors to an ethiopian blood bank between 2009 and 2013 and donation factors that would improve the safety of the blood supply in underdeveloped countries. Lab Med. 2016;47(2):134–139. doi:10.1093/labmed/lmw003
14. Chaudhary V, Agrawal VK, Sexena SK, Upadhyay D, Singh A, Singh SP. Research article seroprevalence of common transfusion transmissible infections among blood donors in Western Uttar Pradesh, India. Int J Med Sci Public Heal. 2014;3(11):1381–1384. doi:10.5455/ijmsph.2014.160820143
15. Tessema B, Yismaw G, Kassu A, et al. Seroprevalence of HIV, HBV, HCV and syphilis infections among blood donors at Gondar University Teaching Hospital, Northwest Ethiopia: declining trends over a period of five years. BMC Infect Dis. 2010;10:111.
16. Nada HA, Atwa M. Seroprevalence of HBV, HCV, HIV and syphilis markers among blood donors at Suez Canal University Hospital Blood Bank. J Blood Disord Transf. 2013;5(1):177.
17. Shiferaw I, Tadilo W, Melkie I, Shiferaw M. Sero-prevalence and trends of transfusion- transmissible infections among blood donors at Bahir Dar district blood bank, northwest Ethiopia: a four year retrospective study. PLoS One. 2019;14(4):e0214755. doi:10.1371/journal.pone.0214755
18. Kebede W, Mekonnen Z, Gerbi A, Abebe G. Transfusion-transmissible infection surveillance among blood donors in Southwest Ethiopia: a six year retrospective study. Asian Pac J Trop Dis. 2017;7(3):156–161. doi:10.12980/apjtd.7.2017D6-384
19. Bisetegen FS, Bekele FB, Ageru TA, Wada FW. Transfusion-transmissible infections among voluntary blood donors at Wolaita Sodo University Teaching Referral Hospital, South Ethiopia. Can J Infect Dis Med Microbiol. 2016;1–6. doi:10.1155/2016/8254343
20. Teklemariam Z, Mitiku H, Weldegebreal F. Seroprevalence of transfusion transmissible viral infections (HIV, HBV and HCV) among voluntary blood donors at University of Gondar Comprehensive Specialized Hospital, Gondar; Northwest Ethiopia. BMC Hematol. 2018;18:24. doi:10.1186/s12878-018-0115-2
21. Tigabu A, Engda T, Mekonnen F. Seroprevalence of transfusion transmissible viral infections (HIV, HBV and HCV) among voluntary blood donors at University of Gondar Comprehensive Specialized Hospital, Gondar; Northwest Ethiopia. BMC Infect Dis. 2019;19:393. doi:10.1186/s12879-019-3950-2
22. Okoroiwu HU, Okafor IM, Asemota EA. Seroprevalence of transfusion-transmissible infections (HBV, HCV, syphilis and HIV) among prospective blood donors in a tertiary health care facility in Calabar, Nigeria; an eleven year evaluation. BMC Public Health. 2018;18:645.
23. Yambasu EE, Reid A, Owiti P, Manzi M, Murray M, Edwin A. Hidden dangers-prevalence of blood borne pathogens, hepatitis B, C, HIV and syphilis, among blood donors in Sierra Leone in 2016: opportunities for improvement: a retrospective, cross-sectional study. Pan Afr Med J. 2018;30:44. doi:10.11604/pamj.2018.30.44.14663
24. Jary A, Dienta S, Leducq V, et al. Seroprevalence and risk factors for HIV, HCV, HBV and syphilis among blood donors in Mali. BMC Infect Dis. 2019;19:1064. doi:10.1186/s12879-019-4699-3
25. Osei E, Lokpo SY, Agboli E. Sero ‑ prevalence of hepatitis B infection among blood donors in a secondary care hospital, Ghana: a retrospective analysis. BMC Res Notes. 2017;10:391. doi:10.1186/s13104-017-2733-3
26. Fessehaye N, Naik D, Fessehaye T. Transfusion transmitted infections – a retrospective analysis from the National Blood Transfusion Service in Eritrea. Pan Afr Med J. 2011;9:40. doi:10.4314/pamj.v9i1.71219
27. Mavenyengwa RT, Mukesi M, Chipare I, Shoombe E. Prevalence of human immunodeficiency virus, syphilis, hepatitis B and C in blood donations in Namibia. BMC Infect Dis. 2014;14:424.
28. Shrestha AC, Ghimire P, Tiwari BR, Rajkarnikar M. Transfusion-transmissible infections among blood donors in Kathmandu, Nepal. J Infect Dev Ctries. 2009;3(10):794–797. doi:10.3855/jidc.311
29. Mojtaba A, Azadbakht M, Ardakani MT, Delirakbariazar M, Kasraian L. Seroprevalence and trend of HBV, HCV, and HIV Infections among Blood Donors of Fars Province, Iran (2006-2018). Ethiop J Heal Sci. 2020;30(3):397–408.
30. Song Y, Bian Y, Petzold M, Ung COL. Prevalence and trends of major transfusion-transmissible infections among blood donors in Western China, 2005 through 2010. PLoS One. 2014;9(4):e94528. doi:10.1371/journal.pone.0094528
31. Habte Y, Seyoum B, Alemayehu T. Hepatitis B virus infection and associated factors among blood donors at Dire Dawa, Eastern Ethiopia. J Antivir Antiretrovir. 2016;8(4):103–106. doi:10.4172/jaa.1000144
32. Deressa T, Birhan W, Enawgaw B, et al. Proportion and predictors of transfusion- transmissible infections among blood donors in North Shewa Zone, Central North Ethiopia. PLoS One. 2018;13(3):e0194083. doi:10.1371/journal.pone.0194083
33. Thursz MR. Host Genetic factors influencing the outcome of hepatitis. J Viral Hepat. 2003;4(4):215–220. doi:10.1046/j.1365-2893.1997.00052.x
34. Siraj N, Achila OO, Issac J, et al. Seroprevalence of transfusion-transmissible infections among blood donors at National Blood Transfusion Service, Eritrea: a seven- year retrospective study. BMC Infect Dis. 2018;18:264. doi:10.1186/s12879-018-3174-x
35. Ayele AG, Gebre-Selassie S. Prevalence and risk factors of Hepatitis B and Hepatitis C virus infections among patients with chronic liver diseases in public Hospitals in Addis Ababa, Ethiopia. ISRN Trop Med. 2013;1–7. doi:10.1155/2013/563821
36. Bahadar S, Hayat A, Daud M, et al. Prevalence of Hepatitis B and C Infection in District Dir, Khyber Paktunkhwa, Pakistan. World J Zool. 2015;10(2):142–146.
37. Kposowa AJ. Marital status and HIV/AIDS mortality: evidence from the US National Longitudinal Mortality Study. Int J Infect Dis. 2013;17(10):e868–74. doi:10.1016/j.ijid.2013.02.018
38. Dongdem JT, Kampo S, Soyiri IN, Asebga PN, Ziem JB, Sagoe K. Prevalence of hepatitis B virus infection among blood donors at the Tamale Teaching Hospital, Ghana (2009). BMC Res Notes. 2012;5(1):115. doi:10.1186/1756-0500-5-115
39. Behal R, Jain R, Behal KK, Bhagoliwal A, Aggarwal N, Dhole TN. Seroprevalence and risk factors for hepatitis-B virus infection among general population in northern India. Arq Gastroenterol. 2008;45(2):137–140. doi:10.1590/S0004-28032008000200009
40. Hong Z, et al. Seroprevalence and risk factors for hepatitis B infection in an adult population in Northeast China. Int J Med Sci. 2011;8(4):321–331. doi:10.7150/ijms.8.321
41. de Lindenberg ASC, Motta-Castro ARC, Puga MA, et al. Decrease in hepatitis B prevalence among blood donors in Central-West Brazil. J Venom Anim Toxins Incl Trop Dis. 2013;19(1):7. doi:10.1186/1678-9199-19-7
42. Zenebe Y, Mulu W, Yimer M, Abera B. Sero-prevalence and risk factors of hepatitis C virus infection among pregnant women in Bahir Dar city, Northwest Ethiopia: cross sectional study. Pan Afr Med J. 2015;21:158. doi:10.11604/pamj.2015.21.158.6367
43. Bazie EA, Ali MMA, Hamza HB, Magzoub OS, Salih MSM, Haroun BE. Sero-prevalence of viral transfusion-transmissible infections among blood donors at Kosti Teaching Hospital, White Nile State/Sudan. Int J Curr Microbiol App Sci. 2015;4(5):1132–1138.
44. Matee MIN, Magesa PM, Lyamuya EF. Seroprevalence of human immunodeficiency virus, hepatitis B and C viruses and syphilis infections among blood donors at the Muhimbili National Hospital in Dar Es Salaam, Tanzania. BMC Public Health. 2006;6:21. doi:10.1186/1471-2458-6-21
45. Adu-poku F, Agboli E, Tarkang EE, Region HV, Region HV. Seroprevalence of transfusion-transmissible infections among blood donors in the Hohoe Municipal Hospital, Ghana: 2015-2016: a retrospective hospital-based cross-sectional study. PAMJ Clin Med. 2020;2:12.
46. Omran D, Hussein EA, Nagib M. Safety of blood transfusion: an Egyptian study. J Infect Dis. 2013;2(1):124.
47. Reid M, Price JC, Tien PC. Hepatitis C virus infection in the older patient. Infect Dis Clin North Am. 2017;31(4):827–838. doi:10.1016/j.idc.2017.07.014
48. Tserenpuntsag B, Ouynbileg L, Nelson K, McNutt LA. Prevalence of infectious diseases among Mongolian blood donors. J Infect Dev Ctries. 2008;2(1):73–75. doi:10.3855/jidc.326
49. Malaju MT, Asale GA. Association of Khat and alcohol use with HIV infection and age at first sexual initiation among youths visiting HIV testing and counseling centers in Gamo-Gofa Zone, South West Ethiopia. BMC Int Health Hum Rights. 2013;13:10. doi:10.1186/1472-698X-13-10
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
