Back to Journals » Pharmacogenomics and Personalized Medicine » Volume 13
Prevalence of CYP2C8*2 and *3 among Eritreans and its Potential Impact on Artesunate/Amodiaquine Treatment
Authors Habtemikael L, Russom M , Bahta I, Mihreteab S, Berhane A, Mårtensson A, Gil JP
Received 14 August 2020
Accepted for publication 16 October 2020
Published 12 November 2020 Volume 2020:13 Pages 571—575
DOI https://doi.org/10.2147/PGPM.S276215
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Martin H Bluth
Lidia Habtemikael,1 Mulugeta Russom,2 Iyassu Bahta,2 Selam Mihreteab,3 Araia Berhane,4 Andreas Mårtensson,1 Jose Pedro Gil1,5,6
1Department of Women’s and Children’s Health, International Maternal and Child Health (IMCH), Uppsala University, Uppsala, Sweden; 2National Medicines and Food Administration, Ministry of Health, Asmara, Eritrea; 3National Malaria Control Programme, Ministry of Health, Asmara, Eritrea; 4Communicable Diseases Control Division, Ministry of Health, Asmara, Eritrea; 5Department of Microbiology and Tumour Cell Biology, Karolinska Institute, Stockholm, Sweden; 6Biosystems & Integrative Sciences Institute, Faculty of Sciences, University of Lisbon, Lisbon, Portugal
Correspondence: Mulugeta Russom
Eritrean Pharmacovigilance Centre, National Medicines and Food Administration, Asmara, Eritrea
Tel +291-7197450
Fax +291-1-122899
Email [email protected]
Background: In Eritrea, artesunate–amodiaquine is the first-line treatment against uncomplicated malaria. Amodiaquine, which is mainly bio-transformed by CYP2C8, is known to be associated with adverse events of different severity. Extrapyramidal events are among the less common but have been reported with non-negligible frequency in Eritrea. This study was conducted to investigate the allele frequencies of CYP2C8*2 and *3, both associated with decreased amodiaquine metabolism, among the Eritrean population.
Methods: During September–November 2018, dried blood samples from 380 participants and 17 patients who previously had experienced extrapyramidal symptoms following treatment of artesunate–amodiaquine were collected and PCR-RFLP genotyped for CYP2C8*2 and *3.
Results: The allele frequencies of CYP2C8*2 and *3 were determined as 5.9% (95% CI: 4.4– 7.8) and 4.6% (95% CI: 3.2– 6.3), respectively. Four out of the 17 patients with extrapyramidal reactions showed to be carriers of the alleles.
Conclusion: CYP2C8*2 and *3 frequencies among Eritreans were found to be intermediate between the documented for Caucasian and African populations. These findings, along with the alleles not being decisive for the occurrence of extrapyramidal events, might be of importance regarding the amodiaquine-containing malaria treatment in Eritrea. Furthermore, it suggests a significant proportion of slow amodiaquine metabolizers in the Sahel region, information of potential interest in the context of amodiaquine-involving seasonal malaria chemoprevention.
Keywords: malaria, uncomplicated, artesunate/amodiaquine, CYP2C8, slow metabolizers
Plain Language Summary
Of the globally reported artesunate–amodiaquine (AS-AQ)-related extrapyramidal symptoms (2018), >50% were submitted from Eritrea. The causes/factors for this population-specific increased incidence of extrapyramidal symptoms are not known. We hypothesized that mutations in CYP2C8, associated to reduced amodiaquine metabolism, might be more frequent among Eritreans, potentially leading to the adverse effects. In 2018, dried blood samples were collected from 380 non-malaria patients and 17 people who experienced extrapyramidal symptoms following treatment with AS-AQ. Molecular analysis of the samples collected from all parts of Eritrea was performed in Uppsala University, Sweden. The results reveal that allele frequencies of CYP2C8*2 and *3, both associated with decreased AQ metabolism, were determined as 5.9% and 4.6%, respectively. These results reflect that the Eritrean population is significantly different from other African populations. Most of the 17 cases did not carry CYP2C8 minor alleles, clearly showing that such adverse effects can occur independently of the patient genotype status for this gene. This pilot study was however too small for testing possible associations with these variants; thus, warrants further larger studies. Our results further fuel the intriguing question if the specific CYP2C8 profile in the Eritrean population drives an overall increased risk of AQ-related extrapyramidal reactions.
Introduction
Malaria is a key public health issue in Eritrea, with 70% of the national population considered to be in high risk of the disease and >50,000 new cases being reported every year, mostly Plasmodium falciparum infections.1 The fixed-dose artesunate–amodiaquine (AS-AQ) combination is the first-line of treatment for uncomplicated malaria. In recent years, extrapyramidal symptoms associated with AS-AQ have been frequently observed in Eritrea,2 representing >50% of such reports in VigiBase, the WHO global database of individual case safety reports (December 2018).3 The causes/factors for this population-specific incidence of AS-AQ related extrapyramidal symptoms are not known.
Amodiaquine is mainly metabolized by the hepatic cytochrome P450 (CYP) 2C8.4 The CYP2C8 gene harbours a number of sequence polymorphisms leading to decreased biotransformation capacity of amodiaquine. Globally, CYP2C8*2 and *3 are the most prevalent, with *3 suggested to result in significantly impaired metabolism.5 CYP2C8*2, defined by an I269F change (exon 5, 805A>T), is the most frequent minor allele present in sub-Saharan regions (10–20%), while *3 (R139K, exon 3, 416G>A and 399, exon 8 in absolute linkage) is dominant among Caucasians6 but rare in Africans (<0.5%).7
Eritrea is located in the Eastern Sahel region - a natural interface between African and Arabian speaking Caucasian populations – where CYP2C8 allele frequencies are not known. This study was conducted to determine the prevalence of CYP2C8 polymorphism for the first time in Eritrea, part of the Sahel belt. In parallel, we conducted a pilot analysis of a group of previous malaria patients who have experienced extrapyramidal effects upon AS-AQ treatment.
Materials and Methods
A hospital-based cross-sectional study was conducted between September and November 2018 in hospitals covering all (six) administrative regions of Eritrea (Figure 1). A total of 380 unrelated subjects (160 males, 220 females, mean age: 31 years), covering the major Eritrean ethnic groups, were randomly recruited from the outpatient departments. None of these subjects were malaria patients or, to our knowledge, exposed to amodiaquine in connection with the study.
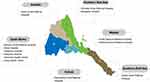 |
Figure 1 Map of Eritrea, its six administrative regions and the geographic distribution of the sampling points (health facilities in each zone) covering all of the regions of the country. |
In total, 17 previous malaria patients with experience of AS-AQ related extrapyramidal symptoms, were retrospectively retrieved from the Eritrean Pharmacovigilance Centre, were recruited and analysed separately. Upon informed consent, a blood sample (ca. 200µL) was obtained for each enrolled patient and preserved as dried blood spots in filter paper. All procedures and interactions with subjects followed the recommendations of the Declaration of Helsinki Convention in its latest iteration.
DNA-extractions were performed through a rapid boiling-based method as previously described,8 with minor modifications. Polymerase chain reaction primers were as follows: for 805A>T analysis (*2): 5ʹ-ATGTTGCTCTTACACGAAGTTACA-3ʹ (fw) and 5ʹ-ATCTTACCTGCTCCATTTTGA-3ʹ (rev). For 416G>A (*3), 5ʹ-CTTCCGTGCTACATGATGACg-3ʹ (fw, incl. 3ʹ mismatch) and 5ʹ-TGCTGAGAAAGGCATGAAG-3ʹ (rev).9 This was followed by digestions with BclI (805A>T) and XmnI (416G>A). Restriction results were visualized through 2.5% agarose electrophoresis. Fisher’s exact test was applied for proportion comparisons.
Results
The 380 subjects were successfully genotyped for both CYP2C8*2 and *3. Approximately 10.5% were carriers of *2 and 8.2% of *3, resulting in allele frequencies of 5.9% (95% CI: 4.4–7.8) and 4.6% (95% CI: 3.2–6.3) respectively (Table 1). Both alleles were found to be in Hardy–Weinberg equilibrium (Fisher’s exact test, p>0.2). Concerning the 17 cases that have experienced AS-AQ-associated extrapyramidal effects, four showed to be carriers of the *2 and/or *3 allele (Table 1).
 |
Table 1 CYP2C8 Genotype and Allele Frequencies Among Selected Eritrean Population, 2018 |
Discussion
This is the first study investigating CYP2C8 polymorphism in the Eastern Sahel region. Compared to other Africans,5,9–18 the *2 allele frequency among Eritreans is markedly lower, while the *3 frequency was found to be second highest in Africa; following a regional Tanzanian study.18 The prevalence of CYP2C8*3 polymorphism in most African countries was reported to be negligible, less than 0.5%, except in Zanzibar (2.1%)9 and Tanzania (10%).18 This finding might therefore be of concern since amodiaquine is a key antimalarial used for the treatment of uncomplicated malaria, presently pivotal in the Eritrean malaria control program. Extrapolating from the obtained data, one could expect at least one *3/*3 carrier for every 200–400 individuals, which are potentially in higher risk of amodiaquine-related adverse effects including extrapyramidal reactions.
Sulfadoxine-pyrimethamine/amodiaquine seasonal malaria chemoprevention, though not implemented in Eritrea, is mostly targeted for populations in the Sahel belt during seasonal malaria. Assuming data as representative of populations from these African/Caucasian interface regions, the reported CYP2C8*3 frequency raises a moderate apprehension, as this allele has been previously associated with accelerated selection of parasite carrying resistance markers.19 Furthermore, the optimal use of amodiaquine should carefully consider the characteristics of the targeted setting and population.20
Concerning the specific issue of amodiaquine-driven extrapyramidal effects and CYP2C8, this pilot study could only conclude that the presence of *2 or *3 is not an essential condition for their occurrence. Other key genetic factors are likely to be involved and thus, future larger associative studies are required for their identification.
CYP2C8 is involved in the biotransformation of a range of relevant therapeutics, including widely used antidiabetics (eg repaglinide), cytostatics (eg paclitaxel) and statins (eg cerivastatin).21 Our data suggests as such the potential for specific drug exposures beyond amodiaquine in this population. The future of precision medicine initiatives in Africa is dependent on a better understanding of native pharmacogenetic specificities.22 Studies like the present one showcases the pharmacogenetic diversity of the continent and the need of its better characterization.
Conclusion
CYP2C8*2 and *3 are present among the Eritreans in prevalence intermediate between the Caucasian and sub-Saharan populations. To our knowledge, this is the first population reported with such a profile, suggesting that populations in the Sahel region include a significant proportion of amodiaquine low-metabolizers. This represents potentially valuable information when monitoring amodiaquine-containing therapeutic strategies in these regions, including seasonal malaria chemoprevention.
Data Sharing Statement
Data used for this study are all available on the manuscript.
Ethics Approval and Informed Consent
Ethical approval to conduct the study was obtained from the health research ethics and protocol review committee of the Ministry of Health of Eritrea (reference number: 8/2018). Besides, informed consent was obtained from all study subjects to participate in the study.
Acknowledgments
The authors sincerely thank all Medical Directors, laboratory experts, enrolled patients and Zonal Medical Officers of the respective Zones and study sites for their commendable collaboration in identifying eligible people and taking the dried blood samples. We also thank Fitui Habtemikael and Saba Haile for their support in transportation and procurement of materials.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was funded by the Swedish Research Council Grant (VR-2014-3134). LH received a scholarship through the Minor Fields Studies program, financed by the Swedish International Development Cooperation Agency. The sponsors, however, had no involvement in the design, conduct and write-up of the study.
Disclosure
The authors declare that they have no competing interests.
References
1. World Health Organization, Global Malaria Programme. World Malaria Report 2019; 2019.
2. Russom M, Tesfai D, Gebregiorgis S, et al. Artesunate/amodiaquine-induced acute extrapyramidal reactions in children and younger adults: case series assessment. Drug Saf. 2016;39(8):763–768. doi:10.1007/s40264-016-0429-6
3. Lindquist M. VigiBase, the WHO global ICSR database system: basic facts. Drug Inf J. 2008;42:409–419.
4. Li X-Q, Björkman A, Andersson TB, Ridderström M, Masimirembwa CM. Amodiaquine clearance and its metabolism to N-desethylamodiaquine is mediated by CYP2C8: a new high affinity and turnover enzyme-specific probe substrate. J Pharmacol Exp Ther. 2002;300(2):399–407. doi:10.1124/jpet.300.2.399
5. Parikh S, Ouedraogo J-B, Goldstein JA, Rosenthal PJ, Kroetz DL. Amodiaquine metabolism is impaired by common polymorphisms in CYP2C8: implications for malaria treatment in Africa. Clin Pharmacol Ther. 2007;82(2):197–203. doi:10.1038/sj.clpt.6100122
6. Cavaco I, Piedade R, Gil JP, Ribeiro V. CYP2C8 polymorphism among the Portuguese. Clin Chem Lab Med. 2006;44(2):168–170. doi:10.1515/CCLM.2006.030
7. Rajman I, Knapp L, Morgan T, Masimirembwa C. African genetic diversity: implications for cytochrome P450-mediated drug metabolism and drug development. EBioMedicine. 2017;17:67–74.
8. Strøm GEA, Tellevik MG, Hanevik K, Langeland N, Blomberg B. Comparison of four methods for extracting DNA from dried blood on filter paper for PCR targeting the mitochondrial plasmodium genome. Trans R Soc Trop Med Hyg. 2014;108(8):488–494.
9. Cavaco I, Strömberg-Nörklit J, Kaneko A, et al. CYP2C8 polymorphism frequencies among malaria patients in Zanzibar. Eur J Clin Pharmacol. 2005;61(1):15–18. doi:10.1007/s00228-004-0871-8
10. Röwer S, Bienzle U, Weise A, et al. Short communication: high prevalence of the cytochrome P450 2C8*2 mutation in Northern Ghana. Trop Med Int Health. 2005;10(12):1271–1273. doi:10.1111/j.1365-3156.2005.01525.x
11. Kudzi W, Dodoo AN, Mills JJ. Characterisation of CYP2C8, CYP2C9 and CYP2C19 polymorphisms in a Ghanaian population. BMC Med Genet. 2009;10:124. doi:10.1186/1471-2350-10-124
12. Adjei GO, Kristensen K, Goka BQ, et al. Effect of concomitant artesunate administration and cytochrome P4502C8 polymorphisms on the pharmacokinetics of amodiaquine in Ghanaian children with uncomplicated malaria. Antimicrob Agents Chemother. 2008;52(12):4400–4406. doi:10.1128/AAC.00673-07
13. Yates AD, Achuthan P, Akanni W, et al. Ensembl 2020. Nucleic Acids Res. 2020 Jan 8;48(D1):D682–D688. doi:10.1093/nar/gkz966.
14. Paganotti GM, Gramolelli S, Tabacchi F, et al. Distribution of human CYP2C8*2 allele in three different African populations. Malar J. 2012;11:125. doi:10.1186/1475-2875-11-125
15. Arnaldo P, Thompson RE, Lopes MQ, Suffys PN, Santos AR. Frequencies of cytochrome P450 2B6 and 2C8 allelic variants in the mozambican population. Malays J Med Sci. 2013;20(4):13–23.
16. Adehin A, Bolaji OO, Kennedy MA. Polymorphisms in CYP2C8 and CYP3A5 genes in the Nigerian population. Drug Metab Pharmacokinet. 2017;32(3):189–191. doi:10.1016/j.dmpk.2016.09.001
17. Staehli Hodel EM, Csajka C, Ariey F, et al. Effect of single nucleotide polymorphisms in cytochrome P450 isoenzyme and N-acetyltransferase 2 genes on the metabolism of artemisinin-based combination therapies in malaria patients from Cambodia and Tanzania. Antimicrob Agents Chemother. 2013;57(2):950–958. doi:10.1128/AAC.01700-12
18. Marwa KJ, Schmidt T, Sjögren M, Minzi OMS, Kamugisha E, Swedberg G. Cytochrome P450 single nucleotide polymorphisms in an indigenous Tanzanian population: a concern about the metabolism of artemisinin-based combinations. Malar J. 2014;13:420. doi:10.1186/1475-2875-13-420
19. Cavaco I, Mårtensson A, Fröberg G, Msellem M, Björkman A, Gil JP. CYP2C8 status of patients with malaria influences selection of Plasmodium falciparum pfmdr1 alleles after amodiaquine-artesunate treatment. J Infect Dis. 2013;207(4):687–688. doi:10.1093/infdis/jis736
20. Greenwood B. New tools for malaria control - using them wisely. J Infect. 2017;74(Suppl 1):S23–S26. doi:10.1016/S0163-4453(17)30187-1
21. Tornio A, Backman JT. Cytochrome P450 in Pharmacogenetics: an Update. Adv Pharmacol. 2018;83:3–32. doi:10.1016/bs.apha.2018.04.007.
22. Peñas-LLedó E, Terán E, Sosa-Macías M, et al. Challenges and opportunities for clinical pharmacogenetic research studies in resource-limited settings: conclusions from the council for international organizations of medical sciences-Ibero-American Network of Pharmacogenetics and Pharmacogenomics Meeting. Clin Ther. 2020;42(8):1595–1610.e5. doi:10.1016/j.clinthera.2020.06.008.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
