Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 14
Prevalence of cardiac comorbidities, and their underdetection and contribution to exertional symptoms in COPD: results from the COSYCONET cohort
Authors Alter P , Mayerhofer BA , Kahnert K, Watz H, Waschki B , Andreas S , Biertz F , Bals R, Vogelmeier CF, Jörres RA
Received 19 March 2019
Accepted for publication 10 July 2019
Published 20 September 2019 Volume 2019:14 Pages 2163—2172
DOI https://doi.org/10.2147/COPD.S209343
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Richard Russell
Peter Alter,1 Barbara A Mayerhofer,2 Kathrin Kahnert,3 Henrik Watz,4 Benjamin Waschki,5,6 Stefan Andreas,7,8 Frank Biertz,9 Robert Bals,10 Claus F Vogelmeier,1 Rudolf A Jörres2
1Department of Medicine, Pulmonary and Critical Care Medicine, Philipps University of Marburg (UMR), Member of the German Center for Lung Research (DZL), Marburg, Germany; 2Institute and Outpatient Clinic for Occupational, Social and Environmental Medicine, University Hospital, LMU Munich, Comprehensive Pneumology Center Munich (CPC-M), Member of the Center for Lung Research (DZL), Munich, Germany; 3Department of Internal Medicine V, University Hospital, LMU Munich, Comprehensive Pneumology Center Munich (CPC-M), Member of the German Center for Lung Research (DZL), Munich, Germany; 4Pulmonary Research Institute at Lungen Clinic Grosshansdorf, Airway Research Center North (ARCN), Member of the German Center for Lung Research (DZL), Grosshansdorf, Germany; 5Department of Pneumology, LungenClinic Grosshansdorf, Airway Research Center North (ARCN), Member of the German Center for Lung Research (DZL), Grosshansdorf, Germany; 6Department of General and Interventional Cardiology, University Heart Center, Hamburg, Germany; 7Department of Cardiology and Pneumology, University Medical Center, Goettingen, Germany; 8Lung Clinic, Immenhausen, Germany; 9Institute for Biostatistics, Center for Biometry, Medical Informatics and Medical Technology, Hannover Medical School, Hannover, Germany; 10Department of Internal Medicine V - Pulmonology, Allergology, Intensive Care Medicine, Saarland University Hospital, Homburg, Germany
Correspondence: Peter Alter
Department of Medicine, Pulmonary and Critical Care Medicine, Philipps University of Marburg (UMR), Member of the German Center for Lung Research (DZL), Baldingerstrasse 1, Marburg 35033, Germany
Tel +49 6 421 586 6140
Email [email protected] 
Rudolf A Jörres
Institute and Outpatient Clinic for Occupational, Social and Environmental Medicine, University Hospital, LMU Munich, Comprehensive Pneumology Center Munich (CPC-M), Member of the Center for Lung Research (DZL), Ziemssenstrasse 1, Munich 80336, Germany
Tel +49 8 944 005 2466
Email [email protected]  
Background: A substantial prevalence of cardiovascular disease is known for COPD, but detection of its presence, relationship to functional findings and contribution to symptoms remains challenging. The present analysis focusses on the cardiovascular contribution to COPD symptoms and their relationship to the patients’ diagnostic status, medication and echocardiographic findings.
Methods: Patients from the COPD cohort COSYCONET with data on lung function, including FEV1, residual volume/total lung capacity (RV/TLC) ratio, diffusing capacity TLCO, and echocardiographic data on left ventricular ejection fraction (LVEF) and end-diastolic diameter (LVEDD), medical history, medication, modified British Medical Research Council dyspnea scale (mMRC) and Saint Georges Respiratory Questionnaire (SGRQ) were analyzed.
Results: A total of 1591 patients (GOLD 0–4: n=230/126/614/498/123) fulfilled the inclusion criteria. Ischemic heart disease, myocardial infarction or heart failure were reported in 289 patients (18.2%); 860 patients (54%) received at least one cardiovascular medication, with more than one in many patients. LVEF<50% or LVEDD>56 mm was found in 204 patients (12.8%), of whom 74 (36.3%) had neither a cardiovascular history nor medication. Among 948 patients (59.6%) without isolated hypertension, there were 21/55 (38.2%) patients with LVEF<50% and 47/88 (53.4%) with LVEDD>56 mm, who lacked both a cardiac diagnosis and medication. LVEDD and LVEF were linked to medical history; LVEDD was dependent on RV/TLC and LVEF on FEV1. Exertional COPD symptoms were best described by mMRC and the SGRQ activity score. Beyond lung function, an independent link from LVEDD on symptoms was revealed.
Conclusion: A remarkable proportion of patients with suspicious echocardiographic findings were undiagnosed and untreated, implying an increased risk for an unfavorable prognosis. Cardiac size and function were dependent on lung function and only partially linked to cardiovascular history. Although the contribution of LV size to COPD symptoms was small compared to lung function, it was detectable irrespective of all other influencing factors. However, only the mMRC and SGRQ activity component were found to be suitable for this purpose.
Keywords: COPD, heart failure, echocardiography, medication, dyspnea, symptoms
Introduction
A substantial prevalence of cardiovascular disease has been reported for patients with COPD, comprising a range from 28% to 70%,1–4 with poor agreement between chart-based and objectively identified comorbidities.5 Beyond shared risk factors, a variety of potential causal interactions has been discussed.6–10 This is important as the concomitance of cardiovascular disease and COPD is associated with an unfavorable prognosis.11–14 Regarding clues from symptoms, the diagnosis of the predominant underlying disease and its contribution to COPD symptoms remains challenging. The situation is complicated by the fact that, even when objective measures of heart function are available, they do not necessarily correlate with the magnitude of symptoms. In addition, patients may receive cardiovascular medication, which may alleviate symptoms to a variable degree, depending on the type and severity of cardiac disease. Moreover, the indication that led to medication in the past might not be verifiable at a later time.
This opens the possibility to encounter patients with and without cardiac disease as a comorbidity of COPD, as well as with and without medication in all conceivable combinations. A further complication is the fact that the medication can be more or less specific, which may render its attribution to a specific disorder difficult.15,16 The distribution and frequency of these conditions, including abnormalities of common echocardiographic measures, are not well known so far, and it would be valuable to obtain this information from a sufficiently characterized population of COPD patients.
We therefore examined the history of ischemic heart disease including myocardial infarction, and heart failure, cardiovascular medication, moreover two left ventricular echocardiographic measures, lung function and symptom scores in the large COPD cohort COSYCONET. For the quantification of symptoms, the modified British Medical Research Council dyspnea scale (mMRC) and the Saint George's Respiratory Questionnaire (SGRQ) were used. The study had two specific aims, first the description of the patterns observed, second the analysis and quantification of the relationship between parameters of lung and heart function and their relative role for exertional COPD symptoms.
Methods
Study cohort and participants
We used data of the baseline visit of the German COPD cohort COSYCONET (COPD and Systemic Consequences – Comorbidities Network), a prospective, multi-center cohort study in patients with stable COPD of spirometric GOLD grades 0–4.17,18 GOLD 0 refers to patients with chronic bronchitis not fulfilling the spirometric criterion FEV1/FVC <0.7. All study participants provided their written informed consent, and the study was approved by the Ethics Committee of the University of Marburg as coordinating center and the Ethics Committees of all study centers; it is in accordance with the declaration of Helsinki and registered on ClinicalTrials.gov (registration number NCT01245933). For details of the selection process, see Supplemental methods and Figure S1.
Assessments
Postbronchodilator17 spirometry and body plethysmography were performed as recommended by the American Thoracic Society/European Respiratory Society19 and Deutsche Gesellschaft für Pneumologie und Beatmungsmedizin.20–23 As a measure of airway obstruction, we used the FEV1; for lung hyperinflation, we chose the residual volume (RV), total lung capacity (TLC) and their ratio RV/TLC. The diffusing capacity for carbon monoxide (TLCO) was determined via duplicate assessments of the single-breath method, and the transfer coefficient (KCO) as ratio of TLCO and alveolar volume (VA). Predicted values of FEV1 and diffusing capacity were calculated as recommended by the Global Lung Function Initiative,24–26 those of RV and TLC were taken from the ECSC.21,24 Spirometric COPD grades and the categorization of symptoms and exacerbation risk were based on the GOLD recommendations,18 choosing the mMRC27 for ABCD groups. For the assessment of COPD symptoms, the SGQR score including its activity, impact and symptoms components,28 the mMRC29 and the COPD Assessment Test (CATTM)30 were evaluated. Echocardiography followed standard techniques to obtain the left ventricular end-diastolic diameter (LVEDD) and left ventricular ejection fraction (LVEF).31 A reduced LVEF was assumed for LVEF <50%, in addition <40% for defining a more severe subgroup. LV dilatation, as a further marker of systolic cardiac impairment, was assumed for LVEDD >56 mm.32–34 Regarding cardiovascular medication, we examined diuretic agents, beta-blockers, angiotensin-converting enzyme inhibitors (ACE inhibitor) and angiotensin receptor blockers (ARB), and mineralocorticoid receptor antagonists.33
Data analysis
For descriptive purposes, mean values and SDs were calculated. For categorical data, the number (percentage) of respective patients is given. We defined the combined criterion “any cardiac history” by the presence of at least one report on ischemic heart disease, myocardial infarction or heart failure symptoms. Analogously, “any cardiovascular medication” was defined by taking at least one compound out of the four classes mentioned above. To illustrate the number and proportion of patients belonging to the different categories and their overlap, Euler diagrams were drawn.35
The association cohort was defined by having complete data for a more comprehensive set of parameters, as completeness of the data is required for structural equation modeling (SEM) if one wants to avoid imputation procedures.8,9 Differences between this group and the patients excluded were analyzed using ANOVA for continuous and chi-square tests for categorical variables (see Supplemental data analysis).
To eliminate trivial dependences from anthropometric measures and reduce the complexity of the model, all variables in the SEM analyses were adjusted for age, sex and BMI. Due to the very high correlation between the mMRC and the SGRQ activity component, we introduced a latent variable (construct) named “Exertional COPD Symptoms” having these two variables as indicators. All other variables were manifest (observed). The goodness of fit was quantified via the comparative fit index (CFI) and the root mean square error of approximation (RMSEA), as well as the standard chi-square statistics, although it is known to be oversensitive in large data sets via indicating an inadequate fit. For all computations, the software IBM SPSS Statistics 25.0.0.1 and Amos 25.0.0 (Wexford, PA, USA) was used. Statistical significance was assumed for p<0.05.
Results
Study population
Overall, 1591 COPD patients of GOLD grades 0–4 fulfilled the inclusion criteria for the descriptive analysis, and among these 1468 patients for the association analysis. Details of the selection process are shown in Figure S1, and baseline characteristics of both groups are given in Table 1. The subgroup of 1468 patients exhibited a slightly less severe airway obstruction and less COPD symptoms compared to the 123 patients excluded for the association analysis due to missing data.
 |
Table 1 Baseline characteristics |
Descriptive analysis
Figure 1A shows the prevalence of the three selected cardiac disorders and the combined history defined by the presence of any of the three disorders (18.2% of patients). The fact that the height of the bar for the combined history was not the sum of those for the single diagnoses indicates the simultaneous presence of several diagnoses in many patients. Figure 1A moreover shows the prevalence of abnormal echocardiographic findings defined as LVEDD >56 mm, or LVEF <50% or <40%. Requiring at least one of these criteria, an impaired left heart function occurred in 204 patients (12.8%). The overlap between the combined history and the echocardiographic criteria LVEDD >56 mm and LVEF <50% is shown in the Euler diagram of Figure 1B. Obviously, the overlap between these three variables was rather small, and the majority of patients with abnormal echocardiographic findings reported no history of cardiac disease.
In an analogous manner, the prevalence of cardiovascular medication including the combined score defined by the presence of any of the respective medications is shown in Figure 2A. More than half of the patients received at least one cardiovascular drug (n=860, 54%). Most frequent were compounds of the ACE inhibitor/ARB group, followed by diuretics and beta-blockers. Again, the fact that the sum of the single components markedly exceeded the combined bar, reflects the fact that quite a number of patients received several drugs. The overlap of medication with the two echocardiographic measures is depicted in Figure 2B. Approximately half of the patients with suspicious echocardiographic findings received cardiovascular medication. The criterion LVEF <50%, or LVEDD >56 mm, or both, was fulfilled in 204 patients (12.8%), of whom 74 (36.3%) had neither a cardiovascular history nor medication. Conversely, the large majority of patients with medication exhibited normal echocardiographic findings, suggesting an indication for treatment other than systolic heart failure. This could be hypertension, which was reported by 860 patients (54.1%). Of 335 patients receiving beta-blocker treatment, the majority (n= 279, 83%) reported arterial hypertension, which appeared as most frequent indication. There was a trend toward higher rates of beta-blocker use in GOLD groups B and D (A-D: 19.2%, 25.3%, 16.3%, 23.3%; p =0.051).
The overlap between the combined scores of medication and medical history, and the criterion LVEF <50% is shown in Figure 3A. In order to focus on systolic heart failure, we then excluded all patients with isolated hypertension, ie, hypertension in the absence of other cardiovascular disorders (n=643, 40.4%). There remained 948 patients (59.6%) without isolated hypertension. The Euler diagram for this reduced data set regarding LVEF <50% is given in Figure 3B, regarding LVEDD >56 mm in Figure 3C. These figures illustrate that there were 21/55 patients (38.2%) and 47/88 patients (53.4%), respectively, with suspicious echocardiographic findings lacking both a respective diagnosis and medication. In order to clinically characterize these patients, we tested in the groups with suspicious echocardiographic findings, whether there were differences in FEV1, RV/TLC, TLCO, mMRC, total SGQR, its activity, impact and symptom components, and the CAT score, when comparing the complementary subgroups defined by the absence of both history and medication and either history or medication or both. The LVEF <50% group did not show significant differences between these subgroups, while in the LVEDD >56 mm group mMRC (p=0.021) and the SGRQ activity component (p=0.002) were worse in the second subgroup. We did not extend these comparisons due to the relatively small sample sizes.
Association analysis by SEM
The aim was to reveal, to which extent lung function, echocardiographic measures, a history of cardiac disorders, or medication directly and indirectly contributed to COPD symptoms. Preliminary analyses revealed that with regard to the other variables, the explanatory power of the SGRQ activity component was superior to that of its other components or the total score; we therefore restricted the analysis to this component. The SGRQ activity score and the mMRC were highly correlated with each other, which allowed their combination into a latent variable termed “Exertional COPD Symptoms”. On the other hand, the CAT could not be consistently embedded into the model, possibly due to its internal heterogeneity as indicated by the fact that an exploratory factor analysis revealed its division into two factors. Thus, regarding COPD symptoms, we restricted the analysis to the latent variable indicated by the mMRC and the SGRQ activity component. Analogous findings were obtained regarding spirometric lung function, for which FEV1 turned out to be the most informative measure compared to FVC and FEV1/FVC. Similarly, the ratio RV/TLC was superior to the separate measures, and TLCO was superior to KCO, as judged from the degree of association with other variables in the multiple regression analyses. These observations were the basis for the construction of the SEM (Figure 4).
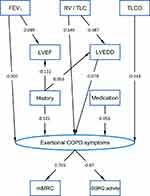 |
Figure 4 Structural equation model (SEM) describing the relationships between indices of lung function including the FEV1% predicted, the ratio of residual volume to total lung capacity (RV/TLC) and carbon monoxide diffusing capacity (0% predicted), echocardiographic measures including the left ventricular end-diastolic diameter (LVEDD, mm) and left ventricular ejection fraction (LVEF, %), the combined medication score, the combined history score, the modified British Medical Research Council dyspnea scale (mMRC) and the activity component of the Saint George's Respiratory Questionnaire (SGRQ). All measured (manifest) variables are indicated by rectangles. A latent variable (indicated by an oval) named “Exertional COPD Symptoms” with indicator variables mMRC and the SGRQ activity component was defined to summarize symptoms. The lines with one arrow describe unidirectional effects, and standardized regression coefficients are given at these arrows. Correlations between a number of variables were introduced to improve the fit. Lung function parameters were correlated with each other, moreover history and medication, furthermore, the error terms of LVEDD and LVEF. These correlations, which were not relevant for the validity of the structure, and the error terms needed for mathematical reasons for all dependent variables have been omitted for the sake of clarity. The numerical values of the respective unstandardized regression coefficients as well as measures of statistical significance are given in Table S1. |
Consistent with our previous findings,8,9 we considered lung function as determinant of other indices, especially echocardiographic findings and symptoms, and “Exertional COPD Symptoms” as final outcome. The effects of lung function on exertional symptoms were dominant, but still there was a significant direct link from LVEDD, but not from LVEF, suggesting that cardiac morphology was more important than cardiac function. Moreover, the summary variables of cardiac history and medication were directly linked to COPD symptoms. Cardiac history was associated with both, LVEDD and LVEF, while LVEDD was dependent on the RV/TLC ratio only, LVEF on FEV1 only. In the multivariate approach, there remained no independent direct links to medication, which was highly correlated with history and thereby explained. In addition, lung function parameters and the error terms of LVEDD and LVEF were highly correlated with each other. We introduced these correlations into the SEM in order to describe the data as well as possible. As they did not affect the validity (in terms of significant links) of the structure that was finally identified, they are omitted in Figure 4 for the sake of clarity, as well as the error terms of dependent variables.
The model fitted the data with a chi-square value of 47.36 and 19 degrees of freedom (p<0.001), corresponding to a CFI of 0.974 and RMSEA of 0.032 (10/90% confidence limits: 0.021, 0.043), ie, values >0.95 and <0.05, respectively, which are considered as indicators of an adequate fit. Taking into account the well-known limitations of the chi-square statistics in large data sets, these results indicated a sufficient fit of the data. The regression coefficients, both unstandardized and standardized, are shown in Table S1, and the standardized coefficients are additionally depicted at the arrows in Figure 4.
Discussion
The present study revealed a substantial prevalence of cardiac disease in patients with stable COPD, in accordance with previous findings.2 At least one of the three selected cardiac disorders was reported by about one-fifth of the patients, while the rate of suspicious echocardiographic left heart findings was lower. The overlap between reported cardiac history and abnormal echocardiography was low, and only a minority of patients with a respective history exhibited an impaired left ventricular systolic function or dilatation. Conversely, less than half of those with impairments reported a cardiac disease, which is in line with other studies.36 A similar pattern was obtained in the partial overlap between cardiovascular medication and suspicious echocardiographic findings. Due to the high prevalence of hypertension and the fact that the majority of cardiovascular medication is also used for the treatment of hypertension, it appeared not unlikely that a large proportion of treatment was due to this different indication. The majority of patients receiving beta-blocker treatment had hypertension. There was a trend toward higher beta-blocker rates in patients with increased COPD symptoms, but there was no specific dependence on exacerbation risk. It is still unclear whether beta-blockers bear the potential to prevent exacerbations,37–39 but in the observational study COSYCONET, the treating physicians did not appear to choose beta-blocker treatment with regard to exacerbation risk.
When excluding patients with isolated hypertension, still a relevant proportion of individuals with LVEF <50% or LVEDD >56 mm did not receive appropriate medication for heart failure treatment (38% and 53%, respectively). This raises the question for the reasons underlying the discordances, in view of the fact that patients with COPD and heart failure appear less likely to receive an appropriate heart failure therapy and thus experience a worse prognosis.40
One of the reasons could be the lack of sufficiently specific symptoms leading to further cardiac examinations. For the assessment of COPD symptoms, several questionnaires have been established and are in clinical use, eg, the mMRC, SGRQ and CAT. This raises the question to which extent these questionnaires are capable of detecting an aggravation of symptoms, by cardiac disorders. We thus investigated in a detailed association analysis to which extent cardiac findings were related to COPD symptoms, especially exertional dyspnea.
In patients with COPD, it is likely that exertional dyspnea, the most frequent symptom of both COPD and left heart failure, is mainly attributed to the respiratory disease, while cardiac causes may be overlooked. We thus addressed the question, to which extent COPD symptom scores are related to echocardiographic findings in groups with and without reported cardiac history and/or medication. It turned out that both history and medication were nearly equivalent with regard to symptoms, as reflected in their high correlation, but that history was superior with regard to its association to echocardiography. Regression analyses revealed a multitude of relationships. Details are given in Supplemental discussion 1.
In the present analysis, hyperinflation in terms of RV/TLC was associated with reduced LVEDD, and airway obstruction in terms of FEV1 with reduced LVEF (analytical details are discussed in the Supplement). One might speculate that RV/TLC more reflects a chronic, structural alteration and FEV1 more a dynamic alteration related to the pressure conditions in the chest. A reduction of left ventricular volume and mass has been described for lung hyperinflation or emphysema,41 while bronchodilation can improve pulmonary perfusion42 and left heart function.10
Remarkably enough and despite all the other links, a reduction in LVEDD was directly associated with increased symptoms, whereas a reduced LVEF was not (see Supplemental discussion 2). This also nicely illustrated the presence of both an indirect (via LVEDD) and direct effect of RV/TLC on COPD symptoms. The question to which extent a reduced cardiac forward volume, an impaired filling9 or increased wall stress are crucial for increased symptoms,8,32 is not yet answered and was not the topic of the present study. These findings were obtained, although in the majority of cases the measures were in the normal range of left ventricular diameters. Taken together, our results indicate that left heart disorders have a statistically significant although small effect on “Exertional COPD Symptoms”, even in patients with adequate medication, and that in particular LVEDD bears objective additional information. Although an impaired LVEF has been previously associated with reduced physical activity and psychological status in COPD,43 there was no such direct link to COPD symptoms in the present study (see Supplemental discussion 3). Nevertheless, the present findings reach beyond other studies that showed a limited impact of echocardiographic abnormalities on health status only.36 Limitations are discussed in the Supplement.
The results of the association analyses show that among standardized COPD scores the mMRC and the SGRQ activity component confer the highest chance to get a hint on a concomitant cardiac disorder. In addition, the findings illustrated in the descriptive Euler diagrams demonstrate that an echocardiographic examination focusing on left heart size and function could be informative in a significant proportion of patients with suspicious findings but no respective diagnosis or medication. There are several options for the assessment of cardiac function. Since the imaging quality of echocardiography may be limited in COPD, alternative methods such as cardiac magnetic resonance imaging or computed tomography could be used. Further procedures such as ischemic testing and assessment of coronary calcification could also be helpful to prevent underdiagnosis.44 The long-term follow-up of COSYCONET might reveal whether undetected cardiac abnormalities and lack of treatment have impact on the course of disease.
Conclusion
In a large observational COPD cohort, we found a remarkable proportion of patients with suspicious echocardiographic findings in whom cardiovascular disease was undiagnosed and untreated, implying an increased risk for an unfavorable prognosis. On the other hand, cardiovascular history was only partially linked to echocardiographic parameters. The effect of COPD was reflected in the dependence of cardiac size or function as well as symptoms on lung function. In addition, there was a small direct contribution of left ventricular size to COPD symptoms, irrespective of all other influencing factors. Specifically, size was inversely related to both exertional COPD symptoms and lung hyperinflation, thereby amplifying the effect of hyperinflation on symptoms. Although the mMRC and SGRQ activity component reflected clinical symptoms related to cardiac function, direct assessment of heart size and function is probably more reliable in the detection of concomitant cardiac disease in COPD.
Data sharing statement
COSYCONET is an ongoing long-term multi-center observational study and data are not intended to be freely available to the public. If, however, there is interest in analyzing specific questions, a formal request can be submitted to the study office, which will be evaluated by the steering committee on scientific grounds. There is no limitation except proven expertise in COPD studies.
Acknowledgments
This work was supported by the German Federal Ministry of Education and Research (BMBF) Competence Network Asthma and COPD (ASCONET) and performed in collaboration with the German Center for Lung Research (DZL). The project is funded by the BMBF with grant number 01 GI 0881, and is supported by unrestricted grants from AstraZeneca GmbH, Bayer Schering Pharma AG, Boehringer Ingelheim Pharma GmbH & Co. KG, Chiesi GmbH, GlaxoSmithKline, Grifols Deutschland GmbH, MSD Sharp & Dohme GmbH, Mundipharma GmbH, Novartis Deutschland GmbH, Pfizer Pharma GmbH, Takeda Pharma Vertrieb GmbH & Co. KG, and Teva GmbH for patient investigations and laboratory measurements. The funding bodies had no involvement in the design of the study, or the collection, analysis or interpretation of the data.
Disclosure
Peter Alter, Barbara A Mayerhofer, Kathrin Kahnert, Henrik Watz, Benjamin Waschki, Frank Biertz, and Rudolf A Jörres report no conflicts of interest in this work. Stefan Andreas report grants and personal fees from Boehringer Ing and Pfizer, and personal fees from Novartis, Astra Zeneca, GSK, Chiesi, and Merini, outside the submitted work. Robert Bals report grants from German Federal Ministry of Education and Research (BMBF) Competence Network Asthma and COPD (ASCONET), during the conduct of the study, and grants and personal fees from AstraZeneca, Novartis, and Boehringer Ingelheim, and personal fees from GlaxoSmithKline, Grifols, and CSL Behring, outside the submitted work. Claus F Vogelmeier report grants and personal fees from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Grifols, and Novartis, personal fees from CSL Behring, Chiesi, Menarini, Mundipharma, Teva, and Cipla, and grants from Bayer Schering Pharma AG, MSD, and Pfizer, outside the submitted work. The authors report no other conflicts of interest regarding this work.
References
1. Vanfleteren LE, Spruit MA, Groenen M, et al. Clusters of comorbidities based on validated objective measurements and systemic inflammation in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;187(7):728–735. doi:10.1164/rccm.201209-1665OC
2. Mullerova H, Agusti A, Erqou S, Mapel DW. Cardiovascular comorbidity in COPD: systematic literature review. Chest. 2013;144(4):1163–1178. doi:10.1378/chest.12-2847
3. Gershon AS, Mecredy GC, Guan J, Victor JC, Goldstein R, To T. Quantifying comorbidity in individuals with COPD: a population study. Eur Respir J. 2015;45(1):51–59. doi:10.1183/09031936.00061414
4. Greulich T, Weist BJD, Koczulla AR, et al. Prevalence of comorbidities in COPD patients by disease severity in a German population. Respir Med. 2017;132:132–138.
5. Triest FJ, Franssen FM, Spruit MA, Groenen MT, Wouters EF, Vanfleteren LE. Poor agreement between chart-based and objectively identified comorbidities of COPD. Eur Respir J. 2015;46(5):1492–1495.
6. Miller J, Edwards LD, Agusti A, et al. Comorbidity, systemic inflammation and outcomes in the ECLIPSE cohort. Respir Med. 2013;107(9):1376–1384.
7. Stone IS, Barnes NC, James WY, et al. Lung deflation and cardiovascular structure and function in chronic obstructive pulmonary disease. A randomized controlled trial. Am J Respir Crit Care Med. 2016;193(7):717–726.
8. Alter P, Jorres RA, Watz H, et al. Left ventricular volume and wall stress are linked to lung function impairment in COPD. Int J Cardiol. 2018;261:172–178.
9. Alter P, Watz H, Kahnert K, et al. Airway obstruction and lung hyperinflation in COPD are linked to an impaired left ventricular diastolic filling. Respir Med. 2018;137:14–22.
10. Hohlfeld JM, Vogel-Claussen J, Biller H, et al. Effect of lung deflation with indacaterol plus glycopyrronium on ventricular filling in patients with hyperinflation and COPD (CLAIM): a double-blind, randomised, crossover, placebo-controlled, single-centre trial. Lancet Respir Med. 2018;6(5):368–378. doi:10.1016/S2213-2600(18)30054-7
11. Hawkins NM, Huang Z, Pieper KS, et al. Chronic obstructive pulmonary disease is an independent predictor of death but not atherosclerotic events in patients with myocardial infarction: analysis of the Valsartan in Acute Myocardial Infarction Trial (VALIANT). Eur J Heart Fail. 2009;11(3):292–298. doi:10.1093/eurjhf/hfp001
12. Iversen KK, Kjaergaard J, Akkan D, et al. The prognostic importance of lung function in patients admitted with heart failure. Eur J Heart Fail. 2010;12(7):685–691. doi:10.1093/eurjhf/hfq050
13. Lawson CA, Mamas MA, Jones PW, et al. Association of medication intensity and stages of airflow limitation with the risk of hospitalization or death in patients with heart failure and chronic obstructive pulmonary disease. JAMA Network Open. 2018;1(8):e185489. doi:10.1001/jamanetworkopen.2018.5489
14. Sato Y, Yoshihisa A, Oikawa M, et al. Prognostic impact of chronic obstructive pulmonary disease on adverse prognosis in hospitalized heart failure patients with preserved ejection fraction - A report from the JASPER registry. J Cardiol. 2019;73(6):459–465. doi:10.1016/j.jjcc.2019.01.005
15. Lucke T, Herrera R, Wacker M, et al. Systematic analysis of self-reported comorbidities in large cohort studies - a novel stepwise approach by evaluation of medication. PLoS One. 2016;11(10):e0163408. doi:10.1371/journal.pone.0163408
16. Graf J, Lucke T, Herrera R, et al. Compatibility of medication with PRISCUS criteria and identification of drug interactions in a large cohort of patients with COPD. Pulm Pharmacol Ther. 2018;49:123–129. doi:10.1016/j.pupt.2018.01.011
17. Karch A, Vogelmeier C, Welte T, et al. The German COPD cohort COSYCONET: aims, methods and descriptive analysis of the study population at baseline. Respir Med. 2016;114:27–37. doi:10.1016/j.rmed.2016.03.008
18. Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary. Am J Respir Crit Care Med. 2017;195(5):557–582. doi:10.1164/rccm.201701-0218PP
19. Celli BR, Decramer M, Wedzicha JA, et al. An Official American Thoracic Society/European Respiratory Society Statement: research questions in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2015;191(7):e4–e27. doi:10.1164/rccm.201501-0044ST
20. Vogelmeier C, Buhl R, Criee CP, et al. [Guidelines for the diagnosis and therapy of COPD issued by Deutsche Atemwegsliga and Deutsche Gesellschaft fur Pneumologie und Beatmungsmedizin]. Pneumologie. 2007;61(5):e1–e40. doi:10.1055/s-2007-959200
21. Criee CP, Sorichter S, Smith HJ, et al. Body plethysmography–its principles and clinical use. Respir Med. 2011;105(7):959–971. doi:10.1016/j.rmed.2011.02.006
22. Criee CP, Baur X, Berdel D, et al. [Standardization of spirometry: 2015 update. Published by German Atemwegsliga, German Respiratory Society and German Society of Occupational and Environmental Medicine]. Pneumologie. 2015;69(3):147–164. doi:10.1055/s-0034-1391345
23. Vogelmeier C, Buhl R, Burghuber O, et al. [Guideline for the diagnosis and treatment of COPD patients - issued by the German Respiratory Society and the German Atemwegsliga in Cooperation with the Austrian Society of Pneumology]. Pneumologie. 2018;72(4):253–308. doi:10.1055/s-0043-125031
24. Quanjer PH, Tammeling GJ, Cotes JE, Pedersen OF, Peslin R, Yernault JC. Lung volumes and forced ventilatory flows. Eur Respir J. 1993;6(Suppl 16):5–40. doi:10.1183/09041950.005s1693
25. Quanjer PH, Stanojevic S, Cole TJ, et al. Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40(6):1324–1343. doi:10.1183/09031936.00080312
26. Stanojevic S, Graham BL, Cooper BG, et al. Official ERS technical standards: global lung function initiative reference values for the carbon monoxide transfer factor for Caucasians. Eur Respir J. 2017;50(3). doi:10.1183/13993003.00711-2017.
27. Fletcher CM, Elmes PC, Fairbairn AS, Wood CH. The significance of respiratory symptoms and the diagnosis of chronic bronchitis in a working population. Br Med J. 1959;2(5147):257–266. doi:10.1136/bmj.2.5147.257
28. Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self-complete measure of health status for chronic airflow limitation. The St. George’s respiratory questionnaire. Am Rev Respir Dis. 1992;145(6):1321–1327. doi:10.1164/ajrccm/145.6.1321
29. Fletcher CM. Standardised questionnaire on respiratory smyptoms: a statement prepared and approved by the MRC Committee on the Aetiology of Chronic Bronchitis (MRC breathlessness score). BMJ. 1960;2:1665.
30. Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD assessment test. Eur Respir J. 2009;34(3):648–654.
31. Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16(3):233–270.
32. Alter P, van de Sand K, Nell C, et al. Airflow limitation in COPD is associated with increased left ventricular wall stress in coincident heart failure. Respir Med. 2015;109(9):1131–1137.
33. Ponikowski P, Voors AA, Anker SD, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37(27):2129–2200.
34. Mathew T, Williams L, Navaratnam G, et al. Diagnosis and assessment of dilated cardiomyopathy: a guideline protocol from the British Society of Echocardiography. Echo Res Pract. 2017;4(2):G1–G13.
35. Micallef L, Rodgers P. eulerAPE: drawing area-proportional 3-Venn diagrams using ellipses. PLoS One. 2014;9(7):e101717.
36. Houben-Wilke S, Spruit MA, Uszko-Lencer N, et al. Echocardiographic abnormalities and their impact on health status in patients with COPD referred for pulmonary rehabilitation. Respirology. 2017;22(5):928–934.
37. Duffy S, Marron R, Voelker H, et al. Effect of beta-blockers on exacerbation rate and lung function in chronic obstructive pulmonary disease (COPD). Respir Res. 2017;18(1):124.
38. Kubota Y, Asai K, Furuse E, et al. Impact of beta-blocker selectivity on long-term outcomes in congestive heart failure patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2015;10:515–523.
39. Suissa S, Ernst P. Beta-blockers in COPD: a methodological review of the observational studies. COPD. 2018;15(5):520–525.
40. Fisher KA, Stefan MS, Darling C, Lessard D, Goldberg RJ. Impact of COPD on the mortality and treatment of patients hospitalized with acute decompensated heart failure: the Worcester heart failure study. Chest. 2015;147(3):637–645.
41. Barr RG, Bluemke DA, Ahmed FS, et al. Percent emphysema, airflow obstruction, and impaired left ventricular filling. N Engl J Med. 2010;362(3):217–227.
42. Vogel-Claussen J, Schonfeld CO, Kaireit TF, et al. Effect of Indacaterol/Glycopyrronium on Pulmonary Perfusion and Ventilation in Hyperinflated Patients with Chronic Obstructive Pulmonary Disease (CLAIM). A Double-Blind, Randomized, Crossover Trial. Am J Respir Crit Care Med. 2019;199(9):1086–1096.
43. Mesquita R, Franssen FM, Houben-Wilke S, et al. What is the impact of impaired left ventricular ejection fraction in COPD after adjusting for confounders? Int J Cardiol. 2016;225:365–370.
44. Williams MC, Murchison JT, Edwards LD, et al. Coronary artery calcification is increased in patients with COPD and associated with increased morbidity and mortality. Thorax. 2014;69(8):718–723.
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



