Back to Journals » Infection and Drug Resistance » Volume 14
Prevalence of Bacterial Urinary Tract Infection and Antimicrobial Susceptibility Patterns Among Diabetes Mellitus Patients Attending Zewditu Memorial Hospital, Addis Ababa, Ethiopia
Authors Yenehun Worku G, Belete Alamneh Y, Erku Abegaz W
Received 31 December 2020
Accepted for publication 25 March 2021
Published 15 April 2021 Volume 2021:14 Pages 1441—1454
DOI https://doi.org/10.2147/IDR.S298176
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Professor Suresh Antony
Gebremdhin Yenehun Worku,1 Yerega Belete Alamneh,2 Woldaregay Erku Abegaz3
1Department of Microbiology, Addis Ababa Public Health Research and Emergency Management Directorate, Addis Ababa, Ethiopia; 2Department of Microbiology, Immunology and Parasitology, St. Paul’s Hospital Millennium Medical College, Addis Ababa, Ethiopia; 3Department of Microbiology, Immunology and Parasitology, Faculty of Medicine, Addis Ababa University, Addis Ababa, Ethiopia
Correspondence: Yerega Belete Alamneh Tel +251920735677
Email [email protected]
Background: Urinary tract infection (UTI) is caused by colonization and growth of microorganisms within the urinary system. Diabetic patients are more prone to bacterial UTI due to impaired host defense and high glucose concentration in urine. Surveillance of uropathogens and their antibiogram is a key to patient management.
Methods: A hospital-based cross-sectional study was conducted from May to July, 2018. Urine samples were collected for culture and identification based on the standard protocol. An antimicrobial susceptibility test (AST) was done for all isolates using the Kirby–Bauer disk diffusion method. Data were entered into Epi-data version 3.2.1 and exported to the Statistical Package for the Social Science (SPSS) version 20.
Results: Out of 225 participants, significant bacteriuria was reported in 9.8% of the cultures. Five species of bacteria were isolated and E. coli (63.6%) was the leading uropathogen, followed by K. pneumoniae (13.6%). Duration of diabetes, previous history of UTIs and symptomatic UTI were found to be strongly associated with significant bacteriuria. Gram-negative bacterial isolates showed high sensitivity to nitrofurantoin and meropenem (100%). In contrast, a high level of resistance to ampicillin, doxycycline and cefuroxime (100%) and to amoxicillin-clavulanate (94.4%) was observed. Gram-positive bacteria showed high level of resistance to penicillin (100%). Multiple-drug resistance (MDR) was high for Gram-negative bacteria (100%).
Conclusion: Previous history of UTIs and duration of diabetes were found to be important factors that increase the prevalence of UTI among diabetes patients. This study also showed a high prevalence of drug resistance to doxycycline, amoxicillin-clavulanate, cefuroxime and penicillin for both Gram-negative and Gram-positive bacteria. Since therapeutic selection for empirical treatment and management should be based on knowledge of the local bacterial profile and antimicrobial response, we suggest physicians take this high resistance profile in to consideration when prescribing antimicrobials against the pathogens in question.
Keywords: UTI, antibiotic susceptibility, diabetes mellitus, significant bacteriuria
Background
Urinary tract infection is caused by colonization and growth of microorganisms such as bacteria, fungi and viruses within the urinary tract (UT).1,2 However, UTI due to virus and fungi has a low incidence.1 The primary etiological agents of UTIs are Gram-negative bacteria; however, Gram-positive bacteria may also be involved in UT infections.3,4 The common uropathogens are Escherichia coli, Staphylococcus saprophyticus, Klebsiella pneumoniae, Proteus mirabilis, Enterococcus species, Pseudomonas aeruginosa1,2,5–7 and group B streptococcus.2 The most common cause of UTI in men and women with and without DM is E. coli. Some reports have noted that a lower proportion of UTIs is caused by this organism in diabetic patients as compared with age-matched non-diabetic patients.8
Under normal circumstances, the UT is resistant to long-term colonization and growth of microorganisms.9 This resistance emanates from various physiological processes, one of which is emptying out urine that flushes out harmful microbes that temporarily colonize urine in the bladder.3,5,9,10 Additionally, innate immunity such as cytokines, chemokines,3,5 secretory immunoglobulin,5,9 mucous production, prostatic secretion, barrier formation and high concentration of urea3 prevent persistent microbial colonization and infection of the UT. Nevertheless, structural and functional abnormality that blocks urine flow11 and other risk factors that damage host immunity may lead to UTI.6 When such damage occur, the UT can be struck by uropathogens like Escherichia coli, Klebsiella pneumoniae and Proteus mirabilis, which possess virulent factors that enable them to colonize urinary epithelial cells.10
Due to their anatomic and physiologic nature, UTI is more common in women than men.1,12 Nearly half of all women have been affected by this infection at least once in their lifetime.13 Other important risk factors that enhance UTI include: diabetes mellitus (DM), hypertension, allergies, catheterization, use of diaphragms, birth control pills and spermicidal agents, age, delays in micturition, abuse of antibiotics and other immune suppressive conditions.6
Chronic diseases are emerging as one of the leading causes of worldwide morbidity and mortality. Among these, DM is one of the dominant non-communicable, chronic and endocrine diseases.14,15 According to estimates from the 2017 International Diabetics Federation, 451 million (8.4%) people, globally, between 18–99 years of age were living with DM and 5 million people aged 20–79 years of age died due to this disease. However, the number of people with DM is projected to rise to 693 million (9.9%) by 2045. From the African region, in 2017, Ethiopia had the highest number of people (2.6 million) with diabetes, with a 5.2% national prevalence.16 Similarly, a higher prevalence of DM was also found in a retrospective study between January 2010 and December 2013 in Addis Ababa.14
Host immune system abnormalities due to DM such as impaired migration, chemotaxis, phagocytosis and intracellular killing potential of polymorphonuclear cells, local complications related to neuropathy like impaired bladder emptying and higher glucose concentration of urine in diabetic patients enhance UTI. Patients with DM have a higher prevalence of asymptomatic bacteriuria (ASB) and a higher incidence of symptomatic UTIs, which more often lead to complications compared with those without DM.17 It appears that the increased prevalence of urinary tract infections in diabetic patients is not the result of a difference in the bacteria but is due to changes in the uro-epithelial cells leading to an increased adherence of E. coli expressing type 1 fimbriae. Hypothetically, these changes are in the glycosylation of the infected cells.18 Research shows that UTI is more common,19–21 severe and produces more serious outcomes in patients with DM.22–24
The increased risk of UTI among diabetic patients, coupled with the increase in the incidence of DM worldwide in recent years, may impose a substantial burden on medical costs.23 In addition, the high rates of antibiotic prescription, including broad-spectrum antibiotics, for UTI in this group of patients may further induce the development of antibiotic-resistant urinary pathogens25. Moreover, the inappropriate use of antibiotics often results in increased resistance of UT pathogens to the most commonly used antimicrobial drugs.26 Appropriate antibiotic use in patients with a complicated UTI seems to reduce length of hospital stay and therefore has a favorable effect on patient outcomes and health care costs.27
Studies regarding UTI in DM patients are limited in Ethiopia, particularly in the study area. Therefore, this study was designed to determine bacterial profile and antimicrobial susceptibility patterns among DM patients attending Zewditu Memorial Hospital (ZMH) in Addis Ababa, Ethiopia, with the aim of generating reliable information on which to base treatment of diabetic patients with UTIs in this area.
Methods and Materials
Study Setting
The study was conducted from May to July, 2018 at Zewditu Memorial Hospital, Addis Ababa, Ethiopia. Addis Ababa is the capital city of Ethiopia. According to the 2007 census report,28 Addis Ababa city had a total population of 2,738,248, with a growth rate of 2.1%. Based on this figure, the central statistical agency of Ethiopia estimated that the population of Addis Ababa was projected to reach around 3.95 million in 2018. ZMH is one of the biggest hospitals in Ethiopia and is located in central Addis Ababa. It was built, owned and operated by the Seventh-Day Adventist Church, but was later nationalized during the Derg regime in 1976. It provides all-round health care services and is the leading hospital providing antiretroviral treatment (ART) and addressing other chronic diseases, including DM.
Study Design and Participants
A hospital-based cross-sectional study was conducted to determine the prevalence of urinary tract bacterial infection and antimicrobial resistance patterns of bacterial uropathogens among diabetes mellitus patients at Zewditu Memorial Hospital, Addis Ababa, Ethiopia.
Source Population
The source population was all diabetic patients visiting ZMH.
Study Population
Diabetic patients who came for follow-up at ZMH were included.
Inclusion Criteria
The study involved DM patients above 18 years and those who came to ZMH for follow-up during the study period and were willing to participate.
Exclusion Criteria
DM patients who had been taking antibacterial drugs for the previous two weeks, pregnant DM women and DM patients previously exposed to catheterization were excluded from the study.
Operational Definition
Asymptomatic bacteriuria: the presence of significant bacteriuria (≥105cfu/mL) in urine culture without signs and symptoms of urinary tract infection.
MDR: non-susceptibility to at least one agent in three or more antimicrobial categories.
Symptomatic bacteriuria: the presence of significant bacteria in urine culture (≥105cfu/mL) accompanied by at least two complaints of UTI symptoms such as dysuria, urgency for urination, frequent urination, suprapubic pain, flank pain, fever and chills.
Sample Size and Sampling Technique
Sample size was calculated by applying the single population proportion formula, n=(Zα/2)2 × p (1−p)/d2), where, n = sample size, z = statistic for a level of confidence, d = margin of error, and p = expected or proportional prevalence, 95% confidence level with a margin of error of 5% and 17.8% prevalence from a previous study29 conducted at Gondar University Hospital. The calculation resulted in 225 samples. Therefore, 225 study participants were selected using a systematic random sampling technique via the lottery method from DM patients’ follow-up records, and data and urine sample were collected when they came for follow-up during the study period. Hospital DM follow-up records showed that the total DM patients attending ZMH for treatment follow-up during the sample collection period was 750.
Data Collection and Laboratory Methods
Clinical Examination
After obtaining an informed consent from the patients, socio-demographic data and clinical data were collected from patient using pre-structured questioners by nurses.
Specimen Collection
A freshly voided midstream urine sample (10–20 mL) was collected in a wide-mouthed sterile, dry and leak-proof container after instructing the enrolled DM patients to clean their genitals with soap and water.4
Culturing and Identification Procedure
Urine specimens obtained from DM patients were inoculated onto MacConkey agar (HKM, China) and Blood agar (Biomark, India)30 using a calibrated loop (0.001mL). Cultures were incubated in an aerobic atmosphere at 37°C for 24 hours. A positive urine culture was defined as a colony count of ≥105CFU/mL for midstream urine.31 A Stuart scientific colony counter was used for counting. All positive cultures were further identified by their colony characteristics and Gram staining. Further identification was done using different biochemical tests, including catalase, mannitol salt agar and PYR test for Gram-positive bacteria and mannitol utilization, hydrogen sulphide production (H2S), indole production, citrate utilization, lysine iron agar test, gas production, hydrolysis of urea, and motility tests and carbohydrate metabolism for Gram-negative bacteria.4
Antimicrobial Susceptibility Testing
Antimicrobial susceptibility testing was done on Mueller–Hinton agar (Oxoid, England) using the Kirby–Bauer disk diffusion method based on Clinical and Laboratory Standard Institute (CLSI) guidelines.32 When pure culture was obtained, a loopful of bacteria (3–5 pure colonies) was taken and emulsified in 5mL sterile normal saline and mixed gently until it formed a homogenous suspension. Then, the turbidity of the suspension was adjusted to the density of a McFarland 0.5 standard (Mary-l’Etoil, France). A sterile cotton swab was dipped into the suspension, and excess suspension was removed by gentle rotation of the swab against the surface of the tube in order to standardize the inoculum size. The swab was then used to distribute the bacteria evenly over the entire surface of Mueller–Hinton agar (Oxoid, England). The inoculated plates were left at room temperature to dry for 3–5 minutes. Next, selected antimicrobial disks were placed on the plate by a disk dispenser and incubated at 35°C–37°C for 16–18 hours.4,30 The antimicrobial agents tested with respective concentrations were: nitrofurantoin (30 µg), ciprofloxacin (15 µg), doxycycline (30µg), ampicillin (10µg), vancomycin (30µg), co-trimoxazole (25µg), gentamycin (10µg), cefoxitin (30µg), penicillin (10 units), meropenem (10µg), ceftazidime (30µg), cefuroxime (5µg), cefepime (30µg), and amoxicillin-clavulanate (30µg). These antimicrobial agents were selected based on recommended drugs for treatment of UTIs from CLSI guidelines32 and Ethiopian hospital treatment guidelines, 2014. Diameters of the zone of inhibition around the disks were measured using a digital caliper. The result was interpreted as sensitive, intermediate and resistant based on CLSI guidelines.30 Multiple-drug resistance is defined as non-susceptibility of tested bacteria to at least one antimicrobial agent in three or more antimicrobial categories.33 Positive results from urine culture and antimicrobial sensitivity test results were reported to the attending physicians for subsequent treatment and follow-up.
Quality Assurance
Completeness of the questionnaires was properly checked by applying a pre-test before the actual data collection. Collection and examination of the specimens were done following the standard operating procedures (SOPs) for urinalysis, culture and antimicrobial susceptibility patterns. Proper specimen labeling and matching with respective identification numbers was checked. Sterility and performance of culture media was tested prior to the actual work. Sterility of media was checked by incubating overnight at 37°C. In addition, E. coli (ATCC 25922), K. pneumoniae (ATCC 700603), P. mirabilis (ATCC 35699) and S. aureus (ATCC 25923) were used as reference strains. Training was given for data collectors, who were all nurses.
Data Analysis and Interpretation
The data were cleaned, edited and entered onto Epi-data version 3.2.1 and analyzed using SPSS version 20 statistical software for further analysis. Different variables were described and characterized by frequency distribution. Binary and multivariate logistic regressions were used and a p-value of less than or equal to 0.05 with a 95% confidence interval was considered to test statistically significant associations.
Results
Socio-Demographic Characteristics
A total of 225 diabetic patients with and without symptoms of UTI were investigated during the study period. Of these, 75 (33.3%) were males and 150 (66.7%) were females. The age of the patients ranged from 20 to 80 years, with a mean age of 45.52 years and a standard deviation (SD) of ±14.079. The majority of participants 158 (70.2%) were married. All study participants were from an urban area and the majority (206 (91.6%)) had type II diabetes mellitus. Nearly half of the study participants earned a monthly income of 1651–5250 Ethiopian birr. About 80% of the study participants were literate (Table 1).
 |
Table 1 Socio-Demographic Characteristics of Diabetes Mellitus Patients Investigated for Urinary Tract Infection at Zewditu Memorial Hospital, Addis Ababa, Ethiopia, May to July, 2018 |
Clinical Features of Urinary Tract Infection
Symptoms suggestive of UTI were observed in 97 (43.11%) of the study subjects. The most frequently observed complaints were flank/loin pain, which was observed among 90 (40%), frequent urination, 80 (35.56%), and urgent urination, 66 (29.3%). Fever, dysuria, suprapubic pain, nausea and vomiting were also observed among 52 (23.2%), 42 (18.7%), 38 (16.9%), 26 (11.6%) and 5 (2.2%) participants, respectively (Table 2).
 |
Table 2 Frequency of Symptoms Suggestive of UTI in Diabetes Mellitus Patients in Zewditu Memorial Hospital, Addis Ababa, Ethiopia, May to July, 2018 |
Culture Results
Significant bacteriuria was observed in 22 of 225 (9.8%) urine samples cultured. Of these, 15 (68.2%) were from symptomatic and 7 (37.8%) were from asymptomatic UTI DM patients. Out of s total 22 positives, 3 (13.6%) were from males and 19 (86.4%) from females. Bivariate logistic regression analysis determined significant bacteriuria to be strongly associated with duration of diabetes, previous UTIs and current symptoms of UTI (p <0.05). The results of the study indicate that the chance of getting a UTI among DM patients who have had diabetes for more than 10 years was more than four times greater (COR; 4.364 [95% CI, 1.637–11.629]) than among DM patients who have had diabetes for 10 years or less. Similarly, DM patients with previous UTIs had a 4.709 times greater chance of developing a UTI (COR; 4.709 [95% CI; 1.873–11.842]) than those patients without previous UTIs; and those patients with a symptomatic UTI were at three times greater risk of having significant bacteriuria than asymptomatic patients (COR; 3.162 [95% CI, 1.235–8.094]).
However, other factors like gender, educational status, DM type, marital status, frequency of previous UTIs and fasting blood glucose levels were not statistically associated with significant bacteriuria. The odds of getting significant bacteriuria were not statistically significant between age groups (p >0.05), as significant bacterial isolates were distributed in all age groups of the study participants. However, the highest (20.0%) and the lowest (4.7%) prevalent significant bacteriuria were observed among the age groups ≥64 years and 50–64 years, respectively. Regarding marital status, the highest (14.3%) significant bacteriuria was isolated from single DM patients but this association was not statically significant (p >0.050). Similarly, 19/22 (86.4%) of the bacteria were isolated from female diabetics. Even though the risk of getting a UTI was higher (3.481 times) in female patients than in male patients, no statistically significant association was observed between gender and significant bacteriuria, albeit the failure was marginal (p = 0.051).
In multivariate logistic regression analysis, duration of diabetes and previous UTIs were significantly statistically associated with significant bacteriuria, with adjusted odds ratios (AORs) of (95% CI) 3.477 [1.266–9.554] and 3.645 [1.403–9.473], respectively. Symptomatic condition was not shown to have a significant association with AOR (95% CI) 2.354 [0.881, 6.294] (Table 3).
Bacterial Etiologies
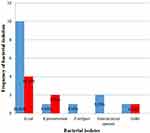
A total of 22 bacterial uropathogens were isolated. Of these, 4 (18.2%) were Gram-positive bacteria and 18 (81.8%) were Gram-negative bacteria. E. coli was the most frequently isolated uropathogen [14 (63.6%)] followed by K. pneumoniae [3 (13.7%)] and P. rettgeri [1 (4.5%)]. Among Gram-positive bacteria, only Enterococcus spp. [2 (9.1%)] and coagulase negative staphylococcus (CoNs) [2 (9.1%)] were isolated (Figure 1).
Antimicrobial Susceptibility Testing
The antimicrobial susceptibility test was performed on all culture-positive urine samples using the disk diffusion method. Gram-negative bacterial isolates (n=18) were tested against 12 antibiotics while Gram-positive isolates were tested against 9 antibiotics (Tables 4 and 5, respectively). Sensitivity to Gram-negative isolates was highest for meropenem, with a rate of 18/18 (100%), nitrofurantoin 18/18 (100%) and gentamicin 16/18 (88.9%), whereas the highest level of resistance was observed for doxycycline, with a rate of 18/18 (100%), ampicillin 18/18 (100%), cefuroxime 18/18 (100%) and amoxicillin-clavulanate 17 (94.4%). E. coli, the most frequently isolated bacteria, was highly resistant to doxycycline (100%), ampicillin (100%) and amoxicillin-clavulanate (92.9%) (Table 4). Overall, Gram-positive bacterial isolates (n = 4) showed 100% sensitivity to six of the tested antibiotics, whereas a high level of resistance was observed to penicillin (100%) (Table 5).
Multiple Drug Resistance Patterns
The frequency of MDR was found in all Gram-negative bacteria (100%), whereas none of the Gram-positive bacteria showed MDR (Table 6).
Discussion
In the present study, the overall prevalence of UTIs in both symptomatic and asymptomatic diabetic patients was 9.8%. This rate is comparable to the rates reported from other studies in Ethiopia, such as in Debre Tabor (10.9%),20 Hawassa (13.8%)34 and Addis Ababa (10.9%)35), and in India (12.2%)36 and Romania (10.7%).37 However, much higher prevalence rates were reported in Bahir Dar (30.5%)38 and Arbaminch (33.8%)39 in Ethiopia, and in Sudan (19.5%),40 India (22%),21 Pakistan 52.76%),41 Egypt (52.2%)19 and Nepal (54.25%).42 Inclusion of asymptomatic DM patients and exclusion of catheterized DM patients, which is a known determinant factor that may increase the prevalence of UTIs,19,42 could be reasons for detecting a lower prevalence of UTIs in our study compared to these other reports.
In the present study, 22 bacterial uropathogens belonging to five species were isolated. Gram-negative bacteria were more prevalent (81.8%) than Gram-positive bacteria (18.2%), as has been the case in most studies conducted elsewhere in the world. Our finding of high Gram-negative bacterial isolates in DM patients is in concordance with reports from Mekelle (83%),43 India (92%)44 and Sudan (87.2%).40 However, relatively lower prevalence rates were reported from similar studies in Ethiopia, such as in Debre Tabor (41.9%)20 and Bahir Dar (61.9%).45
Generally, in the present study (63.6%) and in most other studies conducted elsewhere, such as Pakistan (60.0%),24 India (67.6%),46 Dessie (63%),47 Sudan (54.6%)40 and Romania (68.9%),37 E. coli was the most frequently isolated uropathogen. This dominance of E. coli among UTI patients may not be surprising since it is the commonest flora of the gastrointestinal tract and bowel from which it ascends to the urinary tract and uses its well characterized virulence factors to colonize the urinary tract. The second leading bacterial isolate in our study was K. pneumoniae (13.7%), as was true for reports from Bahir Dar,38 Dessie,47 Sudan40 and Nigeria.22 In contrast, Proteus species, Pseudomonas species and CoNs were reported to be the second most abundant bacterial isolates from UTI in studies in Addis Ababa,48 Bahir Dar49 and Pakistan,24 respectively.
In the present study, small proportions of Enterococcus spp. and CoNs were isolated, each with a rate of 9.1% among the overall bacterial isolates. The 9.1% prevalence rate for Enterococcus spp. was also reported in Sudan.40 However, S. aureus and S. saprophyticus in Debre Tabor,20 S. saprophyticus in Bahir Dar,49 S. aureus in Arbaminch39 and CoNs in Dessie47 were the leading Gram-positive isolates, showing variations in the dominance of UTI bacterial isolates among the different geographic locations in Ethiopia.
In the present study, duration of DM was shown to be an important risk factor for UTI development (p = 0.003), as evidenced by the proportion of significant bacteriuria being observed more in those with a diabetic history of >10 years (17.2%) than in those who were diabetic for only <10 years (4.5%). This is likely to be because of the progressive nature of diabetes that may damage the genitourinary system (neuropathy) leading to a dysfunctional bladder thereby creating micturition abnormality, a condition important for the developments of UTIs.19,50 In fact, previous studies from Egypt19 and India51 reached the same conclusion, that the longer the duration of DM, the higher the rate of UTIs observed. However, some studies from Iran52 and Addis Ababa26 reported no statistically significant association between duration of diabetes and UTIs.
Another identified risk factor associated with significant bacteriuria in this study was previous history of UTIs. Sixty-nine of the study participants had a history of previous UTIs. However, 14 of the 22 (63.63%) participants with significant bacteriuria were among those who had a previous history of UTIs. In fact, the likelihood of developing significant bacteriuria was 4.709 times greater among participants with a history of UTIs than those without (p <0.05). Other in-country studies have reported the same trend of a higher rate of significant bacteriuria among those with a previous history of UTIs.26,29,53 However, two studies, from Hawassa34 and Arbaminch,39 reported a contradictory finding, whereby they found a statistically significant association of significant bacteriuria among DM patients with no history of UTIs. Possibly these lstudies introduced real biases during recruitment of participants.
Only 97/225 (43.1%) of the participants in our study had symptoms of UTI. However, the majority (15/22; 68%) of participants with significant bacteriuria were those with symptoms (p <0.05). This is not unexpected, as was the case in other studies,26,35 because long duration of DM and delayed medical intervention are likely to result in renal defects that, in turn, lead to occurrence of UTI symptoms. In contrast, our finding disagrees with the studies conducted in Hawassa,34 Sudan40 and Italy,8 where symptomatic UTI had no significant association with significant bacteriuria. The difference might be due to respondent bias in accurately describing the symptoms characterizing symptomatic UTI.
Of the three variables described above that were found in bivariate analysis to have had significant associations with significant bacteriuria in this study, only previous history of UTIs and current symptoms of UTI were found by multivariate analysis to be persistently associated with significant bacteriuria. Surprisingly, however, this finding contradicted reports from Sudan,40 whereby duration of diabetes and previous history of UTIs had no significant association with significant bacteriuria in multivariate analysis. Moreover, in reports of other studies in Ethiopia, multivariate analysis showed previous history of UTIs to have had statistically significant associations with significant culture positive bacteria in diabetic patients.34,53 However, other variables, such as gender in Harar53 and educational status in Hawassa,34 were equally important in this association but were not even significant by bivariate analysis in our study.
Regarding antimicrobial sensitivity test results, the Gram-negative uropathogens were highly sensitive to meropenem (100%), nitrofurantoin (100%) and gentamicin (88.9%). The latter two antimicrobials were also shown previously to be highly effective against Gram-negative bacteria with sensitivities of 95.5% and 73.3% for nitrofurantoin and gentamicin, respectively, in Dessie, Ethiopia.47 A 100% sensitivity to nitrofurantoin was also recorded from a study in Arbaminch39 and Gondar.29
In contrast, high level resistance was exhibited by the Gram-negative isolates against a number of tested antimicrobials that are commonly used against bacterial UTI: ampicillin (100%), doxycycline (100%), cefuroxime (100%), amoxicillin-clavulanate (94.4%), co-trimoxazole (72.2%) and ciprofloxacin (61.1%). The most disturbing observation in this connection is that all Gram-negative isolates had shown resistance to more than at least one antimicrobial in three classes tested (100% MDR level). Such high levels of resistance among uropathogens against these same antimicrobials seems widespread both in Ethiopia (eg 91.4% against ampicillin and 79.2% against amoxicillin-clavulanate,38 100% against co-tromoxazole, greater than 75% against amoxicillin-clavulanate,53 and between 60% to 100% against ampicillin29,34–36) and elsewhere in the world.36 Still more studies have confirmed that detection of high MDR among Gram-negative uropathogens is common both in Ethiopia and elsewhere.20,34,47,49,53 The remarkably higher prevalence of resistance, including MDR, against these commonly prescribed antibiotics may be due to: their wider availability and ease of access outside treatment centers and, thus, indiscriminate use of the drugs without prescription;54 fake drugs, sub-standard or expired drugs circulating that are likely to be used for self-treatment; frequent use of broad-spectrum antibiotics as prophylactics; and lack of laboratory tests for both AST and pathogen identification.4
E. coli, the leading uropathogen in the present study, was 100% sensitive to meropenem and nitrofurantoin, and 87.5% to gentamicin. In the contrast, a very high level of resistance was observed to doxycycline (100%), ampicillin (100%), cefuroxime (100%) and amoxicillin-clavulanate (92.9%). Such high sensitivity of E. coli to the former two antibiotics was also reported previously from Sudan,40 where it was 100% and 86.3% sensitive to gentamicin and nitrofurantoin, respectively; and in Ethiopia, where it was observed to be 100% sensitive to nitrofurantoin in Addis Ababa26 and Hawassa.34 However, this pathogen has been found to exhibit very high resistance (100%) to ampicillin, which is also supported by the reports from Hawassa (100%)34 and Bahir Dar,49 Iran (86.6%).55 In contrast, in our study the 92.2%, 100% and 100% resistance of E. coli to amoxicillin-clavulanate, ampicillin and doxycycline, respectively, disagrees with the report from Sudan,40 in that amoxicillin-clavulanate (sensitivity rate, 90.9%), ampicillin (sensitivity rate, 72.7%) and, in Tehran,56 doxycycline (sensitivity rate, 100%) were presented as effective drugs. The deterioration in the effectiveness of amoxicillin-clavulanate and ampicillin against E. coli from UTIs is a matter of concern, given the facts that the primary etiology of UTI infection for DM patients is this uropathogen and these antimicrobials are among the most commonly used drugs in Ethiopia.
In the present study, the overall percentage of antimicrobial sensitivity of Gram-positive bacterial isolates to the majority of the tested antibiotics was high. For instance, Enterococcus spp. were 100% sensitive to nitrofurantoin, ampicillin and vancomycin. Previous studies from Ethiopia and tSudan20,29,40 also reported this high sensitivity rate for the former two antibiotics. Similarly, CoNs were highly sensitive (100%) to six of the tested antimicrobials, namely, nitrofurantoin, ciprofloxacin, co-trimoxazole, gentamicin, cefoxitin and doxycycline, which was also observed in previous studies in Ethiopia, where 100% sensitivity was reported to doxycycline,20 gentamicin34 and ampicillin.40
Mixed results were observed in regard to resistance among the two Gram-positive bacterial species isolated, Enterococcus spp. and CoNs. On the one hand, Enterococcus isolates were 100% resistant to doxycycline, but CoNs were 100% sensitive to this drug. On the other hand, resistance to ciprofloxacin was 50% for Enterococcus spp. while it was 0% to CoNs. Moreover, CoNs isolates were 100% resistant to penicillin, as was the case in other studies elsewhere.34,53 Overall, Gram-positive isolates presented better options for empiric treatment than Gram-negative isolates since they were 100% sensitive to the majority of tested antimicrobial drugs except penicillin and doxycycline resistance for CoNs and Enterococci, respectively.
Conclusion
Significant bacteriuria was obtained from 9.8% of participants, and Escherichia coli (63.6%) was the leading uropathogen. Presence of previous urinary tract infections and duration of diabetes were found to be important factors responsible for increased prevalence of laboratory-confirmed urinary tract infection among the diabetes patients. Nitrofurantoin and gentamicin were effective against both Gram-positive and Gram-negative bacterial uropathogens in the current study, which may be used for empirical therapy when urine culture is unavailable. This study also showed a high prevalence of drug resistance to common antimicrobials, particularly to co-trimoxazole, ciprofloxacin, doxycycline, ampicillin, amoxicillin-clavulanate, cefuroxime and penicillin. The prevalence of MDR was also high for Gram-negative bacteria. Therefore, cautious use of antibiotic therapy and immediate treatment of urinary tract infections in DM patients is mandatory. Moreover, therapeutic selection for empirical treatment and management should be based on knowledge of the local bacterial profile and antimicrobial response, and there should be continuous monitoring and review of antimicrobial policy in hospitals and the country at large.
Limitations of the Study
The present study did not include non-diabetic patients as a control group, which makes it difficult to indicate how much DM may increase the prevalence of UTIs compared with non-DM patients. In addition, the present study did not address UTIs caused by bacterial pathogens that are difficult to culture in the ordinary culture media and anaerobic bacterial pathogens which might change the prevalence of UTIs.
Abbreviations
AAU, Addis Ababa University; ATCC, American type culture collection; ASB, asymptomatic bacteriuria; CLSI, Clinical Laboratory Standard Institute; CoNs, coagulase negative staphylococci; COR, crude odds ratio; DMIP, Department of Microbiology, Immunology and Parasitology; DM, diabetes mellitus; MSU, midstream urine; NDM, non-diabetes mellitus; PYR, pyrrolidonyl acrylamides, SPSS, Statistical Package for the Social Science; SB, symptomatic bacteriuria; UTI, urinary tract infection; WHO, World Health Organization; ZMH, Zewditu Memorial Hospital.
Data Sharing Statement
Data is available upon request.
Ethics Approval and Consent
Ethical clearance was obtained from the Research and Ethical Review Committee and was approved by the Department of Microbiology, Immunology and Parasitology, School of Medicine, Addis Ababa University. Ethical clearance was also secured from Addis Ababa Public Health Research and Emergency Management Directorate. Official permission was also obtained from ZMH. In addition, written informed consent was obtained from the study participants before the initiation of data collection and all participants were informed about the purpose of the study, and told that it was conducted in accordance with the Declaration of Helsinki. The individual results of investigations remained confidential. The laboratory findings of the study participants were communicated to the responsible health professional assigned to the diabetic clinic for the purpose of managing the cases accordingly.
Consent for Publication
Not applicable. This study does not contain any individual or personal data.
Acknowledgments
The authors would like to thank Addis Ababa University, Department of Microbiology, Immunology and Parasitology, Faculty of Medicine for sponsoring the research. Our special thanks and appreciation go to the staff members of Zewditu Memorial Hospital, the medical director, physicians, nurses and laboratory staff working in the diabetic clinic for their support in screening of symptomatic and asymptomatic study participants and organizing the preconditions of sample and data collection. Our deepest thanks also go to the Addis Ababa Public Health Research and Emergency Management Directorate for their unreserved material and reagent supply that made this study possible. We also extend our profound gratitude to the study participants for their willingness to engage, without whom this research would not have been possible.
Author Contributions
All authors made a significant contribution to the work reported, whether that was in the conception, study design, execution, acquisition of data, analysis and interpretation or in all of these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; agreed on the journal to which the article was submitted; and agree to be accountable for all aspects of the work.
Funding
This study was sponsored by Addis Ababa University, Addis Ababa, Ethiopia.
Disclosure
The authors declare that they have no conflicts of interest for this work.
References
1. Vasudevan R. Urinary tract infection: an overview of the infection and the associated risk factors. J Microbiol Exp. 2014;1(2):00008.
2. Flores-Mireles AL, Walker JN, Caparon M, Hultgren SJ. Urinary tract infections: epidemiology, mechanisms of infection and treatment options. Nat Rev Microbiol. 2015;13(5):269–284. doi:10.1038/nrmicro3432
3. Fauci AS. Harrison’s Principles of Internal Medicine. Vol. 2. McGraw-Hill, Medical Publishing Division New York; 2008.
4. Cheesbrough M. District Laboratory Practice in Tropical Countries, Part 2. Cambridge University Press; 2006:464.
5. Moura A, Nicolau A, Hooton T, Azeredo J. Antibiotherapy and pathogenesis of uncomplicated UTI: difficult relationships. J Appl Microbiol. 2009;106(6):1779–1791. doi:10.1111/j.1365-2672.2008.04115.x
6. Jatileni NJV, Maposa I, Mavenyengwa RT. A Retrospective Study of the variability in etiological agents of urinary tract infections among patients in Windhoek-Namibia. Open J Med Microbiol. 2015;05(04):184. doi:10.4236/ojmm.2015.54023
7. Odoki M, Bazira J, Agwu E. Health-point survey of bacteria urinary tract infections among suspected diabetic patients attending clinics in Bushenyi district of Uganda. Spec Bacterial Pathog J. 2015;1(1):1–5.
8. Bonadio M, Costarelli S, Morelli G, Tartaglia T. The influence of diabetes mellitus on the spectrum of uropathogens and the antimicrobial resistance in elderly adult patients with urinary tract infection. BMC Infect Dis. 2006;6(1):54. doi:10.1186/1471-2334-6-54
9. Hanson LA, Fasth A, Jodal U, Kaijser B, Svanborg Edén C. Biology and pathology of urinary tract infections. J Clin Pathol. 1981;34(7):695–700. doi:10.1136/jcp.34.7.695
10. Sussman M, Gally DL. The biology of cystitis: host and bacterial factors. Annu Rev Med. 1999;50(1):149–158. doi:10.1146/annurev.med.50.1.149
11. Hickling DR, Sun -T-T, Wu X-R. Anatomy and physiology of the urinary tract: relation to host defense and microbial infection. In: Mulvey MA, Klumpp DJ, Stapleton AE, editors. Urinary Tract Infections. John Wiley & Sons; 2017:1–25.
12. Hooton TM. Pathogenesis of urinary tract infections: an update. J Antimicrob Chemother. 2000;46(suppl_1):1–7. doi:10.1093/jac/46.suppl_1.1
13. François M, Hanslik T, Dervaux B, et al. The economic burden of urinary tract infections in women visiting general practices in France: a cross-sectional survey. BMC Health Serv Res. 2016;16(1):365. doi:10.1186/s12913-016-1620-2
14. Gizaw M, Harries AD, Ade S, et al. Diabetes mellitus in Addis Ababa, Ethiopia: admissions, complications and outcomes in a large referral hospital. Public Health Action. 2015;5(1):74–78. doi:10.5588/pha.14.0107
15. Bloom DE, Cafiero E, Jané-Llopis E, et al. The Global Economic Burden of Non-communicable Diseases. PGDA Working Papers. Program on the Global Demography of Aging; January, 2012 [cited June 3, 2020]. Report No.: 8712.
16. Zibaeenezhad MJ, Sayadi M, Mohammadi SS, et al. Clinical outcomes after percutaneous coronary intervention in patients with and without history of diabetes mellitus with different stent size; 2020
17. Geerlings SE. Urinary tract infections in patients with diabetes mellitus: epidemiology, pathogenesis and treatment. Int J Antimicrob Agents. 2008;31:54–57. doi:10.1016/j.ijantimicag.2007.07.042
18. Taganna J, de Boer AR, Wuhrer M, Bouckaert J. Glycosylation changes as important factors for the susceptibility to urinary tract infection. Biochem Soc Trans. 2011;39(1):349–354. doi:10.1042/BST0390349
19. El-Nagar MMG-A, Abd El-Salam AE-D, Gabr HM, Abd El EE-DM. Prevalence of urinary tract infection in Damietta diabetic patients. Menoufia Med J. 2015;28(2):559. doi:10.4103/1110-2098.163918
20. Worku S, Derbie A, Sinishaw MA, Adem Y, Biadglegne F. Prevalence of bacteriuria and antimicrobial susceptibility patterns among diabetic and nondiabetic patients attending at Debre Tabor Hospital, Northwest Ethiopia. Int J Microbiol. 2017;2017:1–8. doi:10.1155/2017/5809494
21. Sharma E, Thakuriya R. Clinical profile of urinary tract in diabetes. Int J Recent Trends Sci Technol. 2015;15(3):448–450.
22. Abubakar E-M-M. Antimicrobial susceptibility pattern of pathogenic bacteria causing urinary tract infections at the Specialist Hospital, Yola, Adamawa state, Nigeria. JCMR. 2009;1(1):1–8.
23. Yu S, Fu AZ, Qiu Y, et al. Disease burden of urinary tract infections among type 2 diabetes mellitus patients in the U.S. J Diabetes Complications. 2014;28(5):621–626. doi:10.1016/j.jdiacomp.2014.03.012
24. Kumar R, Kumar R, Perswani P, Taimur M, Shah A, Shaukat F. Clinical and microbiological profile of urinary tract infections in diabetic versus non-diabetic individuals. Cureus. 2020;11(8).
25. Nitzan O, Elias M, Chazan B, Saliba W. Urinary tract infections in patients with type 2 diabetes mellitus: review of prevalence, diagnosis, and management. Diabetes Metab Syndr Obes. 2015;8:129–136. doi:10.2147/DMSO.S51792
26. Woldemariam HK, Geleta DA, Tulu KD, et al. Common uropathogens and their antibiotic susceptibility pattern among diabetic patients. BMC Infect Dis. 2019;19(1):43. doi:10.1186/s12879-018-3669-5
27. Spoorenberg V, Hulscher MEJL, Akkermans RP, Prins JM, Geerlings SE. Appropriate antibiotic use for patients with urinary tract infections reduces length of hospital stay. Clin Infect Dis. 2014;58(2):164–169. doi:10.1093/cid/cit688
28. Schmidt E, Kedir M Urbanization and spatial connectivity in Ethiopia: urban growth analysis using GIS [Internet]. Africa Portal. International Food Policy Research Institute. 2009 [cited 2020 Jun 3].
29. Yismaw G, Asrat D, Woldeamanuel Y, Unakal CG. Urinary tract infection: bacterial etiologies, drug resistance profile and associated risk factors in diabetic patients attending Gondar University Hospital, Gondar, Ethiopia. Eur J Exp Biol. 2012;2(4):889–898.
30. Vandepitte J, Engbaek K, Rohner P, Piot P, Heuck CC; Organization WH. Basic laboratory procedures in clinical bacteriology/J. Vandepitte … [et al.]. Basic Lab Proced Clin Bacteriol. 2003.
31. Graham JC, Galloway A. ACP Best Practice No 167: the laboratory diagnosis of urinary tract infection. J Clin Pathol. 2001;54(12):911–919. doi:10.1136/jcp.54.12.911
32. Wayne PA Clinical and laboratory standards institute. Performance standards for antimicrobial susceptibility testing; 2011.
33. Magiorakos A-P, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268–281. doi:10.1111/j.1469-0691.2011.03570.x
34. Nigussie D, Amsalu A. Prevalence of uropathogen and their antibiotic resistance pattern among diabetic patients. Turk J Urol. 2017;43(1):85–92. doi:10.5152/tud.2016.86155
35. Yeshitela B, Gebre-Selassie S, Feleke Y. Asymptomatic bacteriuria and symptomatic urinary tract infections (UTI) in patients with diabetes mellitus in Tikur Anbessa Specialized University Hospital, Addis Ababa, Ethiopia. Ethiop Med J. 2012;50(3):239–249.
36. Senthamarai S, Sivasankari S, Anitha C, et al. A study on clinical presentation, bacterial profile and its antibiotic sensitivity pattern in urinary tract infections among diabetic patients attending tertiary care hospital. Tamilnadu Infect. 2016;2:4.
37. Chiţă T, Licker M, Sima A, et al. Prevalence of urinary tract infections in diabetic patients. Rom J Diabetes Nutr Metab Dis. 2013;20(2):99–105. doi:10.2478/rjdnmd-2013-0012
38. Derbie A, Hailu D, Mekonnen D, Abera B, Yitayew G. Antibiogram profile of uropathogens isolated at Bahir Dar Regional Health Research Laboratory Centre, Northwest Ethiopia. Pan Afr Med J. 2017;26. doi:10.11604/pamj.2017.26.134.7827
39. Mama M, Manilal A, Gezmu T, Kidanewold A, Gosa F, Gebresilasie A. Prevalence and associated factors of urinary tract infections among diabetic patients in Arba Minch Hospital, Arba Minch province, South Ethiopia. Turk J Urol. 2019;45(1):56–62. doi:10.5152/tud.2018.32855
40. Hamdan HZ, Kubbara E, Adam AM, Hassan OS, Suliman SO, Adam I. Urinary tract infections and antimicrobial sensitivity among diabetic patients at Khartoum, Sudan. Ann Clin Microbiol Antimicrob. 2015;14(1). doi:10.1186/s12941-015-0082-4
41. Zubair KU, Shah AH, Fawwad A, Sabir R, Butt A. Frequency of urinary tract infection and antibiotic sensitivity of uropathogens in patients with diabetes. Pak J Med Sci. 2019;35(6):1664–1668. doi:10.12669/pjms.35.6.115
42. Kumar Jha P, Baral R, Khanal B. Prevalence of uropathogens in diabetic patients and their susceptibility pattern at a tertiary care center in Nepal-a retrospective study. Int J Bio Lab Sci. 2014;3:29–34.
43. Tesfahunegn Z, Asrat D, Woldeamanuel Y, Estifanos K. Bacteriology of surgical site and catheter related urinary tract infections among patients admitted in Mekelle Hospital, Mekelle, Tigray, Ethiopia. Ethiop Med J. 2009;47(2):117–127.
44. Akram M, Shahid M, Khan AU. Etiology and antibiotic resistance patterns of community-acquired urinary tract infections in J N M C Hospital Aligarh, India. Ann Clin Microbiol Antimicrob. 2007;6(1):4. doi:10.1186/1476-0711-6-4
45. Melaku S, Kibret M, Abera B, Gebre-Sellassie S. Antibiogram of nosocomial urinary tract infections in Felege Hiwot referral hospital, Ethiopia. Afr Health Sci. 2012;12(2):134–139. doi:10.4314/ahs.v12i2.9
46. Sharma N, Gupta A, Walia G, Bakhshi R. Pattern of antimicrobial resistance of Escherichia coli isolates from urinary tract infection patients: a three year retrospective study. J Appl Pharm Sci. 2016;6(01):062–065. doi:10.7324/JAPS.2016.600110
47. Kibret M, Abera B. Prevalence and antibiogram of bacterial isolates from urinary tract infections at Dessie Health Research Laboratory, Ethiopia. Asian Pac J Trop Biomed. 2014;4(2):164–168. doi:10.1016/S2221-1691(14)60226-4
48. Mamuye Y. Antibiotic resistance patterns of common Gram-negative uropathogens in St. Paul’s Hospital Millennium Medical College. Ethiop J Health Sci. 2016;26(2):93–100. doi:10.4314/ejhs.v26i2.2
49. Belete Y, Asrat D, Woldeamanuel Y, Yihenew G, Gize A. Bacterial profile and antibiotic susceptibility pattern of urinary tract infection among children attending Felege Hiwot Referral Hospital, Bahir Dar, Northwest Ethiopia. Infect Drug Resist. 2019;12:3575–3583. doi:10.2147/IDR.S217574
50. Fu AZ, Iglay K, Qiu Y, Engel S, Shankar R, Brodovicz K. Risk characterization for urinary tract infections in subjects with newly diagnosed type 2 diabetes. J Diabetes Complications. 2014;28(6):805–810. doi:10.1016/j.jdiacomp.2014.06.009
51. Janifer J, Geethalakshmi S, Satyavani K, Viswanathan V. Prevalence of lower urinary tract infection in South Indian type 2 diabetic subjects. Indian J Nephrol. 2009;19(3):107. doi:10.4103/0971-4065.57107
52. Raoofi A, Ghavami M, Shahhamzeh M, Ghasemi M, Hedartabar R, Salehi L. The impact of demographic factors and blood sugar control on the incidence of urinary tract infections in Khorramabad in 2013. Iran Red Crescent Med J. 2016;18(5). doi:10.5812/ircmj.21942
53. Abate D, Kabew G, Urgessa F, Meaza D. Bacterial etiologies, antimicrobial susceptibility patterns and associated risk factors of urinary tract infection among diabetic patients attending diabetic clinics in Harar, Eastern Ethiopia. East Afr J Health Biomed Sci. 2017;1(2):11–20.
54. Damtew E Prevalence of Diabetes Mellitus Among Active Pulmonary Tuberculosis Patients at St. Peter Specialized Hospital, Addis Ababa Ethiopia [Internet] [Thesis]. Addis Ababa University; 2014 [cited June 3, 2020].
55. Ranjbar R, Nazari S, Farahani O. Phylogenetic analysis and antimicrobial resistance profiles of Escherichia coli strains isolated from UTI-suspected patients. Iran J Public Health. 2020;49(9):1743–1749. doi:10.18502/ijph.v49i9.4094
56. Raeispour M, Ranjbar R. Antibiotic resistance, virulence factors and genotyping of Uropathogenic Escherichia coli strains. Antimicrob Resist Infect Control. 2018;7(1):118. doi:10.1186/s13756-018-0411-4
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.