Back to Journals » Journal of Multidisciplinary Healthcare » Volume 11
Prevalence of antigliadin IgA antibodies in psoriasis vulgaris and response of seropositive patients to a gluten-free diet
Authors Kolchak NA, Tetarnikova MK, Theodoropoulou MS, Michalopoulou AP, Theodoropoulos DS
Received 13 September 2016
Accepted for publication 15 December 2016
Published 27 December 2017 Volume 2018:11 Pages 13—19
DOI https://doi.org/10.2147/JMDH.S122256
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Nikolai A Kolchak,1 Maria K Tetarnikova,2 Maria S Theodoropoulou,3 Alexandra P Michalopoulou,4 Demetrios S Theodoropoulos5
1Department of Hematology, Omsk State Medical Academy, Omsk, Russia; 2Dermatology Private Practice, Chelyabinsk, Russia; 3Department of Pharmacy, Trikala General Hospital, Trikala, Greece; 4Department of Philosophy and Social Studies, School of Philosophy, University of Crete, Rethymnon, Greece; 5Allergy Associates of La Crosse, Onalaska, WI, USA
Introduction: The course of psoriasis relies on a variety of metabolic and immunological parameters. Identification of underlying pro-inflammatory conditions and their control is desired for optimal management.
Background: Increased prevalence of serum markers for celiac disease has been reported among patients with psoriasis. The likelihood of occult celiac disease in a subpopulation of patients has been postulated and gluten-free diets have been reported to be effective.
Patients and methods: The prevalence of gliadin IgA antibodies was assessed among patients with psoriasis in an urban population. The clinical effects of a strict gluten-free diet were followed.
Results: Over a 2-year period, 97 patients with Psoriasis Area and Severity Index greater than 2.4 were recruited from a population followed in a dermatology clinic. Gliadin IgA antibodies were assessed in all participants and in 91 controls. Elevated gliadin IgA antibodies were found in 13 patients (14%) and two controls (2%). Values in five patients were assessed as greater than 30.0 U/mL or “strong positive” according to the manufacturer of the assay. All 13 patients were placed on a strict gluten-free diet without any other modifications in their ongoing treatment of psoriasis. Improvement of psoriatic lesions was observed in all patients with positive gliadin IgA antibodies but the decline in the Psoriasis Area and Severity Index score and the scaling down of pharmaceutical treatment was more pronounced in the five patients with strong positive gliadin IgA indicating an immune aberration amenable to diet changes.
Conclusion: Prevalence of antigliadin IgA antibody is significant among patients with psoriasis not diagnosed with celiac disease or non-celiac gluten sensitivity. For all its limitations, antigliadin IgA testing can identify patients likely to benefit from gluten-free diets.
Keywords: psoriasis, celiac disease, antigliadin antibodies
Introduction
Psoriasis vulgaris is a chronic immune disease of the skin, the course of which can be modified by a number of underlying, co-morbid or simply co-existing parameters. Some of them are endocrine (metabolic syndrome), psychological (anxiety), infectious (Streptococcal), pharmaceutical (beta blockers), dietary (vitamin A), and life-style related (insufficient sun exposure), but the involvement of other contributors is also likely given the fine balance of the immune responses which determine the onset and course of psoriasis.1,2
Because of the impact that gluten has on dietary, life-style, and chronic ill-health and the potential to affect the course of psoriasis, a possible association of gluten-related pathology with psoriasis has been hypothesized. Among Scandinavian patients with psoriasis, prevalence of serum antibodies to gliadin has been reported to be as high as 16%.3 This rate appears significant in view of generally accepted prevalence rates of antigliadin seropositivity in Scandinavian general population studies of approximately 1%.4,5 A study, however, of patients from a more diverse racial background in Michigan, USA, has not confirmed this finding and did not show increased prevalence of serum antigliadin in psoriasis.6 Given the relatively high prevalence of celiac disease and seropositivity for celiac disease markers in Scandinavian populations, it is possible that this discrepancy in the prevalence of antigliadin antibodies may simply reflect gene frequencies that may be population specific.
While the prevalence of serological markers for celiac disease, both in general populations and among patients with psoriasis, remains to be determined, the association of the two diseases has been proposed in a small number of patients with either subclinical celiac disease or with “non-celiac gluten sensitivity”. Using anti-tissue transglutaminase IgA antibodies as a marker for detection of celiac disease, a large, multicenter, primary care-based study found a 4% prevalence of seropositivity among patients with psoriasis and no previous diagnosis of celiac disease. The diagnosis of gastrointestinal celiac disease was later confirmed by pathology in all of them.7 Independently, however, of an eventual diagnosis of celiac disease, the detection of patients with suitable serological profile is worthwhile as definite histological proof of celiac disease may not be necessary for improvement of psoriasis to occur. It is now increasingly recognized that gluten sensitivity, unrelated to IgE-mediated allergy and unrelated to celiac disease, can be screened for by antigliadin testing.8,9 Indeed, gluten-free diets (GFDs) in antigliadin seropositive psoriatic patients have been rewarding even though not all of the studied patients had histologically diagnosed celiac disease.10
The present study is seeking to establish: i) the prevalence of serum gliadin IgA antibody titer (antigliadin IgA antibody [AGA]) in the urban population of an industrialized area with negligible Scandinavian or Celtic representation and presumed low prevalence of celiac disease; ii) the prevalence of elevated AGA in patients with psoriasis in the same population; iii) the impact that GFD may have in psoriatic patients who lack gastrointestinal, dermatological or other features of celiac disease; and iv) the usefulness of quantitative analysis of AGA in selecting patients most likely to benefit from a GFD.
For the purposes of this study, it was thought that clinical relevance of positive AGA would be best served by its detection in a population characterized by both low prevalence of celiac disease and low prevalence of celiac disease markers in general, and it was for this reason that a Central Russian city was chosen for the study. The prevalence of celiac disease in Russia is, in itself, a special issue and is reported at rates lower than those in Scandinavian or Western European literature even though larger studies would be needed to establish this fact.11,12
It is understood that prevalence studies addressing the association of psoriasis with serological markers for celiac disease may vary in their outcomes. The current understanding, however, of celiac disease-related immune aberrations is likely to expand. Not only does celiac disease appear to encompass a spectrum of highly varying and poorly demarcated disorders – many of which may not even involve the gastrointestinal tract at all – its immune profile is quite unusual too as it exhibits both Th1 and Th2 features in fairly balanced representations.13 Psoriasis, on the other hand, is an autoimmune disease which has been previously reported to be susceptible to immune modulation addressed to concurrent inflammatory conditions originally unrelated to psoriasis.14 These considerations make it likely that, even in the absence of a pathology-based association of the two diseases, select psoriatic patients may benefit from GFD.
Ethics statement
The study conforms to the principles of the Declaration of Helsinki and the standard National Institutes of Health Office of Extramural Research recommendations. The project was granted approval by the Chelyabinsk Oblast State Health and Social Welfare Department, Division of Clinical Research. Written informed consent was obtained from all participants. No children were included in the study.
Patients and methods
Patients with psoriasis vulgaris were recruited over a 2-year period from a central tertiary care dermatology clinic servicing the greater area of Chelyabinsk, Russia (population 1,130,000). Patients with psoriatic arthritis were excluded. The Psoriasis Area and Severity Index (PASI) was used to assess severity and extent of psoriasis, and patients with a PASI score below 2.4 at the onset of the study were excluded. Patients with diagnosed celiac disease, dermatitis herpetiformis, chronic lower gastrointestinal symptoms, anemia, chronic fatigue or “foggy brain” at the time of the study were also excluded. Also, patients with IgA deficiency were excluded.
AGA was chosen as a single screening test for celiac disease because of significantly lower cost as compared to tissue transglutaminase antibody assays. Antigliadin levels were assessed by the EliA IgA GliadinDP 250 Method of Phadia. According to the manufacturer, patients were considered positive if AGA was greater than 11.4 U/mL (99th percentile).15 Patients with positive AGA were placed on a strict GFD. All patients were followed regularly every 3 to 4 months, and evaluation of outcome for the purposes of the study was at 12 months since recruitment.
Control samples for AGA were obtained from volunteer blood donors matched for age range and gender distribution. Control subjects with psoriasis of any form were excluded. All other exclusion criteria were the same as for patients with psoriasis. Data analysis was performed with the SPSS 13.0 statistical software package (SPSS Inc., Chicago, IL, USA). Categorical data were analyzed with χ2 test. For continuous data, one-way analysis of variance was used.
Results
Over the 2-year period of the study, 97 patients with PASI score >2.4 were identified who fulfilled the recruitment criteria. Ninety-one matching controls were recruited through blood donation programs in the same greater area. Participants’ demographics are presented in Table 1.
  | Table 1 Demographic characteristics of patients and controls Note: ‘–’ indicates not applicable. |
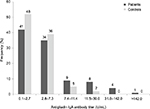
Serum antigliadin IgA levels are shown in Figure 1. The mean, by the manufacturer’s standards, is 2.7 U/mL and the 95th percentile is 3.2 U/mL. Clearly negative results are below 7.4 U/mL. Forty-one patients (42%) had negative AGA below the manufacturer’s established mean value, and 34 (35%) above the mean but below 7.4 U/mL. Consistent distribution results were obtained for controls with 48 (52%) in the negative range below the mean, and 36 (39%) in the negative and above mean.
Nine patients (9%) and five controls (5%) had AGA in the equivocal range 7.4–11.4 U/mL.
Among patients with psoriasis, 13 (14%) tested positive, that is, they had AGA concentrations greater than 11.4 U/mL. Five of them had “strong positive” AGA, that is, values greater than 30.0 U/mL, one having AGA beyond measurable range, that is, greater than 142.0 U/mL. Two controls tested positive at 14.8 U/mL and 17.3 U/mL.
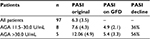
All seropositive patients were offered counseling with a dietitian. Thereafter, they were placed on a GFD and followed regularly. At 12 months past recruitment, PASI scores had declined universally but the effect of the GFD was more noticeable in the five patients with “strongly positive” AGA as opposed to the eight patients with AGA in the 11.5–30.0 U/mL range (Table 2). It is noted that a distinct difference in baseline PASI scores had existed between the “strong positive” and “positive” groups: the group with AGA IgA >30.0 U/mL averaged a PASI score almost double the one of those who presented with AGA IgA in the range 11.5–30.0 U/mL.
The individual characteristics of the five patients with “strong positive” AGA are shown in Table 3. Declining AGA levels were observed, which, at 12 months of follow-up, turned negative or equivocal for four of five patients, indicating both an underlying gluten-related pathology and satisfactory compliance with GFD. Individual PASI score decline rates varied from 43% to 74%. Decreased use or discontinuation of methotrexate (Dava Pharmaceuticals Inc., Fort Lee, NJ, USA) was noted, and one patient was able to discontinue treatment with cyclosporine. Treatment with psoralen plus ultraviolet-A light therapy was discontinued in two out of three patients with “strong positive” AGA. Individual PASI scores for AGA positive subjects and their changes at 4-month intervals over a 12-month period are shown in Figure 2A (strong positive) and B (moderate positive).
Discussion
Embarking on a GFD is a cumbersome undertaking which cannot be recommended without realistic prospects for a substantial gain. As far as the prevalence of serological markers for celiac disease in psoriasis is concerned, the findings of the present study are consistent with previously published works.3–5 They stand at variance, however, with those of others.6 This study presents the prevalence of AGA in Russian patients with psoriasis and absent manifestations of either celiac disease or non-celiac gluten enteropathy, and the response to GFD of patients with high-end AGA. This is the first time a population which is celiac disease-free/gluten sensitivity-free is screened and patients selected for GFD. Given the feasibility and low cost of this approach, a management plan involving early introduction of GFD for certain patients with psoriasis could be considered as a routine measure.
Routine testing for celiac disease in psoriasis is hereby supported independently of history of gastrointestinal symptoms or other evidence suggestive of celiac disease since the positive yield rate for such testing in this study exceeds 10% and may be as high as 19% according to other authors.3 In the present work, a discrimination point was used to separate distinctly high AGA IgA from other positive results. The cut-off limit of 30 U/mL set by the manufacturer for a “strong positive” result may be arbitrary and was obviously never meant to consider the pathology of psoriasis but, in this study, proved helpful in identifying patients more likely than others to benefit from GFD. The establishment of selection criteria for GFD lies beyond the scope of this study, and it is quite likely that the improvement rates in PASI scores in the “strong positive” versus the “positive” group of patients may reflect a selection process that was too rigorous. Yet the underlying psoriasis pathology was clearly responsive to dietary manipulation. Obviously, in order to establish the full effect of GFD on AGA-positive patients with psoriasis, double blind, placebo controlled studies with adequate numbers of participants will be needed. It is not clear how or whether levels of AGA correlate with severity of celiac disease or how faithfully they reflect its activity. Larger studies could possibly find a benefit from GFD applied to patients with lower levels of AGA than those employed in this study, and, possibly, even benefits from a less strict diet. Hereby it is demonstrated that, in patients with AGA in the strong positive range (AGA >30 U/mL), GFD is a meaningful therapeutic trial. These observations are consistent with the seminal observations of Michaëlsson et al.3,10 The need for stratification of screening results and a method to select patients most likely to benefit from long-term diet restrictions is supported.
The sensitivity and specificity of AGA are suboptimal for the diagnosis of celiac disease.16–18 The sensitivity of AGA is considered too low for a single marker.16 In a small one-center study, the sensitivity of AGA in diagnosing celiac disease was reported as 75%, and only as 7% for the diagnosis of non-celiac gluten sensitivity. Other markers, namely, deamidated gliadin peptide antibodies, IgA tissue transglutaminase and IgA endomysial antibodies were far more sensitive than AGA in celiac disease but not satisfactory in the diagnosis of non-celiac gluten sensitivity.17 IgA deficiency also complicates sensitivity issues since it may eventually turn out to be more prevalent among patients with celiac disease than in controls.19 In an attempt to establish the minimum required testing for diagnosis of celiac disease, Swedish authors have argued for the use of a two-marker test, IgA and IgG tissue transglutaminase antibodies, which are reported to have a sensitivity of 96% and specificity of 99.5%.18 There is no question that combined IgA and IgG tissue transglutaminase antibody testing would yield diagnostic results superior to those of single AGA for the diagnosis of celiac disease, but their use in non-celiac gluten sensitivity remains to be assessed. Regarding clinical management of large numbers of patients, containment of cost, and the implementation of a simple single screening test remain top priorities especially in the context of a disease (psoriasis) whose mostly adult patients can safely undergo a very demanding but brief and highly rewarding therapeutic trial of a GFD.
Whether an etiological relation is present between gluten exposure and development of psoriasis in a susceptible subpopulation remains unclear. The two diseases are not likely to share any part of an underlying pathology as far as deduced from their cytokine profiles2,13 Independent of an association with celiac disease, gluten is often postulated as a trigger involved in the expression of auto-immune disorders but again, literature on this subject is scanty. The concept of “Chronic Systemic Inflammation” has emerged as a model that may answer some of the constantly emerging questions on similarities, pathological processes, and therapeutic approaches that are shared by conditions as diverse from each other as cancer, metabolic conditions, and chronic neuropathic pain syndromes. Inflammatory bowel conditions and psoriasis figure prominently in this spectrum making it possible that, in spite of the findings presented here, psoriasis may, after all, have no specific association with either celiac disease or gluten sensitivity except in the context of a constitutional destabilization of the immune system.20 Chronic immune aberrant responses in psoriasis are so pronounced that they may lead to macroscopic organ alterations, as was recently shown in a study which demonstrated that duration of psoriasis in years was inversely correlated with spleen size in a fashion independent of body mass index. In the same study, non-alcoholic hepatic steatosis and other co-morbid systemic conditions were addressed as likely associations of psoriasis.21 The common denominator of so many dissimilar conditions with an inflammatory background is often hypothesized to be lack of regulation due to defective IL-10 production/function.22
Regarding celiac disease, none of the 13 patients or the two controls with positive AGA who are presented here had ever had any evidence of celiac disease prior to this study or during its course. After assessment of AGA, endoscopy-biopsy was offered before initiation of GFD in order to prove the diagnosis of occult celiac disease, but was generally declined and, therefore, the diagnosis of celiac disease was neither confirmed nor ruled out. Similarly, none of the patients or the controls with high AGA had dermatitis herpetiformis. The complete absence of clinical manifestations of celiac disease is certainly intriguing but by no means uncommon in positive test outcomes even when multiple and/or highly specific celiac disease markers are used. The serological evidence of celiac disease, however, which is provided by a positive AGA titer is not negligible.15 After incidental identification of positive AGA IgA, there are no data to allow a safe prediction of clinical course regarding the eventual development of celiac disease or other celiac disease-related pathology.
The prevalence of AGA in patients with psoriasis is clearly elevated when compared to the general population. Response of AGA positive psoriasis to GFD is favorable and may be more rewarding for the select group of patients with higher AGA. This finding indicates that: i) elevation of AGA in these patients is of a specific nature and not a mere result of polyclonal activation and ii) changes in AGA levels over time may be of value in monitoring disease activity. A cause-and-effect model, however, is hard to prove. After all, patients improved significantly but no one became asymptomatic during GFDs. It is possible that, since psoriasis involves a large number of parameters affecting immune integrity and function, removal of one inflammatory stimulus – in this case gluten – in some patients with concurrent occult celiac disease (or a celiac disease-spectrum immune disorder) can lead to improved control of autoimmune aberrations. These patients may be as many as 13% of patients with psoriasis vulgaris according to the present study, or possibly more under more relaxed selection criteria. As with other parameters operative in the course of psoriasis, identification of susceptible subjects and selection for individualized specific intervention may significantly alter management and outcome.7 With this study, along with the benefits of diet changes in selected patients with psoriasis, the highly pleiotropic nature of celiac-related pathology is underlined.
Disclosure
The authors report no conflicts of interest in this work.
References
Griffiths CE, Barker JN. Pathogenesis and clinical features of psoriasis. Lancet. 2007;370(9583): 263–271. | ||
Schlaak JF, Buslau M, Jochum W, et al. T cells involved in psoriasis vulgaris belong to the Th1 subset. J Invest Dermatol. 1994;102(2):145–149. | ||
Michaëlsson G, Gerdén B, Ottosson M, Parra A, Sjoeberg O, Hjelmquist G, Lőőf L. Patients with psoriasis often have increased serum levels of IgA antibodies to gliadin. Br J Dermatol. 1993;129(6):667–673. | ||
Ludwigsson JF, Card TR, Kaukinen K, Bai J, Zingone F, Sanders DS, Murray JA. Screening for celiac disease in the general population and in high-risk groups. United European Gastroenterol J. 2015;3(2):106–120. | ||
Mårild K, Kahrs CR, Tapia G, Stene LC, Størdal K. Infections and risks of celiac disease in childhood: a prospective nationwide cohort study. Am J Gastroenterol. 2015;110(10):1475–1484. | ||
Kia KF, Nair RP, Ike RW, Hiremagalore R, Elder JT, Ellis CN. Prevalence of antigliadin antibodies in patients with psoriasis is not elevated compared with controls. Am J Clin Dermatol. 2007;8(5):301–305. | ||
De Bastiani R, Gabrielli M, Lora L, et al. Association between coeliac disease and psoriasis: Italian primary care multicenter study. Dermatology. 2015;230(2):156–160. | ||
Bardella MT, Elli L, Ferretti F. Non celiac gluten sensitivity. Curr Gastroenterol Rep. 2016;18(12):63. | ||
Mansueto P, Seidita A, D’Alcamo A, Carroccio A. Non-celiac gluten sensitivity: literature review. J Am Coll Nutr. 2014;33(1):39–54. | ||
Michaëlsson G, Gerdén B, Hagforsen E, et al. Psoriasis patients with antibodies to gliadin can be improved by a gluten-free diet. Br J Dermatol. 2000;142(1):44–51. | ||
Repin AA, Bogdarin IuA, Sarantsev BV, Teplov OV, Desiatnikova NV, Parfenov AI. [Prevalence of celiac disease in risk group patients in Nizhny Novgorod region]. Ter Arkh. 2008;80(2):38–44. Russian. | ||
Bykova SV, Sabelnikova EA, Gudkova RB, et al. [Celiac disease detection rate in gastroenterological patients]. Ter Arkh. 2016;88(2):39–43. Russian. | ||
Björck S, Lindehammer SR, Fex M, Agardh D. Serum cytokine pattern in young children with screening detected coeliac disease. Clin Exp Immunol. 2015;179(2):230–235. | ||
Theodoropoulos DS, Morris MS, Morris DL. Sustained improvement of psoriatic lesions in the course of sublingual immunotherapy for airborne allergens; clinical evidence of cross-tolerance. Eur Rev Med Pharmacol Sci. 2015;19(3):392–395. | ||
EliA GliadinDP IgA Fluororenzyme Immunoassay for Antigliadin Antibodies. Directions for use. Phadia GmbH, Freiburg, Germany, 2011. Available from: http://www.phadia.com/en/Products/Autoimmunity-testing-products/Celiac-Disease-Other-Gastrointestinal-Diseases/. Accessed December 07, 2017. | ||
Infantino M, Manfredi M, Meacci F, et al. Diagnostic accuracy of anti-gliadin antibodies in Non Celiac Gluten Sensitivity (NCGS) patients: a dual statistical approach. Clin Chim Acta. 2015;451(Pt B):135–141. | ||
Volta U, Tovoli F, Cicola R, et al. Serological tests in gluten sensitivity (nonceliac gluten intolerance). J Clin Gastroenterol. 2012;46(8):680–685. | ||
Dahlbom I, Nyberg BI, Berntson L, Hansson T. Simultaneous detection of IgA and IgG antibodies against tissue transglutaminase: the preferred pre-biopsy test in childhood celiac disease. Scand J Clin Lab Invest. 2016;76(3):208–216. | ||
Pallav K, Xu H, Leffler DA, Kabbani T, Kelly CP. Immunoglobulin A deficiency in celiac disease in the United States. J Gastroenterol Hepatol. 2016;31(1):133–137. | ||
Uluçkan Ő, Wagner EF. Chronic systemic inflammation originating from epithelial tissues. FEBS J. 2017;284(4):505–516. | ||
Balato N, Napolitano M, Ayala F, Patruno C, Megna M, Tarantino G. Nonalcoholic fatty liver disease, spleen and psoriasis: New aspects of low-grade chronic inflammation. World J Gastroenterol. 2015;21(22):6892–6897. | ||
Asadullah K, Sterry W, Stephanek K, et al. IL-10 is a key cytokine in psoriasis. Proof of principle by IL-10 therapy: a new therapeutic approach. J Clin Invest. 1998;101(4):783–794. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.